Abstract
Staphylococcus aureus infections are a substantial problem for hemodialysis patients. Several vaccine candidates are currently under development, with hemodialysis patients being one possible target population. To determine the potential economic value of a Staphylococcus aureus vaccine among hemodialysis patients, we developed a Markov decision analytic computer simulation model. When Staphylococcus aureus colonization prevalence was 1%, the incremental cost-effectiveness ratio (ICER) of vaccination was ≤$25,217/quality-adjusted life year (QALY). Vaccination became more cost-effective, as colonization prevalence, vaccine efficacy, or vaccine protection duration increased or vaccine cost decreased. Even at 10% colonization prevalence, a 25% efficacious vaccine costing $100 prevented 29 infections, 21 infection-related hospitalizations, and 9 inpatient deaths per 1,000 vaccinated HD patients. Our results suggest that a Staphylococcus aureus vaccine would be cost-effective (i.e., ICERs ≤$50,000/QALY) among hemodialysis patients over a wide range of Staphylococcus aureus prevalence, vaccine costs and efficacies, and vaccine protection durations and delineate potential target parameters for such a vaccine.
Keywords: Staphylococcus aureus, Vaccine, Economics, Hemodialysis, Cost-effectiveness
Introduction
Patients with end-stage renal disease undergoing hemodialysis (HD) treatment have a heightened risk of bacterial infections, particularly from Staphylococcus aureus (S. aureus) [1–3]. This is due in part to the elevated rate of S. aureus nasal colonization among HD patients, an established risk factor for vascular access infections and resulting bacteremic complications that can be more than twice that in the general population [3–4]. Complications of invasive S. aureus infections, including endocarditis, septic arthritis, and osteomyelitis, occur in 5.6% to 16.5% of cases and add to patient mortality and treatment costs [5–7]. Hospitalization rates for infection among HD patients have increased over 40% since the mid-1990s, with the number of admissions for bacteremia/septicemia rising to 112 per 1,000 patient-years in 2008 [8]. Infection continues to be a leading cause of mortality in this patient population, particularly during the first three months of dialysis treatment [8–9].
If an S. aureus vaccine becomes available, vaccination may be a viable approach to preventing S. aureus infections among HD patients. Vaccine developers have already made progress toward advancing S. aureus vaccination. In the past decade, developers completed Phase II (January 2010) and III (July 2006) clinical trials assessing the safety of one S. aureus vaccine and the efficacy of another in the active immunization of adult HD patients [10–11]. Vaccine candidates induced significantly elevated antibody levels to S. aureus antigens in several studies [12–14]. In addition to favorable serological indicators, one study associated vaccination with a lowered incidence of bacteremia in HD vaccinees [15]. Though protective effects began to wane less than a year following vaccination, booster vaccination provided a way to extend vaccine protection duration without increasing serious adverse reactions [16].
Establishing goals and targets for vaccine cost, efficacy, and protection duration and setting thresholds for identifying target populations during the course of vaccine development are of great importance to maximizing vaccine dissemination and utilization [17]. We developed a Markov computer simulation model to evaluate the economic value of vaccinating HD patients with an S. aureus vaccine. Sensitivity analyses varied S. aureus colonization prevalence and vaccine cost, efficacy, and duration of vaccine protection. The results of our model can help funders, policy makers, and vaccine manufacturers establish risk thresholds for and anticipate the impacts of vaccine costs and efficacy levels on the introduction and continued administration of an S. aureus vaccine among HD patients.
Methods
Model Structure
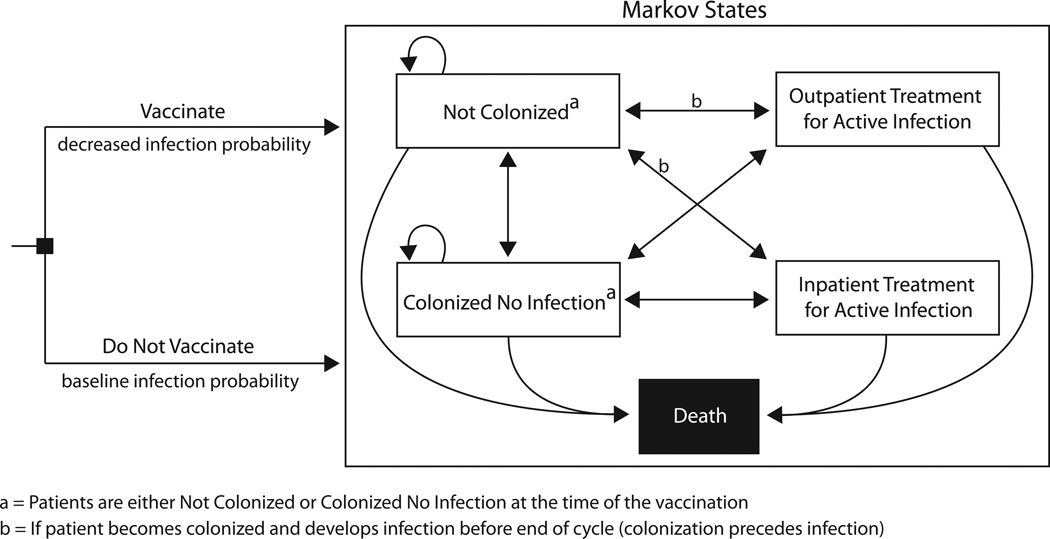
We adapted our previously published Markov model constructed in TreeAge Pro Suite 2009 (TreeAge Software, Williamstown, MA) to determine the potential economic value of S. aureus vaccination among HD patients from the third-party payer perspective [18]. Figure 1 illustrates the model structure, which included five Markov states representing an HD patient’s S. aureus colonization and infection status: (1) not S. aureus colonized, (2) S. aureus colonized without active infection, (3) active S. aureus infection with outpatient treatment, (4) active S. aureus infection with inpatient treatment, and (5) death (absorptive). For each trial, an HD patient went through the model twice—once vaccinated and once not vaccinated—starting off either colonized (S. aureus colonized without active infection Markov state) or not colonized (not S. aureus colonized Markov state) based on the local S. aureus prevalence. Patients regularly received booster vaccinations when undergoing the vaccination option.
FIGURE 1.
Markov model structure
National data from the United States Renal Data System (USRDS) determined the age of the HD patients entering our model (61 years) [8]. Each patient in the model had a specific dialysis vascular access type (i.e., tunneled dialysis catheter, arteriovenous fistula, or arteriovenous graft) and underwent dialysis treatment three times a week [8, 19]. Time steps, or cycles, in the model reflected the duration of the vaccine’s protective effects and corresponded to the frequency of booster vaccination. Each trial used a set cycle length of 3, 6, 9, or 12 months, as based on schedules of HD patient evaluation for ongoing dialysis treatment and existing standards for vaccination [20–23]. The vaccine’s efficacy attenuated the probability of S. aureus infection, but vaccinees could experience vaccine-related side effects.
At the end of every cycle, HD patients could remain in the same Markov state or transition to another. If colonized, patients could stay colonized or develop a clinically apparent S. aureus infection and be treated and medically decolonized. Each patient continued through the model until he or she reached the death state due to death from infection (i.e., inpatient infection-related mortality), from other causes (i.e., general HD patient mortality), or from reaching the end of his or her life expectancy (median: 4.8 years) [24].
