Abstract
Background
Fibroblast growth factor (FGF) signaling controls self renewal of neural stem cells during embryonic telencephalic development. FGF receptor 2 (FGFR2) has a significant role in the production of cortical neurons during embryogenesis, but its role in the hippocampus during development and in adulthood has not been described.
Methods
Here we dissociate the role of FGFR2 in the hippocampus during development and during adulthood with the use of embryonic knock-out and inducible knock-out mice.
Results
Embryonic knock-out of FGFR2 causes a reduction of hippocampal volume and impairment in adult spatial memory in mice. Spatial reference memory, as assessed by performance on the water maze probe trial, was correlated with reduced hippocampal parvalbumin+ cells, whereas short term learning was correlated with reduction in immature neurons in the dentate gyrus. Furthermore, short term learning and newly generated neurons in the dentate gyrus were deficient even when FGFR2 was lacking only in adulthood.
Conclusions
Taken together, these findings support a dual role for FGFR2 in hippocampal short term learning and long-term reference memory, which appear to depend upon the abundance of two separate cellular components, parvalbumin interneurons and newly generated granule cells in the hippocampus.
Keywords: Fgf receptor 2, hippocampus, parvalbumin, neurogenesis, learning, memory
Introduction
Fibroblast growth factor (FGF) signaling promotes forebrain cell survival and regulates dorsoventral patterning at pre-neurogenic stages (1–3). Globally reduced FGF signaling prematurely increases neurogenesis, resulting in an early depletion of stem cells and a profound decrease in cortical surface area and neuron number (4). Throughout neurogenesis, FGF receptors promote self-renewal of radial glial cells, expanding the stem cell population and ultimately increasing neuron production (5, 6). The selective knockout of fgf receptor 2 (fgfr2) produces a more selective defect of cortical neuron number in medial frontal regions. Without FGFR2, proliferating stem cells of the embryonic ventricular zone are less likely to re-enter the cell cycle, decreasing production of excitatory projection neurons in medial prefrontal cortex (PFC) (6, 7).
Like PFC, the hippocampus has been implicated in multiple neuropsychiatric disorders (7). Alterations in hippocampal development are thought to contribute to schizophrenia (8) and decreases in hippocampal volume have been found in youth at risk for depression prior to any clinical presentation (9). Significant changes in hippocampal volume (10–14), gene expression (15–20) and hippocampal-dependent learning (21, 22) have been shown in schizophrenia and mood disorders. In patients with schizophrenia and major depression, impairments in learning and memory have been associated with poor hippocampal functioning (23–26).
In rodents, the hippocampus has been implicated in several forms of cognition: spatial memory (27), context-dependent fear conditioning (28), and trace conditioning (29). While it is clear that postnatal neurogenesis persisting in the hippocampal DG plays a role in learning and memory, what type of learning and memory processes it mediates are yet to be explicated, as studies in which neurogenesis is inhibited by ablative techniques or genetic knockouts have given inconsistent results (30, 31).
FGF signaling has also been implicated in mood disorders and schizophrenia. Levels of FGF ligands and/or receptors are altered in the hippocamous and cortex of patients with both depression and schizophrenia (19, 20, 32, 33) and polymorphism within FGF genes has been found in schizophrenic patients (33). Manipulations of both FGF2 and FGFR1 in animal models show that FGF signaling in the hippocampus affects anxiety behavior and is correlated with response to antipsychotic and antidepressant treatment (34–39).
The loss of fgf receptor 1 (fgfr1) in hGFAP promoter-expressing radial glial cells through Cre recombination results in a volume reduction of both dentate gyrus (DG) and CA fields (40). FGFR1 has been shown to regulate proliferation of hippocampal stem cells during both embryogenesis and early adulthood (40, 41). The hGFAP-Cre fgfr1 knockout mice had smaller postnatal hippocampi, but showed no working memory or fear conditioning alterations (42). Mice lacking fgfr1 via recombination driven by nestin-cre showed no defect in spatial learning, but had alterations in spatial memory consolidation (41). The hippocampus is derived from a region of the dorsomedial telencephalon which expresses fgfr2 during embryonic and postnatal periods; however, the role of FGFR2 in hippocampal development and functioning has not yet been examined. Additionally, the role of hippocampal FGF signaling may not be limited to cell proliferation since recent findings indicate a function for both FGFR1 and FGFR2 in pre-synaptic organization through interactions with FGF7 and/or FGF22 (43, 44).
In this study, we created knockout mice lacking fgfr2 at different stages of pre- and post-natal development to understand the impact of this receptor on hippocampal anatomy, cell proliferation, and learning and memory tasks. Correlations of anatomical findings with behavioral performance in individual animals permitted greater understanding of the role of specific hippocampal processes in learning and memory.
Methods
Animals
Conditional hGFAP-cre fgfr2 knockout mice have been previously described (45, 46) (see Methods in the Supplement). Cre negative mice, littermates when possible, were used as control animals. Behavioral testing was conducted when the mice were 5–8 months old.
To assess the contribution of FGFR2 solely in the postnatal brain, mice homozygous for the fgfr2f alleles were crossed with hGFAP-creERT2 (GCE) mice (47). The latter express a tamoxifen-inducible Cre recombinase-estrogen receptor fusion protein (CreERT2) (48) under the control of the hGFAP promoter (47). These mice received injections of 0.5 mg of tamoxifen dissolved in sunflower seed oil or sunflower seed oil alone twice daily for five consecutive days at 2–4 months of age. Behavioral testing began at least 9 days after the time of the last tamoxifen injection. Behaviorial testing began at 2.5–4.5 months of age and completed at 5.5–7.5 months of age.
Behavior (see Methods in the Supplement for details)
Morris Water Maze
Eight days of training with a probe trial on the ninth day were implemented using a standard, automated water maze (Coulbourn Instruments, Whitehall, PA).
Object Recognition
Over three days, mice were repeatedly presented a familiar object along with a novel object. Time spent with each object was quantified each day and learning was assessed based upon a discrimination index, which quantifies increases in the time spent interacting with the novel object.
Immunocytochemistry and stereology were performed as previously described (6)(see Methods in the Supplement).
Quantitative PCR and In Situ Hybridization
Quantitative PCR was carried out using Taqman Gene Assays (Applied Biosystems) for fgfr2 IIIc isoform, the specific form of the fgfr2 gene knocked out with the Cre-lox system (Mm01269938; context sequence: CAGTTCTGCCAGCGCCTGTGAGAGA), fgfr1 (Mm00438923; context sequence: CCGTTCTGGAAGCCCTGGAAGAGAG) and beta-actin (predeveloped) as previously described (6).
In situ hybridization was carried out as previously described (6) using probes synthesized from linearized plasmids for fgfr1 (4) and fgfr2 IIIc TM (gift of D. Ornitz).
Statistical Analysis
Analysis of variance (ANOVA) was performed with SPSS to identify differences in behavioral performance of KO animals measured over multiple days from controls. Interactions were determined between two variables: day and genotype. Two-tailed student’s t tests were performed with Microsoft Excel comparing regional volumes, stereological cell counts and densities and single behavioral measures. One-tailed student’s t tests were used for anatomical assessments in FGFR2iKO mice. Pearson correlation coefficients were calculated between behavioral and anatomical measures, with significance determined by critical values tables (one-tailed p values).
Results
We examined the role of FGFR2 in the hippocampus in mice heterozygous for the hGFAP-cre transgene and homozygous for a conditional floxed fgfr2 allele (fgfr2f). These conditional FGFR2 knock out mice (FGFR2cKO) exhibited a loss of fgfr2 in radial glia throughout cortex and hippocampus (Fig. 1A,B), as well as in astrocytes of these regions, which has been demonstrated previously (6). The preservation of fgfr1 mRNA, which is expressed in the same regions, was demonstrated with quantitative PCR (average relative expression: hGFAP-cre- negative = 2.5 and hGFAP-cre+ = 3.1).
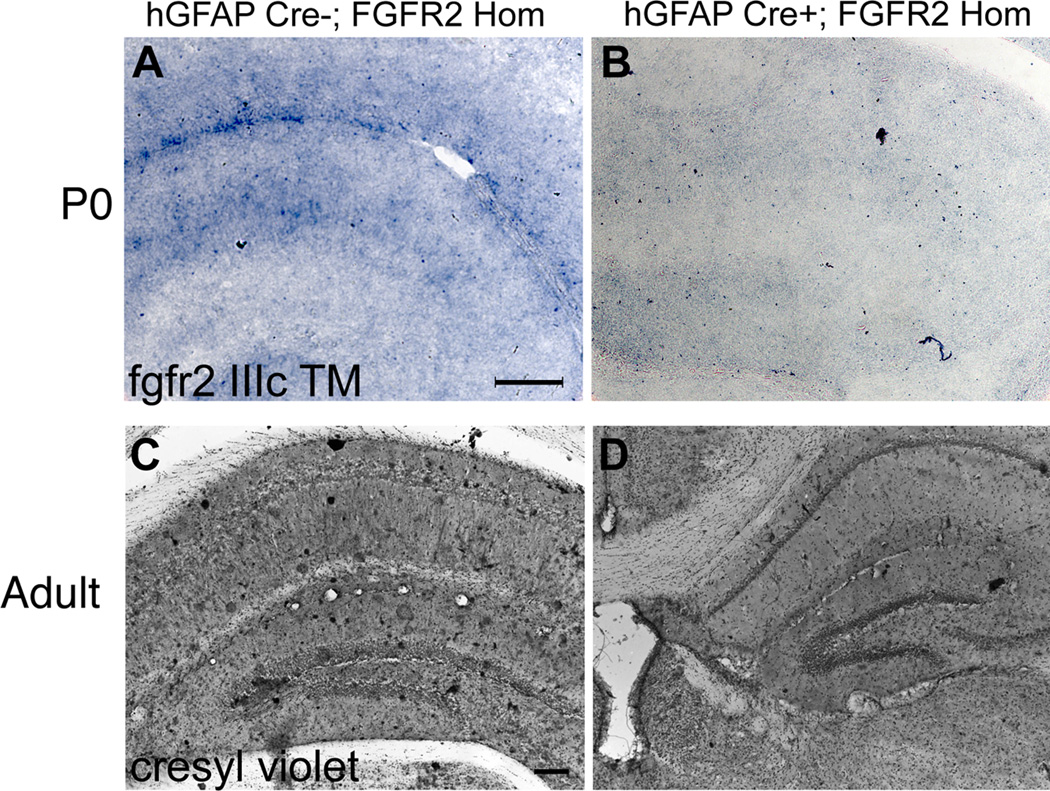
Figure 1.
FGFRcKO mice lacked fgfr2 early in development and had persistent neuroanatomical deficit. A,B: Images of fgfr2 IIIc TM mRNA localization by in situ hybridization in the hippocampus at P0 showing decreased expression in FGFR2cKO mice. C,D: Cresyl violet staining showing decreased volume of hippocampus in a FGFR2cKO compared to a control mouse. Scale bar=200 µm.
Hippocampal morphometry and neural cell populations
In FGFR2cKO mice lacking FGFR2 throughout prenatal and early postnatal life, there were significant changes in volume of DG and CA fields of the hippocampus in adults (8–10 months old) (Fig 1C,D). The volume of DG was 33.9% smaller in FGFR2cKO mice compared to littermate controls. Additionally, FGFR2cKO mice had a 36.9% reduction in total hippocampal volume (CA fields and DG together) (Table 1).
Table 1. Hippocampal changes in FGFR2cKO mice lacking FGFR2 embryonically and in adulthood.
Averages and standard error of the mean (n for each shown) for volumes and cell numbers assessed by stereology. n=number of animals. Differences were assessed with one tailed student’s t-tests based on previous reductions of these volumes and cell types in FGFR2cKO and FGFR1cKO animals (7). BrdU was injected 1 hour prior to analysis.
| Controls | FGFR2cKO | Deficit | |
|---|---|---|---|
| Dentate Gyrus | 4.49 ± 0.21 | 2.97 ± 0.29 | −33.9% |
| Volume (mm3) | n=6 | n=6 | p<0.001 |
| Total | 22.45 ± 1.18 | 14.16 ± 1.06 | −36.9% |
| Hippocampal | n=6 | n=6 | p<0.001 |
| Volume (mm3) | |||
| Total | 43.70 ± 2.92 | 24.30 ± 1.20 | −44.4% |
| Hippocampal | n=5 | n=5 | p<0.0005 |
| Parvalbumin Cell | |||
| Number (×103) | |||
| Dentate Gyrus | 3.55 ± 0.14 | 2.85 ± 0.15 | −19.6% |
| Doublecortin Cell | n=6 | n=6 | p<0.01 |
| Number (×103) | |||
| Dentate Gyrus | 3.43 ± 0.71 | 1.97 ± 0.35 | −42.5 % |
| BrdU+ Cell | n=6 | n=6 | p<0.05 |
| Number (×103) |
Counts were made of parvalbumin+ cells, an abundant sub-class of inhibitory neurons that plays an important role in regulation of neuronal excitability in this region. Parvalbumin neuron number within the entire hippocampus was reduced by 44.4% in adult FGFR2cKO mice (Fig 2A,B,G, Table 1).
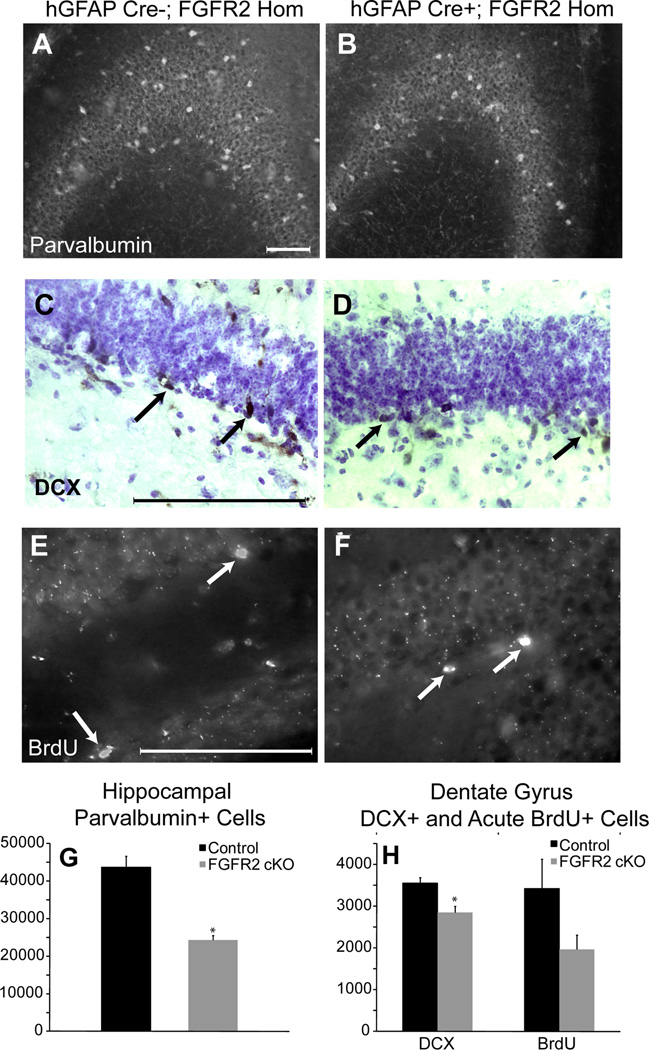
Figure 2.