All patients with an active S. aureus infection received intravenous antibiotic treatment (with costs uniformly distributed between the costs of cefazolin and vancomycin) and underwent medical decolonization (combination of mupirocin, rifampin, and chlorhexidine), which could also cause side effects. Decolonized patients could become recolonized in subsequent cycles. Patients with active infection as determined by the results of agar-based clinical isolates had probabilities of being treated either as outpatients (active S. aureus infection with outpatient treatment Markov state) or as inpatients (active S. aureus infection with inpatient treatment Markov state). Those treated as inpatients could have an invasive infection with or without any combination of the following clinical conditions: abscess, endocarditis, line infection, osteomyelitis, pneumonia, septic arthritis, and septic embolism. Hospitalization costs were condition-specific, age-stratified, and based on data from the Healthcare Cost and Utilization Project (HCUP) [25]. Using the American Medical Association’s CPT Code/Relative Value Search, clinical procedure costs were derived from Medicare’s relative value payment amount for each CPT code [26]. Total costs of invasive infection included costs of access site removal and insertion procedures specific to the patient’s access type.
Each model simulation run involved 1,000 trials of 1,000 HD patients (i.e., 1,000,000 unique outcomes). For each simulation, we evaluated the incremental cost-effectiveness ratio (ICER) of S. aureus vaccination according to the following equation:
where health effects are measured in quality-adjusted life years (QALYs). A $50,000/QALY threshold determined whether the vaccination strategy was cost-effective in a given scenario [27].
Data Inputs
Our model included probability, cost, time, and QALY parameters as shown in Table 1. Input values came from published literature or expert consultation (Dr. Robert R. Muder, Chief, Division of Infectious Diseases, VA Pittsburgh Healthcare System and Dr. Kenneth J. Smith, Section of Decision Sciences and Clinical Systems Modeling, University of Pittsburgh) and assumed distributions or point values based on the available data. An annual discount rate of 3% converted all past and future costs to 2011 US$. Number of antibiotic treatments represented the full course of antibiotics associated with a patient’s clinical condition (derived from MICROMEDX online[28], refined by expert opinion), receiving treatment three times a week according to his or her dialysis schedule (e.g., a condition associated with a 4-week course of antibiotics received 12 antibiotic treatments).
Table 1.
Table of Inputs.
| Description (Units) | Distribution Typea |
Mean | Standard deviation, Standard Errorc, or Range |
Source |
|---|---|---|---|---|
| Costs (US$) | ||||
| Cefazolin, 20mg/kg IV after dialysis (initial) | Gamma | 3.90 | 2.91 | [40–41] |
| Cefazolin 1g Dose (subsequent) | Gamma | 2.93 | 2.19 | [40–41] |
| Vancomycin, 1 g/dose (initial) | Gamma | 10.80 | 7.51b | [41] |
| Vancomycin, 500 mg/dose (subsequent) | Gamma | 5.40 | 3.76b | [41] |
| Decolonization Regimens: | ||||
| Mupirocin, 300 mg Dose | - | 15.91 | - | [42] |
| Rifampin, 2x Day/10 Days | - | 66.77 | 32.35b | [41] |
| Chlorhexidine, 4% Cholorhexidine Gluconate | - | 29.56 | - | [41] |
| Hospitalization: | ||||
| Non-invasive Infection (ages 45–64) | Triangular | 19,010 | 6,819 – 23,694 | [25] |
| Non-invasive Infection (ages 65–84) | Triangular | 14,678.5 | 6,519 – 21,457 | [25] |
| Abscess (ages 45–64) | Gamma | 7,593 | 774c | [25] |
| Abscess (ages 65–84) | Gamma | 8,489 | 1,970c | [25] |
| Bacteremia (ages 45–64) | Gamma | 14,391 | 787c | [25] |
| Bacteremia (ages 65–84) | Gamma | 13,691 | 551c | [25] |
| Endocarditis (ages 45–64) | Gamma | 23,844 | 4,409c | [25] |
| Endocarditis (ages 65–84) | Gamma | 24,379 | 3,648c | [25] |
| Line Infection (ages 45–64) | Gamma | 19,715 | 437c | [25] |
| Line Infection (ages 65–84) | Gamma | 20,562 | 504c | [25] |
| Osteomyelitis (ages 45–64) | Gamma | 26,024 | 6,609c | [25] |
| Osteomyelitis (ages 65–84) | Gamma | 14,767 | 4,309c | [25] |
| Pneumonia (ages 45–64) | Gamma | 22,834 | 860c | [25] |
| Pneumonia (ages 65–84) | Gamma | 20,868 | 589c | [25] |
| Septic Arthritis/Septic Embolism (ages 45–64) | Gamma | 24,693 | 440c | [25] |
| Septic Arthritis/Septic Embolism (ages 65–84) | Gamma | 19,626 | 326c | [25] |
| Clinical Procedures: | ||||
| Transthoracic Echocardiogram | Gamma | 161.66 | 47.02 | [26] |
| Arteriovenous Graft Insertion | - | 722.00 | - | [26] |
| Arteriovenous Graft Removal | - | 606.82 | - | [26] |
| Tunneled Dialysis Catheter Insertion | - | 288.80 | - | [26] |
| Tunneled Dialysis Catheter Removal | - | 143.38 | - | [26] |
| Temporary Catheter | - | 122.65 | - | [26] |
| Physician Consultation | - | 75.77 | - | [26] |
| Agar-based Clinical Culture | - | 12.12 | - | [43] |
| Utility Weights | ||||
| Healthy Year (ages 45–64) | - | 0.92 | - | [44] |
| Healthy Year (ages 65–84) | - | 0.84 | - | [44] |
| Dialysis | - | 0.6528 | 0.095 | [45–50] |
| Non-invasive Infection (Outpatient) | Beta | 0.725 | 0.035 | [51–52] |
| Non-invasive Infection (Inpatient) | Beta | 0.71 | 0.084 | [51, 53–54] |
| Bacteremia | Beta | 0.57 | 0.0566 | [55–56] |
| Abscess | Beta | 0.648 | 0.094 | [51–52, 57] |
| Endocarditis | Beta | 0.587 | 0.0603 | [55, 58–59] |
| Line Infection | Beta | 0.648 | 0.094 | [51–52, 57] |
| Osteomyelitis | Uniform | 0.53 – 0.59 | [60–61] | |
| Pneumonia | Beta | 0.625 | 0.0636 | [62–63] |
| Septic Arthritis | - | 0.600 | - | Expert Opinion |
| Septic Embolism | Triangular | 0.76 | 0.60 – 0.89 | [64] |
| Antibiotic Side Effects | Uniform | - | 0.980 – 0.999 | [65] |
| Vaccine Side Effects | Triangular | 0.950 | 0.710 – 1.00 | [50] |
| Probilities | ||||
| Access Site Type: | ||||
| Arteriovenous Fistula | - | 0.550 | - | [8] |
| Arteriovenous Graft | - | 0.272 | - | [8] |
| Tunneled Dialysis Catheter | - | 0.178 | - | [8] |
| S. aureus Outcomes: | ||||
| Infection given Colonization d | Uniform | - | 0.111 – 0.200 | [66–67] |
| Hospitalization given Infection | - | 0.746 | - | [5] |
| Invasive Infection (Bacteremia) given Hospitalization |
- | 0.428 | - | [5] |
|
Secondary Clinical Outcomes given Invasive Infectione: |
||||
| Abscess | Triangular | 0.078 | 0.032 – 0.191 | [5, 9, 68–69] |
| Endocarditis | Triangular | 0.107 | 0.011 – 0.171 | [5, 9, 68–70] |
| Line Infection | Triangular | 0.0773 | 0.076 – 0.0786 | [71] |
| Osteomyelitis | Triangular | 0.045 | 0.022 – 0.113 | [5, 9, 68–70] |
| Pneumonia | - | 0.160 | - | [72] |
| Septic Arthritis | Triangular | 0.04 | 0.032 – 0.048 | [9, 68–70] |
| Septic Embolism | Triangular | 0.056 | 0.048 – 0.072 | [9, 68] |
| Mortality: | ||||
| All Causes (ages 60–64)e | - | 0.168 | - | [8] |
| All Causes (ages 65–69)e | - | 0.200 | - | [8] |
| All Causes (ages 70–79)e | - | 0.257 | - | [8] |
| Non-invasive Infection (Inpatient) | - | 0.118 | - | [73] |
| Bacteremia | - | 0.202 | - | [5] |
| Endocarditis | - | 0.545 | - | [7] |
| Pneumonia | Beta | 0.368 | 0.174 | [72, 74–76] |
| Septic Arthritis/Septic Embolism | - | 0.222 | - | [77] |
| Side Effects from Vaccination | - | 0.050 | - | Expert Opinion |
| Side Effects from Antibiotic Treatment | - | 0.570 | - | [65] |
| Number of Antibiotic Courses | ||||
| Outpatient Treatment | Uniform | 4 – 6 | Expert Opinion, [28] |
|
| Abscess | - | 12 | - | Expert Opinion, [28] |
| Bacteremia | - | 12 | - | Expert Opinion, [28] |
| Endocarditis | Uniform | - | 12 – 18 | Expert Opinion, [28] |
| Line Infection | Uniform | - | 6 – 12 | Expert Opinion, [28] |
| Osteomyelitis | - | 18 | - | Expert Opinion, [28] |
| Pneumonia | - | 6 | - | Expert Opinion, [28] |
| Septic Arthritis/Septic Embolism | - | 12 | - | Expert Opinion, [28] |
| Duration of Hospitalization (Days)f | ||||
| Drug Treatment Side Effects | - | 7 | [65] | |
| Vaccine Side Effects | Triangular | 0.75 | 0.5 – 1.0 | [78] |
| Non-invasive Infection (Outpatient) (ages 45–64) | Gamma | 4.8 | −0.5c- | [79] |
| Non-invasive Infection (Outpatient) (ages 65–84) | Gamma | 6.2 | −1.5c- | [79] |
| Non-invasive Infection (Inpatient) (ages 45–64) | Gamma | 6.75 | 2.276 | [79] |
| Non-invasive Infection (Inpatient) (ages 65–85) | Gamma | 7.6 | 1.98 | [79] |
| Abscess (ages 45–64) | Gamma | 4.8 | −0.5c | [79] |
| Abscess (ages 65–84) | Gamma | 6.2 | −1.5c | [79] |
| Bacteremia (ages 45–64) | Gamma | 7.1 | 0.2c | [79] |
| Bacteremia (ages 65–84) | Gamma | 7.4 | 0.2c | [79] |
| Endocarditis (ages 45–64) | Gamma | 105. | 1.5c | [79] |
| Endocarditis (ages 65–84) | Gamma | 10.6 | 1.1c | [79] |
| Line Infection (ages 45–64) | Gamma | 9.2 | 0.2c | [79] |
| Line Infection (ages 65–84) | Gamma | 9.8 | 0.4c | [79] |
| Osteomyelitis (ages 45–64) | Gamma | 8.0 | 0.3c | [79] |
| Osteomyelitis (ages 65–84) | Gamma | 9.5 | 0.4c | [79] |
| Pneumonia (ages 45–64) | Gamma | 8.7 | 0.4c | [79] |
| Pneumonia (ages 65–84) | Gamma | 9.0 | 0.3c | [79] |
| Septic Arthritis/Septic Embolism (ages 45–64) | Gamma | 9.8 | 0.1c | [79] |
| Septic Arthritis/Septic Embolism (ages 65–84) | Gamma | 8.7 | 0.1c | [79] |
Based on available data
Standard deviation represents variations in the average wholesale price (AWP) across manufactures
Value is a standard error
Yearly value, modeled as a time dependent parameter
Clinical conditions of HD patient hospitalized for invasive S. aureus infection
Durations used for QALY decrements
Patients had baseline QALYs based on their ongoing dialysis treatment and age for the duration of their lifetime. If a patient experienced infectious complications or side effects from vaccination or treatment, net QALYs were the product of the patient’s baseline QALY and the utility weight associated with those additional conditions [29]. Patients with multiple clinical conditions received the utility weight resulting in the greatest QALY decrement. These utility weights applied to patients’ net QALYs for the duration of each condition, based on the duration of hospitalization for that condition (Table 1). The annual 3% discount rate also applied to future QALYs. Patients accrued the maximum costs of antibiotic treatment and hospitalization from among those associated with their conditions.
Sensitivity Analyses
Sensitivity analyses examined variations in key model parameters by systematically changing their values to determine their effects on the cost-effectiveness of S. aureus vaccination. As studies have shown widely ranging S. aureus nasal colonization prevalence in HD patients (e.g., affected by location-specific or temporal factors) [4, 30], we performed simulations ranging the probability of colonization from 1% to 40%. We also varied the vaccine’s cost ($100 to $300) to represent a wide range based on the Centers of Disease Control and Prevention (CDC) vaccine price list [31], as well as its efficacy (25% to 75%) and protection duration (3 to 12 months). Booster vaccination frequencies of 3, 6, 9, and 12 months corresponded to vaccine protection duration. Probabilistic sensitivity analyses sampled values from all input parameter distributions over the ranges indicated in Table 1.
Results
Table 2 shows the ICERs for vaccination in scenarios with varying S. aureus prevalence and vaccine cost and efficacy when the vaccine’s protective effects lasted 3 to 12 months. ICERs for vaccination were well below $50,000/QALY in all scenarios tested. Systematically varying S. aureus colonization prevalence and vaccine cost, efficacy, and protection duration in sensitivity analyses showed the degree to which each of these parameters affected the ICERs for S. aureus vaccination. In Table 2, “Vaccinate” corresponds to scenarios where vaccination was less costly and more effective than no vaccination, and therefore economically dominant (i.e., negative ICERs). Vaccination became more cost-effective as S. aureus colonization prevalence, vaccine efficacy, and duration of vaccine protection increased. Vaccination quickly became the dominant strategy when the probability of colonization was ≥20% at most vaccine costs, efficacies, and protection durations tested. At a 1% colonization rate, vaccination was not the dominant strategy but still remained cost-effective; ICERs ranged from $1,248/214 48/QALY to $25,217/QALY. At a 5% colonization rate, vaccination became dominant when the vaccine’s cost was ≤$100, efficacy was ≥75%, and protection duration was ≥6 months. For an S. aureus colonization prevalence ≥30%, vaccination generally dominated no vaccination (Table 2).
Table 2.