8–10 month old FGFR2cKO mice lacked cellular elements in hippocampus in adulthood. A,B: Immunocytochemistry for parvalbumin in CA showing decreased parvalbumin neuron density in FGFR2cKO as compared to control mice. C,D: Doublecortin (DCX) immunocytochemistry with cresyl violet counterstaining showing decreased DCX cell number in FGFR2cKO DG as compared to control mice. E, F: Immunocytochemistry for BrdU (1 hour after BrdU tracer injection) in control and FGFR2cKO DG. Arrows indicate DCX+ and BrdU+ cells. G: Histogram of average parvalbumin+ cell number in hippocampus of control and FGFR2cKO mice (n=5; 5). H: Histogram of average DCX+ and BrdU+ cell number in DG of control and FGFR2cKO mice (n=5; 6) (*= p<0.05). Scale bar= 200 µm.
To understand the impact of the FGFR2 loss of function on hippocampal neurogenesis, we examined cells positive for doublecortin (DCX), a protein whose expression levels peaks about 10 days after the birth of a new neuron and declines thereafter (49). FGFR2cKO mice had a significant decrease of 19.6% in DCX+ cell number in the DG (Table 1, Fig 2C,D,H). In conjunction with this finding, counts of acutely incorporated BrdU showed a 42.5% decrease in proliferating cells in the DG (Table 1, Fig 2E,F,H). Together, reduced BrdU+ and DCX+ cells suggested that the ongoing process of neurogenesis was affected by the loss of FGFR2.
These differences were shown in a second cohort of animals examined at six weeks of age that were not behaviorally tested, validating that alterations were consistent throughout development. In this cohort, similar decreases in hippocampal overall volume, volume of DG, and DCX+ cell population were shown with the loss of FGFR2 (Table S1 in the Supplement). Also in these animals, no deficit was shown in another subtype of inhibitory neurons positive for neuropeptide Y (Table S1 in the Supplement).
Behavioral Testing
To assess the behavioral correlates of the cellular defects in the hippocampus and PFC in FGFR2cKO animals, spatial learning and spatial reference memory were both tested using the Morris water maze. Learning was assessed during eight days of training. A probe trial on the ninth day examined spatial memory. FGFR2cKO animals showed significantly slower learning as compared to their control littermates (ANOVA F(2,19) = 15.785; p = 0.001). (Fig 3A). Spatial memory on day nine was also significantly different, with FGFR2cKO mice showing chance levels of preference for the location and quadrant of the hidden platform (Fig 3B).
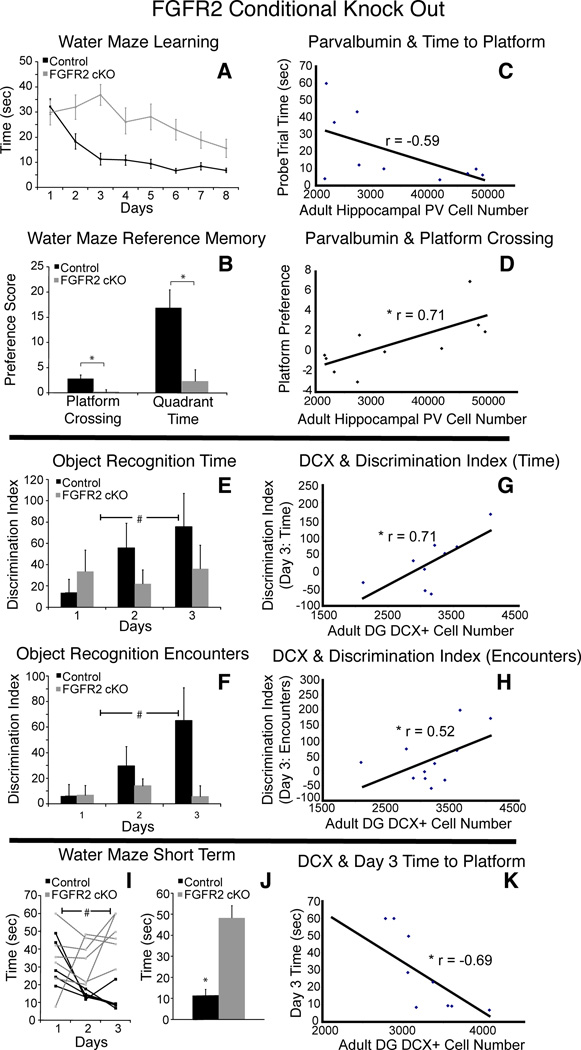
Figure 3.
Cognitive performance in FGFR2cKO mice was correlated with specific hippocampal cellular alterations. A,B: Water maze showing impairment in FGFR2cKO mice (n=10; 11) with average time to find platform over 8 days of training and water maze reference memory in average platform crossing preference score and quadrant preference score. C,D: Significant correlations of hippocampal parvalbumin+ cell number with (C) ninth day time to find platform location (n= 10) and (D) platform crossing preference (n=10). E,F: Average novel object discrimination index based on (E) time spent orienting to objects or (F) number of encounters with objects across three days in object recognition task, showing significant deficit in learning in FGFR2cKO compared to controls (n= 13; 13) . G,H: Significant correlations of DCX+ cell number in DG with (G) third day novel object discrimination index by time and (H) novel object discrimination index by encounters (n= 11). I: Individual animal performance on the first three days of water maze learning showing instability of memory during acquisition in FGFR2cKO. J: Average time to find platform on day 3 was greater in FGFR2cKO animals than control mice (n= 6; 5). K: Day 3 water maze performance was correlated with DCX+ number (n= 11). # p<0.05 ANOVA; * = p<0.05 students t-tests and r = Pearson correlation coefficient. Scale bar=200 µm.
When anatomical findings were compared to the behavioral performance of individual animals on the water maze across control and FGFR2cKO mice, significant correlations were identified between the number of hippocampal parvalbumin cells and three measures of spatial reference memory (Fig 3C,D). The time for each mouse to first reach the previous platform location on the ninth day was negatively correlated with hippocampal parvalbumin neuron number (Fig 3C, r = −0.59, df = 8, p < 0.05), and the preferences for platform crossing and quadrant were positively correlated with hippocampal parvalbumin neuron number (r = 0.71 and r = 0.57 for crossing preference and quadrant preference, respectively, Fig 3D, df = 8, p < 0.05). In contrast, these measures of spatial memory performance were not correlated with BrdU+ cell number (r= −0.42, r =0.11, r =0.32 for time to reach platform, crossing preference and quadrant preference, respectively; df = 9, all NS) or DCX+ immature neuron number (r = −0.19, r =0.20, r =0.24 for time to reach platform, crossing preference and quadrant preference, respectively; df = 9, all NS).