Incremental cost-effectiveness ratio (ICER: US$/QALY) of vaccination compared to no vaccination at different Staphylococcus aureus colonization prevalence, vaccine costs and efficacies, and durations of vaccine protection.
| Vaccine Cost |
Vaccine Efficacy |
Prevalence of SA Colonization (%) |
|||||
|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 20 | 30 | 40 | ||
|
Vaccine Protection for 3 Months | |||||||
| 25% | 8,371 | 7,265 | 5,944 | 3,521 | 1,482 | Vaccinate | |
| $100 | 50% | 7,972 | 5,571 | 2,801 | Vaccinate | Vaccinate | Vaccinate |
| 75% | 7,508 | 3,198 | Vaccinate | Vaccinate | Vaccinate | Vaccinate | |
| 25% | 16,880 | 16,759 | 14,221 | 11,499 | 8,987 | 6,971 | |
| $200 | 50% | 16,382 | 15,920 | 10,854 | 5,495 | 1,038 | Vaccinate |
| 75% | 11,226 | 15,938 | 6,415 | Vaccinate | Vaccinate | Vaccinate | |
| 25% | 25,217 | 24,256 | 22,128 | 19,441 | 16,845 | 14,380 | |
| $300 | 50% | 24,889 | 21,860 | 19,059 | 12,957 | 8,356 | 4,322 |
| 75% | 24,361 | 19,257 | 14,182 | 5,441 | Vaccinate | Vaccinate | |
|
Vaccine Protection for 6 Months | |||||||
| 25% | 4,171 | 3,119 | 1,929 | Vaccinate | Vaccinate | Vaccinate | |
| $100 | 50% | 3,826 | 1,532 | Vaccinate | Vaccinate | Vaccinate | Vaccinate |
| 75% | 3,310 | Vaccinate | Vaccinate | Vaccinate | Vaccinate | Vaccinate | |
| 25% | 8,581 | 7,499 | 6,186 | 3,746 | 1,682 | Vaccinate | |
| $200 | 50% | 8,162 | 5,698 | 2,995 | Vaccinate | Vaccinate | Vaccinate |
| 75% | 7,784 | 3,336 | Vaccinate | Vaccinate | Vaccinate | Vaccinate | |
| 25% | 12,928 | 11,793 | 10,355 | 7,919 | 5,646 | 3,705 | |
| $300 | 50% | 12,411 | 10,003 | 7,202 | 2,064 | Vaccinate | Vaccinate |
| 75% | 12,221 | 7,702 | 2,836 | Vaccinate | Vaccinate | Vaccinate | |
|
Vaccine Protection for 9 Months | |||||||
| 25% | 2,856 | 1,865 | 753 | Vaccinate | Vaccinate | Vaccinate | |
| $100 | 50% | 2,545 | 226 | Vaccinate | Vaccinate | Vaccinate | Vaccinate |
| 75% | 2,007 | Vaccinate | Vaccinate | Vaccinate | Vaccinate | Vaccinate | |
| 25% | 5,848 | 4,893 | 4,978 | 3,693 | Vaccinate | Vaccinate | |
| $200 | 50% | 5,563 | 3,264 | 595 | Vaccinate | Vaccinate | Vaccinate |
| 75% | 2,112 | 865 | Vaccinate | Vaccinate | Vaccinate | Vaccinate | |
| 25% | 8,912 | 7,935 | 6,638 | 4,245 | 2,166 | 259 | |
| $300 | 50% | 8,518 | 6,239 | 3,508 | Vaccinate | Vaccinate | Vaccinate |
| 75% | 8,077 | 3,920 | Vaccinate | Vaccinate | Vaccinate | Vaccinate | |
|
Vaccine Protection for 12 Months | |||||||
| 25% | 2,082 | 1,063 | Vaccinate | Vaccinate | Vaccinate | Vaccinate | |
| $100 | 50% | 1,733 | Vaccinate | Vaccinate | Vaccinate | Vaccinate | Vaccinate |
| 75% | 1,248 | Vaccinate | Vaccinate | Vaccinate | Vaccinate | Vaccinate | |
| 25% | 4,384 | 3,371 | 2,168 | Vaccinate | Vaccinate | Vaccinate | |
| $200 | 50% | 4,056 | 3,430 | Vaccinate | Vaccinate | Vaccinate | Vaccinate |
| 75% | 3,540 | Vaccinate | Vaccinate | Vaccinate | Vaccinate | Vaccinate | |
| 25% | 6,704 | 5,573 | 4,440 | 2,072 | 147 | Vaccinate | |
| $300 | 50% | 6,390 | 3,959 | 1,272 | Vaccinate | Vaccinate | Vaccinate |
| 75% | 5,855 | 1,593 | Vaccinate | Vaccinate | Vaccinate | Vaccinate | |
Vaccinate: vaccination is less costly and more effective than no vaccination (i.e., dominant)
S. aureus vaccination saved costs when economically dominant. Vaccines protecting for 3 months saved between $77 (40% colonization, $100 cost, 25% efficacy) and $3,796 (40% colonization, $100 cost, 75% efficacy) per person vaccinated. Savings ranged from $44 (20% colonization, $100 cost, 25% efficacy) to $4,399 (40% colonization, $100 cost, 75% efficacy), $108 (30% colonization, $200 cost, 25% efficacy) to $4,539 (40% colonization, $100 cost, 75% efficacy), and $5 (10% colonization, $100 cost, 25% efficacy) to $4,635 (40% colonization, $100 cost, 75% efficacy) for vaccines protecting for 6, 9, and 12 months, respectively. Savings increased with colonization prevalence, vaccine efficacy, and protection duration, but decreased with increasing vaccine cost. At a given prevalence, the scenarios with the lowest vaccine cost ($100), highest efficacy (75%), and longest protection duration (12 months) yielded the greatest savings: at 5% colonization prevalence, savings ranged from $76 ($200 cost, efficacy 75%,12 month duration) to $424; at 10% prevalence, savings ranged from $139 ($300 cost, efficacy 75%, 9 month duration) to $1,166; at 20% prevalence, vaccination saved between $44 ($100 cost, efficacy 25%, 6 month duration) and $2,452; at 30% prevalence, savings ranged from $108 ($200 cost, efficacy 25%, 9 month duration) to $3,615; and at 40% prevalence, it ranged from $77 ($100 cost, efficacy 25%, 3 month duration) to $4,635 per vaccinated individual.