To test learning and memory in a different modality, we used an object recognition test in which animals were presented with a previously presented object and three novel objects sequentially over three days. Control mice showed the ability to learn to recognize the familiar object by a proportionate decrease in the time spent orienting to the familiar versus the novel objects (Fig 3E). FGFR2cKO mice showed near chance discrimination indices between novel and familiar objects over three days using exploration time, or number of encounters (Fig 3F).
When anatomical findings were compared to the behavioral performance of individual animals on the object recognition task, no significant correlations were found with hippocampal parvalbumin+ neuron count, in contrast to that shown for spatial reference memory (r = 0.25, df = 8, NS). However, the discrimination index on the third day measured by either encounter number or exploration time, was positively correlated with DCX+ cell number (r = 0.52, r = 0.71, respectively; df= 9, p < 0.05; p < 0.01) (Fig 3G,H). The discrimination index measured by encounters was not significantly correlated with BrdU+ cell number (r = 0.43, df = 10, p = 0.09), but the discrimination index measured by exploration time was significantly correlated with BdrU+ cell number (r = 0.51, df = 10, p < 0.05). Thus, significant correlations were identified for object recognition learning with measures of proliferating cells and immature neurons in DG, but not with parvalbumin cell number.
The above results suggest that object recognition learning and spatial reference memory had independent correlations with hippocampal anatomy. Confirming this, there was no correlation between performance on the two tasks themselves (r = 0.1, df = 19, NS). To analyze this further, we considered the early phase of learning in the water maze, looking at the same time points (days 1–3) analyzed in the object recognition task. Control mice were consistently faster each subsequent day on the water maze task with downward learning slopes over each 24 hour period (Fig 3I). FGFR2cKO mice showed an upward learning slope on days 2–3, reflecting worse performance, likely due to unstable learning during this time period (Fig 3I). As shown in Figure 3I, controls and FGFR2cKO showed significantly different average time to platform over these three days (ANOVA F(2,19) = 15.701; p = 0.001). This difference mainly stems from significantly different learning slopes between groups from day 2 to day 3 (controls = −7.1, FGFR2cKO = 4.9; p < 0.05) with FGFR2cKO mice taking more time to find the platform on day three (Fig 3J). As shown for object recognition, the average time to platform on day three of training showed a significant negative correlation with DG DCX+ cell number (r = −0.69, df = 9, p < 0.01) (Fig 3K) and BrdU+ cell number (r = −0.59, df = 9, p < 0.05; not shown). These correlations suggest that short term learning acquisition and longer term memory on this spatial task may rely on different components of hippocampal functioning.
Adult FGFR2 Knock Out
To further investigate the double-dissociation between short and long-term aspects of learning and memory and the correlation of these cognitive abnormalities with defects in cellular populations of the hippocampus, the role of FGFR2 in hippocampal stem/progenitor cells of adult mice was examined using an inducible knockout strategy.
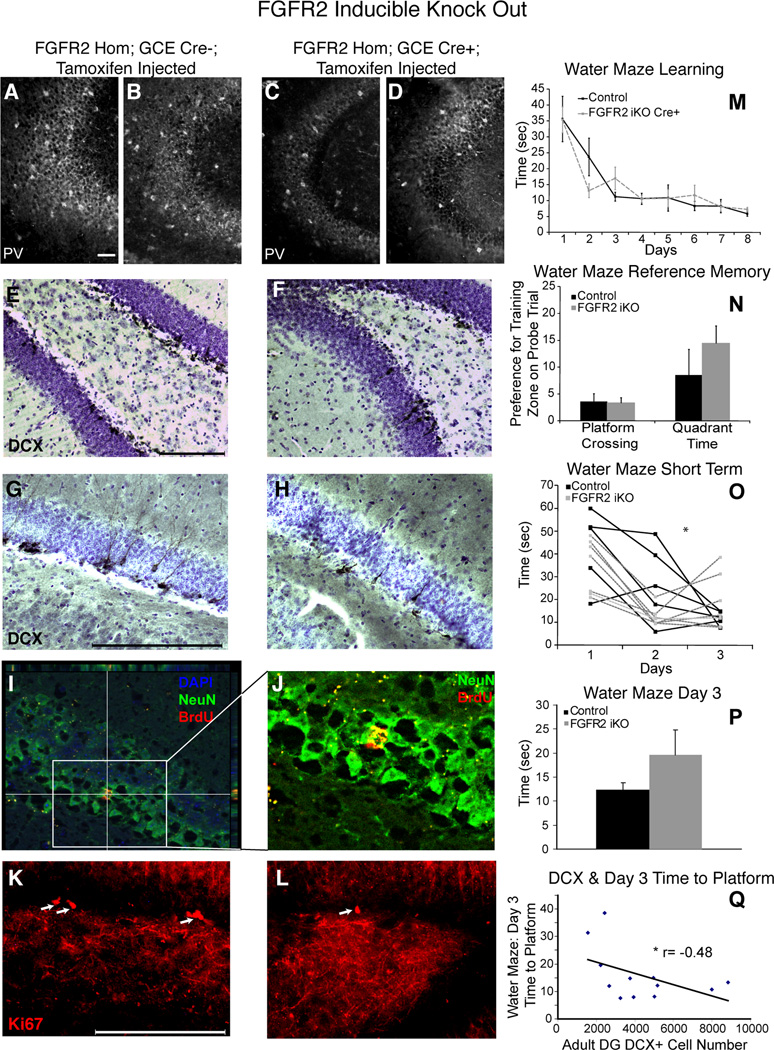
To create inducible FGFR2 knock out mice (FGFR2iKO), we used GCE transgenic mice, which carry a tamoxifen- inducible Cre recombinase under the hGFAP promoter (47). GCE; fgfr2f/f mice were injected with tamoxifen. Two controls were used: tamoxifen-injected GCE-negative animals and vehicle-injected GCE-positive mice. The efficacy of this approach was validated through in situ hybridization and quantitative PCR for the IIIc and transmembrane domains of the fgfr2 gene (Fig 4A,B,E) demonstrating that these mice showed reduced fgfr2 levels only after the tamoxifen injection. In Cre+ mice not injected with tamoxfen, fgfr2 gene expression by qPCR was unchanged compared to Cre- controls (0.89 fold change; n=2,2; NS), confirming that the GCE transgene did not independently impact fgfr2. The preservation of fgfr1 which is expressed in the same regions was validated by in situ hybridization in FGFR2iKO mice (Figure S1 in the Supplement).