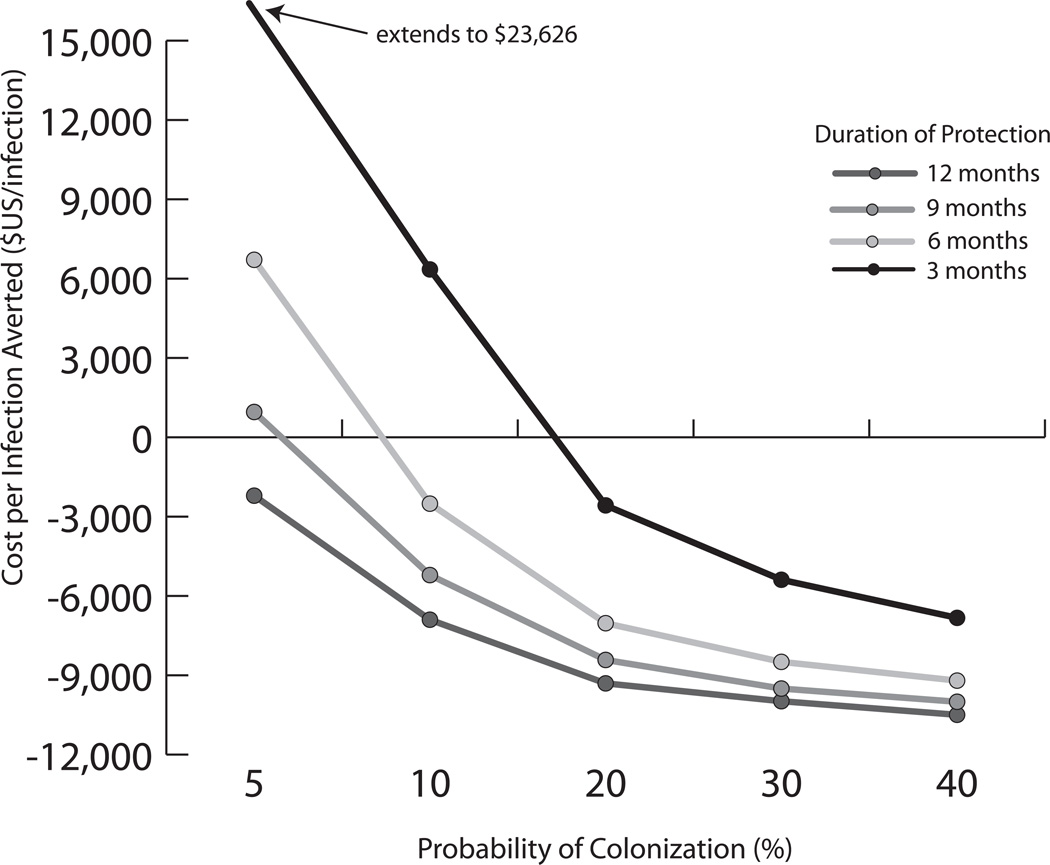
Figure 2 illustrates the cost per infection averted for different colonization rates and durations of protection with a $100, 50% efficacious vaccine. Negative costs imply cost savings per infection averted with vaccination. A vaccine protecting for 12 months provided cost savings per averted infection for all colonization rates ≥5%. Even a 3-month protective vaccine saved costs per averted infection when the colonization rate was ≥20%.
FIGURE 2.
Cost per infection averted for a $100 vaccine with 50% efficacy at varying colonization rates.
Population Level Infection-related Outcomes
Over the course of a vaccinated HD patient’s lifetime in the scenario with a 10% S. aureus colonization prevalence , vaccination prevented 29, 69, and 128 S. aureus infections per 1,000 HD patients vaccinated for vaccine efficacies of 25%, 50%, and 75%, respectively. Additionally, a 25% efficacious vaccine averted 21 hospitalizations and 4 inpatient deaths; a 50% efficacious vaccine averted 52 hospitalizations and 9 inpatient deaths; and a 75% efficacious vaccine prevented 96 hospitalizations and 17 inpatient deaths over the course of 1,000 vaccinated patient lifetimes. At a 30% colonization prevalence, vaccination averted to 340 infections, 61 to 254 hospitalizations, and 11 to 44 inpatient deaths per 1,000 vaccinated HD patients over their lifetime, varying by efficacy (25% to 75%). S. aureus infections, hospitalizations, and inpatient deaths averted increased with increasing vaccine efficacy (i.e., greater vaccine efficacy averted more S. aureus clinical outcomes) and probability of colonization.
DISCUSSION
Forecasting the impact of a vaccine early in its course of development may help increase its adoption and continued utilization [17]. Modeling can help guide investments prior to vaccine licensure, provide benchmarks for vaccine pricing and efficacy, and establish target populations. Our group has previously reported the economic impact of vaccines for various infectious diseases, including S. aureus for other patient populations [32–33] as well as Clostridium difficile [34] and influenza [35]. Results from these studies suggested that an S. aureus vaccine would be cost-effective in high-risk patient populations, such as neonates and orthopedic patients.
Due in part to their reduced immunoresponsiveness associated with chronic renal failure and frequent exposures to invasive devices and healthcare environments, HD patients comprise another group at elevated risk for infections, including S. aureus infections [1–3]. For this reason, higher doses of hepatitis B vaccine are recommended for HD patients in comparison to the general population [21]. According to the CDC, HD patient immunization practices are based on both age and high-risk conditions and include vaccinations for hepatitis B, pneumococcal polysaccharide, and annual inactivated influenza [20, 36]. Additionally, the duration of immunity following an HD patient’s vaccination is shorter than that of a healthy patient. Consequently, the CDC recommends that HD patients have their hepatitis B antibody titers checked annually and that booster vaccination be performed when titers are low [21]. This shorter duration results from both impairments in the immune system and declining antibody levels due to protein loss, as is the case with nephrotic syndrome.
HD patients routinely receive medical care at dialysis centers. Vaccination in these centers is ideal, given the frequency of patient visits. Vaccination programs that use approaches involving system changes, such as standing orders, raise immunization rates more than programs that use other approaches [37]. For vaccines targeted to persons with high-risk conditions, the CDC Task Force on Community Preventive Services recommends multiple interventions in combination [38]. With these considerations in mind, it is not surprising that vaccine developers have already proceeded to pursue an S. aureus vaccine for HD patients. Clinical trials have contributed to advancements in the development of a functioning S. aureus vaccine [10–16]. In addition to the active immunization options being explored, several passive immunization candidates are currently underway, with several having already completed Phase II and/or III clinical trials [39].
Our results suggest that an S. aureus vaccine would be cost-effective among HD patients over a wide range of S. aureus colonization prevalence, vaccine costs and efficacies, and durations of vaccine protection. Alexander et al. reported a 15.9% persistent S. aureus nasal colonization rate among HD outpatients[4], while Kirmani et al. reported a 40% colonization prevalence[30], our results show that vaccination of HD patients at both of these rates can be cost-effective, dominating for many vaccine characteristics. The population-level impact of an S. aureus vaccine among HD patients can be sizable, with vaccination preventing up to 317 hospitalizations and 55 inpatient deaths associated with 425 S. aureus infections per 1,000 vaccinated HD patients at 40% colonization prevalence (75% vaccine efficacy). Though ICERs for S. aureus vaccination remained ≤$50,000/QALY in all scenarios tested, they decreased further with higher S. aureus prevalence, lower vaccine cost, greater vaccine efficacy, or longer duration of vaccine protection. Even for vaccines with the lowest efficacy (25%) and shortest protection duration (3 months), the ICERs for vaccination versus no vaccination were ≤$25,217/QALY. Vaccination quickly became the dominant strategy for vaccines with ≥50% efficacy and ≥6 months of protection when S. aureus prevalence was ≥20% and vaccine cost was ≤$200. These results emphasize the important roles of local prevalence and vaccine cost in the implementation of an S. aureus vaccine. As the distribution of access types represented in our model reflected usage by a heterogeneous cohort of HD patients[8], the effects of 305 vaccination on each type were not compared individually, however access type could have an effect on the probability of infection given colonization and the cost of treatment. When planning an S. aureus vaccination program in HD patients, vaccine developers, insurance payers, and other decision makers involved in the distribution and administration of the vaccine may want to focus particularly on the risk of S. aureus colonization and the cost of the vaccine to the local HD patient community.
Our model tended to be conservative about the potential benefits of an S. aureus vaccine, as it used lower estimates of infection-related procedural costs and excluded rarer S. aureus complications (e.g., meningitis, atrial thrombus, and stroke). Our model required patients to have S. aureus nasal colonization prior to becoming infected, underestimating the rate of overall colonization (e.g. including oropharynx, axilla, and groin colonization) as well as cases of infection without preceding colonization. The model did not consider the additional benefits of reducing transmission, indirectly protecting those not vaccinated.