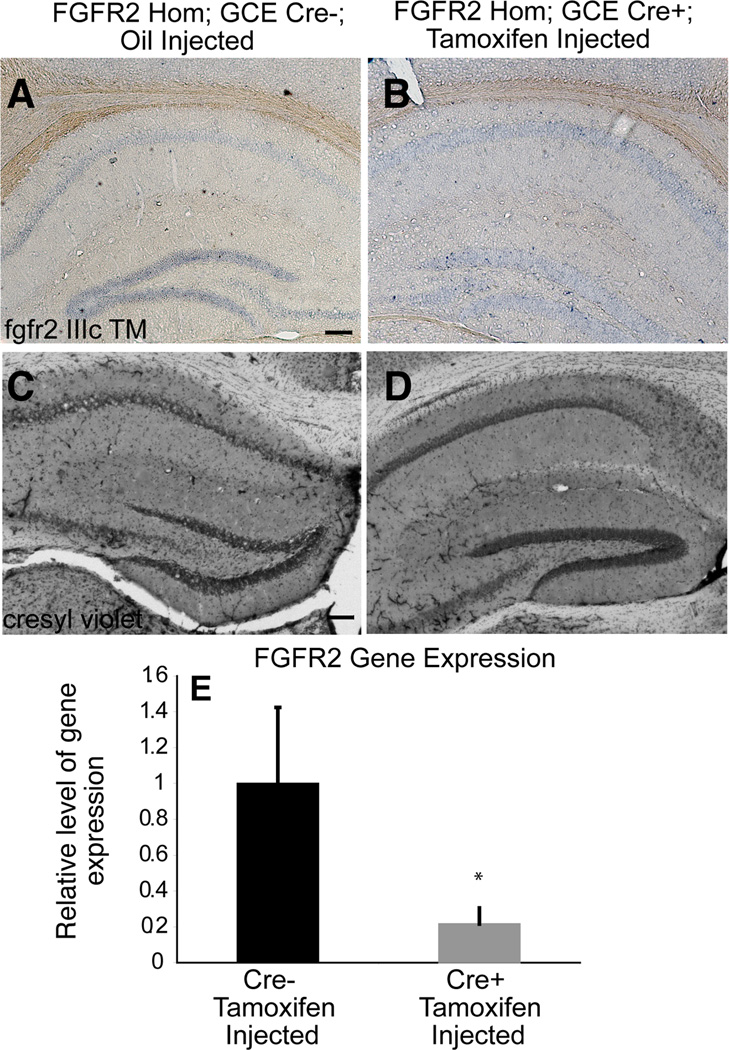
Figure 4.
FGFRiKO mice lacked fgfr2 in adulthood and showed minor alterations in hippocampus. A,B: In situ hybridization of adult hippocampus showing expression of fgfr2 IIIc TM in control mice which was reduced in FGFR2cKO mice. C,D: Cresyl violet staining showing that the overall structure of the adult hippocampus was intact in FGFR2iKO mice. E: fgfr2 IIIc TM gene expression assessed by qPCR with total mRNA showed a significant fold change in FGFR2cKO mice (Cre+) as compared to control littermates (Cre-) (n= 7; 5). Scale bar=200 µm.
When fgfr2 recombination occurred five months before the anatomical analysis at 7–9 months of age, FGFR2iKO mice demonstrated no alteration in overall hippocampal volume (Table 2, Fig 4C,D). However, DG volume in particular was mildly reduced (13% smaller, p=0.06) in FGFR2iKO mice (Table 2), with no difference in the volume of the CA fields.
Table 2. Hippocampal changes in FGFR2iKO mice lacking FGFR2 only in adulthood.
Averages and standard error of the mean (n for each shown) for volumes and cell numbers assessed by stereology. n=number of animals. Differences were assessed with one tailed student’s t-tests. BrdU was injected for six days, three and a half weeks prior to analysis.
| Controls | FGFR2iKO | Deficit | |
|---|---|---|---|
| Dentate Gyrus | 5.47 ± 1.43 | 4.76 ± 0.41 | −13.0% |
| Volume (mm3) | n=6 | n=6 | p=0.06 |
| Total Hippocampal | 23.21 ± 1.47 | 23.19 ± 1.64 | −0.1% |
| Volume (mm3) | n=6 | n=4 | NS |
| Dentate Gyrus | 11.30 ±1.86 | 5.93 ± 1.03 | −47.5% |
| Doublecortin Cell | n=4 | n=6 | p<0.05 |
| Number (×103) | |||
| Dentate Gyrus | 2.36 ± 0.15 | 1.59 ± 0.11 | −32.8 % |
| NeuN/BrdU | n=3 | n=4 | p<0.005 |
| Number (×103) |
FGFR2iKO mice also demonstrated a decrease in the number of newly born, immature neurons. We observed significant decreases in both number (47.5%) and density (42.6%) of DCX+ cells in DG of FGFR2iKO animals compared to littermate controls when the KO was induced at least 120 days prior (Fig 5E–H, Table 2). The generation of new neurons was tested directly by BrdU fate mapping after a six-day incorporation and 3.5 week survival. Neurogenesis was significantly reduced by 32% in FGFR2iKO mice (Fig 5I,J, Table 2). Staining for Ki67 qualitatively showed fewer positive cells in DG of FGFR2iKO mice (Fig 5K,L), suggesting that this deficit in neuronal precursors was likely due to a decrease in the number or proliferation of stem/progenitor cells.
Figure 5.
Adult FGFR2iKO mice lacked DCX+ and proliferating cells in the hippocampus. Cellular findings were correlated with cognitive performance. A,B,C,D: Parvalbumin immunocytochemistry in CA was equivalent in controls (micrographs from two individual brains shown in A,B) and FGFR2iKO mice (micrographs from two individual brains shown in C,D). E,F,G,H: Low (E,F) and high power (G,H) doublecortin (DCX) immunocytochemistry with cresyl violet counterstaining in DG of control and FGFR2 iKO. I,J, Low (I) and high (J) power images of colocalization of BrdU and NeuN staining used for neurogenesis evaluation. K,L: High power images of Ki67 immunocytochemistry demonstrating decreased Ki67+ cells in DG of FGFR2iKO. M: Average time to find platform over 8 days of training in the water maze showed no impairment in FGFR2 iKO mice (n=7; 10). N: Water maze reference memory was intact in FGFR2iKO mice as demonstrated by platform crossing preference score (calculated as the average difference in the number of crossings of the platform area in the target quadrant minus crossings of the same area in each of the other quadrants). O: Individual animal performance on the first three days of water maze learning, showing instability of memory during acquisition in FGFR2iKO mice. P: Average time to find platform on Day 3 was greater in FGFR2iKO animals than control mice (n= 5; 5) (p = 0.10 NS). Q: Day 3 water maze performance was correlated with DCX+ cell number (n= 10) (* = p<0.05 students t-tests and r=Pearson correlation coefficient). Scale bar= 200 µm.
In the Morris water maze, FGFR2iKO mice and littermate controls lacking the GCE transgene but also injected with tamoxifen showed similar times to find the platform over eight days (Fig. 5M). The FGFR2iKO mice also were not significantly different from controls in the amount of time they spent in the platform quadrant or zone on a ninth day probe trial (Fig 5N). Thus, FGFR2iKO mice showed no deficit in the long-term aspects of spatial learning or reference memory. Consistent with this, FGFR2iKO mice also showed no notable differences from controls in parvalbumin immunostaining within the hippocampus (Fig 5A,B vs C,D).
Interestingly, when the early phase of the water maze was analyzed, looking at the same time points (days 1–3) analyzed in the object recognition task, FGFR2iKO animals showed a deficit in shorter-term learning. Control mice had stable, consistent learning with downward, negative slopes over each 24-hour period (Fig 5O). During this same time period, FGFR2iKO mice showed a downward learning slope initially, but then subsequently had worse performance (Fig 5O, ANOVA F(2,15) = 2.830, p = 0.075). The slope of the learning curves were significantly different between groups for these days (controls = −12.8, FGFR2iKO: 3.9; p < 0.05) and a trend for a different average time to find the platform was found for day three (Fig 5P, p = 0.10). Lastly, the time to find the platform on day three showed a trend for a negative correlation with DCX+ cell number in the DG (r = −0.48, df = 10, p = 0.057) (Fig 5Q). This again suggests that distinct components of hippocampal circuitry may underlie different stages of learning.
Discussion
We demonstrate that FGFR2 plays a role in both early hippocampal development and the genesis of new neurons in the adult DG and functionally intervenes in different aspects of learning and memory. Additionally, we found that the FGFR2-dependent losses of different cell types in the adult hippocampus are independently associated with alterations in distinct aspects of learning and memory. Specifically, short-term components of learning and memory that occur after a small number of presentations (within 48 hours) relied on the presence of newly born granule neurons in the adult DG. On the other hand, the long-term encoding of these experiences (reference memory) relied on the functioning of circuitry within the hippocampal fields in which parvalbumin neurons play a significant role.
Early Role of FGFR2 in Hippocampal Development
Hippocampal anatomy and cellular defects in mice lacking FGFR2 in radial glia of the dorsal telencephalon and their daughter cells starting from embryonic development differed from that found in mice with induced knockout of FGFR2 only in adulthood. The decrease in overall hippocampal volume was found only in mice lacking FGFR2 during embryonic development and was similar to the volume defect observed in mice lacking another FGF receptor, FGFR1 (39). These data are consistent with high expression of both FGFR1 and FGFR2 in proliferative zones of the hippocampal primordium (40, 50). Similarly, the 50–65% loss of parvalbumin neurons previously shown in the hippocampus and cortex of FGFR1cKO animals (40, 42) is similar to the 44.4% loss shown here for FGFR2cKO animals and suggests that signaling through both receptors in the embryonic or early postnatal period, perhaps as heterodimers, plays a role in the development of these cells. As the hGFAP-Cre line does not drive a significant amount of Cre recombination in the ventral telencephalon, where the progenitors for these GABAergic neurons are located, and FGFR2 is not expressed in parvalbumin interneurons postnatally (51), these effects may be indirectly mediated through postnatal changes in glial cells expressing FGFR1 and FGFR2. Indeed, postnatal astrocytes have been shown to express both receptors (49, 50), but neither FGFR1 nor FGFR2 may be sufficient alone in the process by which astrocytes signal to parvalbumin cells, regulating their development in an FGFR-dependent way.
The reference memory performance on the water maze, i.e., the ability to learn and remember new spatial information over many days, was impaired when FGFR2 was lacking during early development, but not when FGFR2 was lacking only during adulthood. A possible confound of these experiments is that FGFR2 was not totally lost after inducible cre recombination in adulthood. Nevertheless, adult ongoing FGFR2 functioning was important for hippocampal neurogenesis, while it did not appear to play a major role in reference spatial memory. There is agreement among many models that the process of neurogenesis in DG is not necessary for the retention of spatial memory (52–54). In contrast, the optimal growth of the hippocampus and medial PFC, both of which depend upon FGFR2 functioning during embryonic development, is essential for reference memory. Furthermore, the significant correlation between performance on the water maze probe trial and parvalbumin neuron number within the hippocampus suggests that FGFR2 may influence cognitive behavior by regulating postnatal development of parvalbumin neurons, as suggested above. Within the hippocampus, parvalbumin neuron circuitry has been shown to be critical for synchronous neuronal activity (55, 56) as well as different components of spatial memory (57, 58). Furthermore, parvalbumin neurons are involved in plasticity throughout the brain (59, 60). However, it cannot be ruled out that parvalbumin neuron number also correlates with other FGFR2-dependent neural systems, which more directly influence spatial learning and memory. For example, parvalbumin neuron numbers in the hippocampus may be regulated in part by the presence of an appropriate number of hippocampal glutamatergic cells and their expression of transcription factors such as TBR2 (61). FGFR2 may also affect specific aspects of learning behavior via its role in other elements of hippocampal circuitry including the formation of presynaptic terminals in CA3 (44).
Role of FGFR2 in Adult Hippocampus
Both mouse models studied here demonstrated that the number of newly born granule neurons in the DG was deficient in the absence of FGFR2 signaling in radial glia and astocytes of the adult dorsal telencephalon. Together with the deficit in proliferative cells, these results suggest that FGFR2 plays a role in adult neurogenesis in DG. This is the first evidence supporting the importance of FGFR2 for adult hippocampal neurogenesis. Proliferating precursors and quiescent GFAP+ cells in the subventricular zone (SVZ) and quiescent GFAP+ cells in the subgranular zone (SGZ) in adult rodents have been shown to express FGFR2 (51, 62–64). Adult neurogenesis is also regulated by FGFR1 (41, 65), which is expressed in proliferating precursors of SVZ and SGZ. Although FGFR2 and FGFR1 may play a role in neurogenesis by similar mechanisms, FGFR2 has not been localized within proliferating precursors of the SGZ (64), suggesting that its action may be restricted to quiescent neural stem cells in this region. FGFR2 expression in quiescent GFAP+ cells may also be critical for neurogenesis through non-cell autonomous processes, i.e. a contribution to the environment of the neurogenic niche (16).
Mice lacking FGFR2 only during adult life showed unstable short term learning on the water maze, even though their reference memory over many presentations eventually recovered with no impairment on a standard probe test of spatial memory. Furthermore, the number of newly born neurons in adult FGFR2cKO mice correlated with short-term object recognition and short-term learning on the water maze. Other models of reduced adult hippocampal neurogenesis have shown similar patterns of instability in the acquisition of spatial information early in the course of water maze training (66, 67) and in other forms of short-term learning (68), while spatial reference memory is intact. These findings may reflect a specialized role for the DG in evaluating precise information about spatial distance. Object recognition behavior is typically preserved in models of reduced neurogenesis; however, most of these studies use only short, same-day inter-trial intervals. The object recognition task used here evaluated 24–48 hour retention of memory and the ability to maintain stable short term memories in the face of new memories being formed on subsequent days, a process in which DG neurogenesis has been hypothesized to play a role (69).
A limitation of our study was the evaluation of most anatomical markers in animals that had undergone 2–4 weeks of behavioral testing. Testing experience may interact with the knock out of FGFR2, the results of which would be differences not solely due to the genetic deficit.
However, the deficit in DCX+ cells was similar in six-week old, non-behaviorally tested animals and the testing experience of the animals used for the neurogenesis analysis was much more restricted (three days) than that of the animals used for all other analyses (four weeks), yielding equivalent results.
Conclusion
We have shown that FGFR2 signaling in both the embryonic and adult hippocampus plays crucial roles for different aspects of learning and memory (19, 20, 34, 35, 70, 71). In combination with genetic studies and functional analysis of FGFR gene variants, our data may help elucidate specific variants of affective disorders reflecting different pathobiological origins of illness. Our work and that of others linking different aspects of hippocampal neurophysiology with different time periods of FGF activity (72–75) also suggests mechanisms by which treatment in adulthood may be effective even for disorders with early origins, independent of the role of FGFR2 in development.