Limitations
All computer simulation models are simplified portrayals of the environments and situations they simulate. Our model did not account for all possible outcomes of S. aureus infection in HD patients. Available data restricted the array of clinical conditions included in the model, and parameterization involved derivation from a range of studies and databases of varying quality. Though sensitivity analyses attempted to address a wide range of scenarios, individual case variability may extend beyond the included parameter values. Also, our results may not be applicable to younger patients, as individuals in our HD patient population were ≥61 years of age. Our analysis was limited to HD patients; the cost-effectiveness of vaccination may be different for peritoneal dialysis patients, as their rates of S. aureus colonization and infection are not well defined and varies in the reported literature.
Conclusions
Our results suggest that an S. aureus vaccine for HD patients would be cost-effective over a wide range of estimated S. aureus prevalence, vaccine costs and efficacies, and durations of vaccine protection. Vaccination could reduce the incidence of S. aureus infections in this at-risk population, yielding projected benefits for both individual patients and patient populations that would outweigh the costs of the vaccine and the impact of possible side effects.
Highlights.
We model the potential economic value of a Staphylococcus aureus vaccine in hemodialysis patients
Sensitivity analysis varied colonization prevalence and vaccine characteristics
Vaccination was cost-effective for all tested scenarios and quickly became economically dominant
Vaccination would be cost-effective over a wide range of prevalence rates and vaccine costs, efficacies, and protection durations
Acknowledgements
This work was supported by the National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) grant 5U54GM088491-02 and the Pennsylvania Department of Health (DOH) grant 4100047864. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
We would like to thank Dr. Kenneth J. Smith for his guidance for parameter estimates.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yeohan Song, Email: ysongmd@umich.edu.
Julie H.Y. Tai, Email: juliehy@pitt.edu.
Sarah M. Bartsch, Email: smm168@pitt.edu.
Richard K. Zimmerman, Email: zimmrk@upmc.edu.
Robert R. Muder, Email: robert.muder@va.gov.
REFERENCES
- 1.Fitzgibbons LN, Puls DL, Mackay K, Forrest GN. Management of gram-positive coccal bacteremia and hemodialysis. Am J Kidney Dis. 2011 Apr;57(4):624–640. doi: 10.1053/j.ajkd.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Sexton DJ. Vascular access infections in patients undergoing dialysis with special emphasis on the role and treatment of Staphylococcus aureus. Infect Dis Clin North Am. 2001 Sep;15(3):731–742. doi: 10.1016/s0891-5520(05)70170-7. vii. [DOI] [PubMed] [Google Scholar]
- 3.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998 Aug 20;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 4.Alexander EL, Morgan DJ, Kesh S, Weisenberg SA, Zaleskas JM, Kaltsas A, et al. Prevalence, persistence, and microbiology of Staphylococcus aureus nasal carriage among hemodialysis outpatients at a major New York hospital. Diagn Microbiol Infect Dis. 2011 Feb 17;70(1):37–44. doi: 10.1016/j.diagmicrobio.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Friedman JY, O'Neal BF, Hohenboken MJ, Griffiths RI, Stryjewski ME, et al. Outcomes of Staphylococcus aureus infection in hemodialysis-dependent patients. Clin J Am Soc Nephrol. 2009 Feb;4(2):428–34. doi: 10.2215/CJN.03760708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greiner W, Rasch A, Kohler D, Salzberger B, Fatkenheuer G, Leidig M. Clinical outcome and costs of nosocomial and community-acquired Staphylococcus aureus bloodstream infection in haemodialysis patients. Clin Microbiol Infect. 2007 Mar;13(3):264–268. doi: 10.1111/j.1469-0691.2006.01622.x. [DOI] [PubMed] [Google Scholar]
- 7.Chang FY, MacDonald BB, Peacock JE, Jr, Musher DM, Triplett P, Mylotte JM, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003 Sep;82(5):322–332. doi: 10.1097/01.md.0000091185.93122.40. [DOI] [PubMed] [Google Scholar]
- 8.USRDS 2010 Annual Data Report: atlas of chronic kidney disease and end-stage renal disease in the United States [database on the Internet] National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2010. [cited 2011 May 17]. Available from: http://www.usrds.org/adr.htm. [Google Scholar]
- 9.Inrig JK, Reed SD, Szczech LA, Engemann JJ, Friedman JY, Corey GR, et al. Relationship between clinical outcomes and vascular access type among hemodialysis patients with Staphylococcus aureus bacteremia. Clin J Am Soc Nephrol. 2006 May;1(3):518–524. doi: 10.2215/CJN.01301005. [DOI] [PubMed] [Google Scholar]
- 10.Merck A study to evaluate the safety and immunogenicity of V710 in adults with kidney disease on hemodialysis. 2000 Available from: http://clinicaltrials.gov/show/NCT00572910.
- 11.Nabi Biopharmaceuticals. Study to evaluate the effectiveness of StaphVAX in adults on hemodialysis. 2000 Available from: http://clinicaltrials.gov/show/NCT00071214.
- 12.Harro C, Betts R, Orenstein W, Kwak EJ, Greenberg HE, Onorato MT, et al. Safety and immunogenicity of a novel Staphylococcus aureus vaccine: results from the first study of the vaccine dose range in humans. Clin Vaccine Immunol. 2010 Dec;17(12):1868–1874. doi: 10.1128/CVI.00356-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creech CB, 2nd, Johnson BG, Alsentzer AR, Hohenboken M, Edwards KM, Talbot TR., 3rd Vaccination as infection control: a pilot study to determine the impact of Staphylococcus aureus vaccination on nasal carriage. Vaccine. 2009 Dec 10;28(1):256–260. doi: 10.1016/j.vaccine.2009.09.088. [DOI] [PubMed] [Google Scholar]
- 14.Fattom A, Schneerson R, Watson DC, Karakawa WW, Fitzgerald D, Pastan I, et al. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect Immun. 1993 Mar;61(3):1023–1032. doi: 10.1128/iai.61.3.1023-1032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002 Feb 14;346(7):491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 16.Fattom A, Fuller S, Propst M, Winston S, Muenz L, He D, et al. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine. 2004 Dec 16;23(5):656–663. doi: 10.1016/j.vaccine.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine. 2010 Apr 1;28(16):2806–2809. doi: 10.1016/j.vaccine.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BY, Song Y, McGlone SM, Bailey RR, Feura JM, Tai JH, et al. The economic value of screening haemodialysis patients for methicillin-resistant Staphylococcus aureus in the USA. Clin Microbiol Infect. 2011 Apr 4; doi: 10.1111/j.1469-0691.2011.03525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowrie EG, Li Z, Ofsthun N, Lazarus JM. Measurement of dialyzer clearance, dialysis time, and body size: death risk relationships among patients. Kidney Int. 2004 Nov;66(5):2077–2084. doi: 10.1111/j.1523-1755.2004.00987.x. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Recommended adult immunization schedule, United States, 2011. 2011 Available from: http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. [PubMed]
- 21.Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. Washington, D.C.: Public Health Foundation; 2011. Available from: http://www.cdc.gov/vaccines/pubs/pinkbook/default.htm#download. [Google Scholar]
- 22.Centers for Disease Control and Prevention. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. 2006 Available from: http://www.cdc.gov/vaccines/pubs/downloads/b_dialysis_guide-508.pdf.
- 23.National Kidney Foundation, Kidney Disease Outcome Quality Initiative (KDOQI) Advisory Board. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. American Journal of Kidney Disease. 2002;39(Suppl 2):s1–s246. [PubMed] [Google Scholar]
- 24.USRDS 2009 Annual Data Report: atlas of chronic kidney disease and end-stage renal disease in the United States [database on the Internet] National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009. [cited 2011 May 17]. Available from: http://www.usrds.org/adr_2009.htm. [Google Scholar]
- 25.HCUP facts and figures: statistics on hospital-based care in the United States, 2008. [database on the Internet] [cited 2011 April 27];Agency for Healthcare Research and Quality. 2010 Available from: http://www.hcup-us.ahrq.gov/reports.jsp. [PubMed]
- 26.American Medical Association. [cited 2011 April 27];CPT Code/Relative Value Search. 2011 Available from: https://ocm.ama-assn.org/OCM/CPTRelativeValueSearch.do.
- 27.Neumann PJ, Sandberg EA, Bell CM, Stone PW, Chapman RH. Are pharmaceuticals cost-effective? A review of the evidence. Health Affairs. 2000;19(2):92–109. doi: 10.1377/hlthaff.19.2.92. [DOI] [PubMed] [Google Scholar]
- 28.MICROMEDX 2.0. Ann Arbor, MI: Thomson Reuters; 2011. [updated 2011] [Google Scholar]
- 29.Flanagan W, McIntosh CN, Le Petit C, Berthelot JM. Deriving utility scores for comorbid conditions: a test of the multiplicative model for combining individual condition scores. Popul Health Metr. 2006;4:13. doi: 10.1186/1478-7954-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirmani N, Tuazon CU, Murray HW, Parrish AE, Sheagren JN. Staphylococcus aureus carriage rate of patients receiving long-term hemodialysis. Arch Intern Med. 1978 Nov;138(11):1657–1659. [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. [cited 2011];Vaccines & Immunizations: VFC: CDC Vaccine Price List. 2011 Available from: http://www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list-archives.htm.
- 32.Lee BY, Wiringa AE, Bailey RR, Lewis GJ, Feura J, Muder RR. Staphylococcus aureus vaccine for orthopedic patients: an economic model and analysis. Vaccine. 2010 Mar 11;28(12):2465–2471. doi: 10.1016/j.vaccine.2009.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee BY, Ufberg PJ, Bailey RR, Wiringa AE, Smith KJ, Nowalk AJ, et al. The potential economic value of a Staphylococcus aureus vaccine for neonates. Vaccine. 2010 Jun 23;28(29):4653–4660. doi: 10.1016/j.vaccine.2010.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee BY, Popovich MJ, Tian Y, Bailey RR, Ufberg PJ, Wiringa AE, et al. The potential value of Clostridium difficile vaccine: an economic computer simulation model. Vaccine. 2010 Jul 19;28(32):5245–5253. doi: 10.1016/j.vaccine.2010.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee BY, Tai JH, Bailey RR, Smith KJ, Nowalk AJ. Economics of influenza vaccine administration timing for children. Am J Manag Care. 2010 Mar;16(3):e75–e85. [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011 Jan 28;60(2):1–64. [PubMed] [Google Scholar]
- 37.Task Force on Community Preventive Services. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000 Jan;18(1 Suppl):92–96. [PubMed] [Google Scholar]
- 38.Guide to Community Preventive Services. Vaccinations to prevent diseases: targeted vaccinations. 2011 Available from: www.thecommunityguide.org/vaccines/targeted/index.html.
- 39.Schaffer AC, Lee JC. Vaccination and passive immunisation against Staphylococcus aureus. Int J Antimicrob Agents. 2008 Nov;32(Suppl 1):S71–S78. doi: 10.1016/j.ijantimicag.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Kuypers D, Vanwalleghem J, Maes B, Messiaen T, Vanrenterghem Y, Peetermans WE. Cefazolin serum concentrations with fixed intravenous dosing in patients on chronic hemodialysis treatment. Nephrol Dial Transplant. 1999 Aug;14(8):2050–2051. doi: 10.1093/ndt/14.8.2050. [DOI] [PubMed] [Google Scholar]
- 41.Physicians Desk Reference. Red Book Pharmacy's Fundamental Reference. Montvale, NJ: Thompson Reuters (Healthcare), Inc.; 2010. [Google Scholar]
- 42.McConeghy KW, Mikolich DJ, LaPlante KL. Agents for the decolonization of methicillin-resistant Staphylococcus aureus. Pharmacotherapy. 2009 Mar;29(3):263–280. doi: 10.1592/phco.29.3.263. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Medicare & Medicaid Services. 2010 Clinical Diagnostic Laboratory Fee Schedule (CLAB) Baltimore, MD: U.S. Department of Health & Human Services; 2010. [updated April 2010; cited 2010 April 27]. Available from: http://www.cms.gov/ClinicalLabFeeSched/ [Google Scholar]
- 44.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998 Jun;36(6):778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Desai AA, Baras J, Berk BB, Nakajima A, Garber AM, Owens D, et al. Management of acute kidney injury in the intensive care unit: a cost-effectiveness analysis of daily vs alternate-day hemodialysis. Arch Intern Med. 2008 Sep 8;168(16):1761–1767. doi: 10.1001/archinte.168.16.1761. [DOI] [PubMed] [Google Scholar]
- 46.Kontodimopoulos N, Niakas D. An estimate of lifelong costs and QALYs in renal replacement therapy based on patients' life expectancy. Health Policy. 2008 Apr;86(1):85–96. doi: 10.1016/j.healthpol.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Nuijten M, Andress DL, Marx SE, Sterz R. Chronic kidney disease Markov model comparing paricalcitol to calcitriol for secondary hyperparathyroidism: a US perspective. Curr Med Res Opin. 2009 May;25(5):1221–1234. doi: 10.1185/03007990902844097. [DOI] [PubMed] [Google Scholar]
- 48.Schweitzer EJ, Perencevich EN, Philosophe B, Bartlett ST. Estimated benefits of transplantation of kidneys from donors at increased risk for HIV or hepatitis C infection. Am J Transplant. 2007 Jun;7(6):1515–1525. doi: 10.1111/j.1600-6143.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- 49.Teerawattananon Y, Mugford M, Tangcharoensathien V. Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand. Value Health. 2007 Jan-Feb;10(1):61–72. doi: 10.1111/j.1524-4733.2006.00145.x. [DOI] [PubMed] [Google Scholar]
- 50.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Medical Care. 2000;38(6):583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Brasel KJ, Borgstrom DC, Weigelt JA. Cost-utility analysis of contaminated appendectomy wounds. J Am Coll Surg. 1997 Jan;184(1):23–30. [PubMed] [Google Scholar]
- 52.Chung KC, Walters MR, Greenfield ML, Chernew ME. Endoscopic versus open carpal tunnel release: a cost-effectiveness analysis. Plast Reconstr Surg. 1998 Sep;102(4):1089–1099. doi: 10.1097/00006534-199809040-00026. [DOI] [PubMed] [Google Scholar]