Supplementary Material
Acknowledgements
We would like to acknowledge Rachael Couture, Michaela Thompson, and Jacob Kravitz for technical assistance, David Ornitz for the fgfr2 plasmid and everyone in the Vaccarino lab for helpful discussion. This work was supported by National Institutes Health Grants R01 MH067715, K08 MH086812-01, T32 MH018268, R25 MH071584, and R25 MH077823 and the National Alliance for Research on Schizophrenia and Depression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Drs. Stevens, Schwartz, and Vaccarino and Ms. Jiang report no biomedical financial interests or potential conflicts of interest.
References
- 1.Paek H, Gutin G, Hebert JM. FGF signaling is strictly required to maintain early telencephalic precursor cell survival. Development. 2009;136:2457–2465. doi: 10.1242/dev.032656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutin G, Fernandes M, Palazzolo L, Paek H, Yu K, Ornitz DM, et al. FGF signalling generates ventral telencephalic cells independently of SHH. Development. 2006;133:2937–2946. doi: 10.1242/dev.02465. [DOI] [PubMed] [Google Scholar]
- 3.Ever L, Zhao RJ, Eswarakumar VP, Gaiano N. Fibroblast Growth Factor Receptor 2 Plays an Essential Role in Telencephalic Progenitors. Dev Neurosci. 2007 doi: 10.1159/000112521. [DOI] [PubMed] [Google Scholar]
- 4.Rash BG, Lim HD, Breunig JJ, Vaccarino FM. FGF signaling expands embryonic cortical surface area by regulating Notch-dependent neurogenesis. J Neurosci. 2011;31:15604–15617. doi: 10.1523/JNEUROSCI.4439-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang W, Wong LC, Shi SH, Hebert JM. The Transition from Radial Glial to Intermediate Progenitor Cell Is Inhibited by FGF Signaling during Corticogenesis. J Neurosci. 2009;29:14571–14580. doi: 10.1523/JNEUROSCI.3844-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens HE, Smith KM, Maragnoli ME, Fagel D, Borok E, Shanabrough M, et al. Fgfr2 is required for the development of the medial prefrontal cortex and its connections with limbic circuits. J Neurosci. 2010;30:5590–5602. doi: 10.1523/JNEUROSCI.5837-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens HE, Smith KM, Rash BG, Vaccarino FM. Neural stem cell regulation, fibroblast growth factors, and the developmental origins of neuropsychiatric disorders. Front Neurosci. 2010;4 doi: 10.3389/fnins.2010.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi K. Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Mol Neurobiol. 2009;39:24–36. doi: 10.1007/s12035-008-8049-5. [DOI] [PubMed] [Google Scholar]
- 9.Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk of depression. Arch Gen Psychiatry. 2010;67:270–276. doi: 10.1001/archgenpsychiatry.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohnuma T, Kimura M, Takahashi T, Iwamoto N, Arai H. A magnetic resonance imaging study in first-episode disorganized-type patients with schizophrenia. Psychiatry Clin Neurosci. 1997;51:9–15. doi: 10.1111/j.1440-1819.1997.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 11.Gur RE, Turetsky BI, Bilker WB, Gur RC. Reduced gray matter volume in schizophrenia. Arch Gen Psychiatry. 1999;56:905–911. doi: 10.1001/archpsyc.56.10.905. [DOI] [PubMed] [Google Scholar]
- 12.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 14.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 15.Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biological Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 16.Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Todtenkopf MS, Benes FM. Distribution of glutamate decarboxylase65 immunoreactive puncta on pyramidal and nonpyramidal neurons in hippocampus of schizophrenic brain. Synapse. 1998;29:323–332. doi: 10.1002/(SICI)1098-2396(199808)29:4<323::AID-SYN4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Arnold SE, Talbot K, Hahn CG. Neurodevelopment, neuroplasticity, and new genes for schizophrenia. Prog Brain Res. 2005;147:319–345. doi: 10.1016/S0079-6123(04)47023-X. [DOI] [PubMed] [Google Scholar]
- 19.Tochigi M, Zhang X, Ohashi J, Hibino H, Otowa T, Rogers M, et al. Association study between the TNXB locus and schizophrenia in a Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:305–309. doi: 10.1002/ajmg.b.30441. [DOI] [PubMed] [Google Scholar]
- 20.Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull. 2006;70:221–227. doi: 10.1016/j.brainresbull.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Gould NF, Holmes MK, Fantie BD, Luckenbaugh DA, Pine DS, Gould TD, et al. Performance on a virtual reality spatial memory navigation task in depressed patients. Am J Psychiatry. 2007;164:516–519. doi: 10.1176/ajp.2007.164.3.516. [DOI] [PubMed] [Google Scholar]
- 22.Exner C, Nehrkorn B, Martin V, Huber M, Shiratori K, Rief W. Sex-dependent hippocampal volume reductions in schizophrenia relate to episodic memory deficits. J Neuropsychiatry Clin Neurosci. 2008;20:227–230. doi: 10.1176/jnp.2008.20.2.227. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- 24.Nissen C, Holz J, Blechert J, Feige B, Riemann D, Voderholzer U, et al. Learning as a model for neural plasticity in major depression. Biol Psychiatry. 2010;68:544–552. doi: 10.1016/j.biopsych.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 25.Toulopoulou T, Grech A, Morris RG, Schulze K, McDonald C, Chapple B, et al. The relationship between volumetric brain changes and cognitive function: a family study on schizophrenia. Biol Psychiatry. 2004;56:447–453. doi: 10.1016/j.biopsych.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Rowland LM, Griego JA, Spieker EA, Cortes CR, Holcomb HH. Neural changes associated with relational learning in schizophrenia. Schizophr Bull. 2010;36:496–503. doi: 10.1093/schbul/sbq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kesner RP, Hopkins RO. Mnemonic functions of the hippocampus: a comparison between animals and humans. Biol Psychol. 2006;73:3–18. doi: 10.1016/j.biopsycho.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Radulovic J, Tronson NC. Molecular specificity of multiple hippocampal processes governing fear extinction. Rev Neurosci. 2010;21:1–17. doi: 10.1515/revneuro.2010.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bangasser DA, Waxler DE, Santollo J, Shors TJ. Trace conditioning and the hippocampus: the importance of contiguity. J Neurosci. 2006;26:8702–8706. doi: 10.1523/JNEUROSCI.1742-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li G, Pleasure SJ. Ongoing interplay between the neural network and neurogenesis in the adult hippocampus. Curr Opin Neurobiol. 2010;20:126–133. doi: 10.1016/j.conb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altar CA, Vawter MP, Ginsberg SD. Target identification for CNS diseases by transcriptional profiling. Neuropsychopharmacology. 2009;34:18–54. doi: 10.1038/npp.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terwisscha van Scheltinga AF, Bakker SC, Kahn RS. Fibroblast growth factors in schizophrenia. Schizophr Bull. 2010;36:1157–1166. doi: 10.1093/schbul/sbp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eren-Kocak E, Turner CA, Watson SJ, Akil H. Short-hairpin RNA silencing of endogenous fibroblast growth factor 2 in rat hippocampus increases anxiety behavior. Biol Psychiatry. 2011;69:534–540. doi: 10.1016/j.biopsych.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riva MA, Molteni R, Bedogni F, Racagni G, Fumagalli F. Emerging role of the FGF system in psychiatric disorders. Trends Pharmacol Sci. 2005;26:228–231. doi: 10.1016/j.tips.