- 53.Gerson L, Kamal A. Cost-effectiveness analysis of management strategies for obscure GI bleeding. Gastrointestinal Endoscopy. 2008;68(5):920–936. doi: 10.1016/j.gie.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 54.Loftus EV, Jr, Johnson SJ, Yu AP, Wu EQ, Chao J, Mulani PM. Cost-effectiveness of adalimumab for the maintenance of remission in patients with Crohn's disease. Eur J Gastroenterol Hepatol. 2009 Nov;21(11):1302–1309. doi: 10.1097/MEG.0b013e32832a8d71. [DOI] [PubMed] [Google Scholar]
- 55.Sackett DL, Torrance GW. The utility of different health states as perceived by the general public. Journal of Chronic Diseases. 1978;31(11):697–704. doi: 10.1016/0021-9681(78)90072-3. [DOI] [PubMed] [Google Scholar]
- 56.Yazdanpanah Y, Goldie SJ, Paltiel AD, Losina E, Coudeville L, Weinstein MC, et al. Prevention of human immunodeficiency virus-related opportunistic infections in France: a cost-effectiveness analysis. Clin Infect Dis. 2003 Jan 1;36(1):86–96. doi: 10.1086/344902. [DOI] [PubMed] [Google Scholar]
- 57.Selai C, Rosser R. Eliciting EuroQol descriptive data and utility scale values from inpatients. A feasibility study. Pharmacoeconomics. 1995;8(2):147–158. doi: 10.2165/00019053-199508020-00006. [DOI] [PubMed] [Google Scholar]
- 58.Caviness AC, Cantor SB, Allen CH, Ward MA. A cost-effectiveness analysis of bacterial endocarditis prophylaxis for febrile children who have cardiac lesions and undergo urinary catheterization in the emergency department. Pediatrics. 2004 May;113(5):1291–1296. doi: 10.1542/peds.113.5.1291. [DOI] [PubMed] [Google Scholar]
- 59.Kermode M, Yong K, Hurley S, Marmion B. An economic evaluation of increased uptake in Q fever vaccination among meat and agricultural industry workers following implementation of the National Q Fever Management Program. Aust N Z J Public Health. 2003;27(4):390–398. doi: 10.1111/j.1467-842x.2003.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 60.Ortegon MM, Redekop WK, Niessen LW. Cost-effectiveness of prevention and treatment of the diabetic foot: a Markov analysis. Diabetes Care. 2004 Apr;27(4):901–907. doi: 10.2337/diacare.27.4.901. [DOI] [PubMed] [Google Scholar]
- 61.Lee BY, Bailey RR, Smith KJ, Muder RR, Strotmeyer ES, Lewis GJ, et al. Universal methicillin-resistant Staphylococcus aureus (MRSA) surveillance for adults at hospital admission: an economic model and analysis. Infection Control and Hospital Epidemiology. 2010;31(6):598–606. doi: 10.1086/652524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Medical Care. 1998;36(6):778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Lee GM, Lebaron C, Murphy TV, Lett S, Schauer S, Lieu TA. Pertussis in adolescents and adults: should we vaccinate? Pediatrics. 2005 Jun;115(6):1675–1684. doi: 10.1542/peds.2004-2509. [DOI] [PubMed] [Google Scholar]
- 64.Spangler EL, Dillavou ED, Smith KJ. Cost-effectiveness of guidelines for insertion of inferior vena cava filters in high-risk trauma patients. J Vasc Surg. 2010 Dec;52(6):1537–1545. e1–e2. doi: 10.1016/j.jvs.2010.06.152. [DOI] [PubMed] [Google Scholar]
- 65.McBrien KA, Kleinman KP, Abrams AM, Prosser LA. Use of outcomes to evaluate surveillance systems for bioterrorist attacks. BMC Medical Informatics and Decision Making. 2010;10(25) doi: 10.1186/1472-6947-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaplowitz LG, Comstock JA, Landwehr DM, Dalton HP, Mayhall CG. Prospective study of microbial colonization of the nose and skin and infection of the vascular access site in hemodialysis patients. J Clin Microbiol. 1988 Jul;26(7):1257–1262. doi: 10.1128/jcm.26.7.1257-1262.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu VL, Goetz A, Wagener M, Smith PB, Rihs JD, Hanchett J, et al. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N Engl J Med. 1986 Jul 10;315(2):91–96. doi: 10.1056/NEJM198607103150204. [DOI] [PubMed] [Google Scholar]
- 68.Engemann JJ, Friedman JY, Reed SD, Griffiths RI, Szczech LA, Kaye KS, et al. Clinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysis. Infect Control Hosp Epidemiol. 2005 Jun;26(6):534–539. doi: 10.1086/502580. [DOI] [PubMed] [Google Scholar]
- 69.Marr KA, Kong L, Fowler VG, Gopal A, Sexton DJ, Conlon PJ, et al. Incidence and outcome of Staphylococcus aureus bacteremia in hemodialysis patients. Kidney Int. 1998 Nov;54(5):1684–1689. doi: 10.1046/j.1523-1755.1998.00134.x. [DOI] [PubMed] [Google Scholar]
- 70.Fowler VG, Jr, Justice A, Moore C, Benjamin DK, Jr, Woods CW, Campbell S, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis. 2005 Mar 1;40(5):695–703. doi: 10.1086/427806. [DOI] [PubMed] [Google Scholar]
- 71.Healthcare Cost and Utilization Project. Nationwide Inpatient Sample. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [Google Scholar]
- 72.Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007 Mar;28(3):273–279. doi: 10.1086/512627. [DOI] [PubMed] [Google Scholar]
- 73.Allon M, Depner TA, Radeva M, Bailey J, Beddhu S, Butterly D, et al. Impact of dialysis dose and membrane on infection-related hospitalization and death: results of the HEMO Study. J Am Soc Nephrol. 2003 Jul;14(7):1863–1870. doi: 10.1097/01.asn.0000074237.78764.d1. [DOI] [PubMed] [Google Scholar]
- 74.DeRyke CA, Lodise TP, Jr, Rybak MJ, McKinnon PS. Epidemiology, treatment, and outcomes of nosocomial bacteremic Staphylococcus aureus pneumonia. Chest. 2005 Sep;128(3):1414–1422. doi: 10.1378/chest.128.3.1414. [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez C, Rubio M, Romero-Vivas J, Gonzalez M, Picazo JJ. Staphylococcus aureus bacteremic pneumonia: differences between community and nosocomial acquisition. Int J Infect Dis. 2003 Jun;7(2):102–108. doi: 10.1016/s1201-9712(03)90004-x. [DOI] [PubMed] [Google Scholar]
- 76.Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003 Nov;124(5):1789–1797. [PubMed] [Google Scholar]
- 77.Slaughter S, Dworkin RJ, Gilbert DN, Leggett JE, Jones S, Bryant R, et al. Staphylococcus aureus septic arthritis in patients on hemodialysis treatment. West J Med. 1995 Aug;163(2):128–132. [PMC free article] [PubMed] [Google Scholar]
- 78.Centers for Disease Control and Prevention. Possible side-effects from vaccines. Atlanta, GA: 2009. [updated August, 2009; cited 2009 August 17]. [Google Scholar]
- 79.United States Department of Health & Human Services. HCUP facts and figures: statistics on hospital-based care in the United States. Rockville, MD: AHRQ: Agency for Healthcare Research and Quality; 2009. [cited 2011 October 5]. Available from: http://www.hcup-us.ahrq.gov/reports.jsp. [PubMed] [Google Scholar]