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Turner CA, Calvo N, Frost DO, Akil H, Watson SJ. The fibroblast growth factor system is downregulated following social defeat. Neurosci Lett. 2008;430:147–150. doi: 10.1016/j.neulet.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H. Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain Res. 2008;1224:63–68. doi: 10.1016/j.brainres.2008.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachis A, Mallei A, Cruz MI, Wellstein A, Mocchetti I. Chronic antidepressant treatments increase basic fibroblast growth factor and fibroblast growth factor-binding protein in neurons. Neuropharmacology. 2008;55:1114–1120. doi: 10.1016/j.neuropharm.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29(19):6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohkubo Y, Uchida AO, Shin D, Partanen J, Vaccarino FM. Fibroblast growth factor receptor 1 is required for the proliferation of hippocampal progenitor cells and for hippocampal growth in mouse. J Neurosci. 2004;24:6057–6069. doi: 10.1523/JNEUROSCI.1140-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao M, Li D, Shimazu K, Zhou YX, Lu B, Deng CX. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry. 2007;62:381–390. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Muller Smith K, Fagel DM, Stevens HE, Rabenstein RL, Maragnoli ME, Ohkubo Y, et al. Deficiency in inhibitory cortical interneurons associates with hyperactivity in fibroblast growth factor receptor 1 mutant mice. Biol Psychiatry. 2008;63:953–962. doi: 10.1016/j.biopsych.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 44.Terauchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature. 2010;465:783–787. doi: 10.1038/nature09041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, et al. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- 46.Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- 47.Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, et al. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feil R, Brochard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. ProcNatl AcadSciUSA. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 50.Bansal R, Lakhina V, Remedios R, Tole S. Expression of FGF receptors 1, 2, 3 in the embryonic and postnatal mouse brain compared with Pdgfralpha, Olig2 and Plp/dm20: implications for oligodendrocyte development. Dev Neurosci. 2003;25:83–95. doi: 10.1159/000072258. [DOI] [PubMed] [Google Scholar]
- 51.Chadashvili T, Peterson DA. Cytoarchitecture of fibroblast growth factor receptor 2 (FGFR-2) immunoreactivity in astrocytes of neurogenic and non-neurogenic regions of the young adult and aged rat brain. J Comp Neurol. 2006;498:1–15. doi: 10.1002/cne.21009. [DOI] [PubMed] [Google Scholar]
- 52.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 54.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonifazi P, Goldin M, Picardo MA, Jorquera I, Cattani A, Bianconi G, et al. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science. 2009;326:1419–1424. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- 56.Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murray AJ, Sauer JF, Riedel G, McClure C, Ansel L, Cheyne L, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 59.Chen YJ, Zhang M, Yin DM, Wen L, Ting A, Wang P, et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci U S A. 2010;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nowicka D, Soulsby S, Skangiel-Kramska J, Glazewski S. Parvalbumin-containing neurons, perineuronal nets and experience-dependent plasticity in murine barrel cortex. Eur J Neurosci. 2009;30:2053–2063. doi: 10.1111/j.1460-9568.2009.06996.x. [DOI] [PubMed] [Google Scholar]
- 61.Sessa A, Mao CA, Colasante G, Nini A, Klein WH, Broccoli V. Tbr2-positive intermediate (basal) neuronal progenitors safeguard cerebral cortex expansion by controlling amplification of pallial glutamatergic neurons and attraction of subpallial GABAergic interneurons. Genes Dev. 2010;24:1816–1826. doi: 10.1101/gad.575410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev Neurosci. 2004;26:181–196. doi: 10.1159/000082136. [DOI] [PubMed] [Google Scholar]
- 63.Frinchi M, Bonomo A, Trovato-Salinaro A, Condorelli DF, Fuxe K, Spampinato MG, et al. Fibroblast growth factor-2 and its receptor expression in proliferating precursor cells of the subventricular zone in the adult rat brain. Neurosci Lett. 2008;447:20–25. doi: 10.1016/j.neulet.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 64.Mudo G, Bonomo A, Di Liberto V, Frinchi M, Fuxe K, Belluardo N. The FGF-2/FGFRs neurotrophic system promotes neurogenesis in the adult brain. J Neural Transm. 2009;116:995–1005. doi: 10.1007/s00702-009-0207-z. [DOI] [PubMed] [Google Scholar]
- 65.Mudo G, Belluardo N, Mauro A, Fuxe K. Acute intermittent nicotine treatment induces fibroblast growth factor-2 in the subventricular zone of the adult rat brain and enhances neuronal precursor cell proliferation. Neuroscience. 2007;145:470–483. doi: 10.1016/j.neuroscience.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 66.Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur J Neurosci. 2002;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 68.Manns M, Bichler Z, Leske O, Heumann R. Neuronal Ras activation inhibits adult hippocampal progenitor cell division and impairs spatial short-term memory. Genes Brain Behav. 2010;9:525–536. doi: 10.1111/j.1601-183X.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- 69.Wiskott L, Rasch MJ, Kempermann G. A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–343. doi: 10.1002/hipo.20167. [DOI] [PubMed] [Google Scholar]
- 70.Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, et al. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci U S A. 2004;101:15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sibille E, Arango V, Galfalvy HC, Pavlidis P, Erraji-Benchekroun L, Ellis SP, et al. Gene expression profiling of depression and suicide in human prefrontal cortex. Neuropsychopharmacology. 2004;29:351–361. doi: 10.1038/sj.npp.1300335. [DOI] [PubMed] [Google Scholar]
- 72.Fumagalli F, Bedogni F, Slotkin TA, Racagni G, Riva MA. Prenatal stress elicits regionally selective changes in basal FGF-2 gene expression in adulthood and alters the adult response to acute or chronic stress. Neurobiol Dis. 2005;20:731–737. doi: 10.1016/j.nbd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 73.Molteni R, Fumagalli F, Magnaghi V, Roceri M, Gennarelli M, Racagni G, et al. Modulation of fibroblast growth factor-2 by stress and corticosteroids: from developmental events to adult brain plasticity. Brain Res Brain Res Rev. 2001;37:249–258. doi: 10.1016/s0165-0173(01)00128-x. [DOI] [PubMed] [Google Scholar]
- 74.Molteni R, Lipska BK, Weinberger DR, Racagni G, Riva MA. Developmental and stress-related changes of neurotrophic factor gene expression in an animal model of schizophrenia. Mol Psychiatry. 2001;6:285–292. doi: 10.1038/sj.mp.4000865. [DOI] [PubMed] [Google Scholar]
- 75.Turner CA, Clinton SM, Thompson RC, Watson SJ, Jr, Akil H. Fibroblast growth factor-2 (FGF2) augmentation early in life alters hippocampal development and rescues the anxiety phenotype in vulnerable animals. Proc Natl Acad Sci U S A. 2011;108(19):8021–8025. doi: 10.1073/pnas.1103732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.