Abstract
We demonstrate a cross-phase modulation measurement technique based on the sensitive detection of modulation transfer in a pump-probe setup. By modulating the amplitude of the pump beam and spectrally analyzing the probe beam, we achieve a rapid, background-free measurement of nonlinear phase modulation using power levels acceptable in biological imaging. This measurement technique would allow the extension of widely employed phase microscopy methods to the nonlinear regime, providing intrinsic and universal nonlinear contrast for biological imaging.
The nonlinear refractive index, n2, is sensitive to a wide range of material properties, such as resonant and non-resonant electronic or molecular reorientation effects, yet unlike its linear counterpart, it is not commonly utilized as a microscopy contrast. The reason for this underutilization is the paucity of a robust and sensitive measurement method. The Z-scan technique [1,2] is considered the gold standard for the measurement of self-phase modulation (SPM), which causes self-induced modulation of the index of refraction by an amount proportional to the instantaneous intensity of a propagating light pulse. The Z-scan technique has been used to study the nonlinear properties of many transparent optical materials, but it is inherently a bulk measurement rather than a point measurement technique, and is restricted to clear samples. The use of nonlinear refractive index as a microscopy contrast, however, requires a point measurement method that is insensitive to sample inhomogeneities.
Fischer et al. have recently demonstrated a spectral reshaping technique to sensitively measure SPM using very modest power levels [3–5]. This technique has, for example, been shown to provide functional contrast in chemically activated neurons [6]. This spectral reshaping technique encodes the nonlinear phase modulation signature in the frequency spectrum of the pulse, which is inherently robust with respect to degradation due to scattering [7]. High measurement sensitivity is achieved by employing femtosecond laser pulse shaping. Specifically, a narrow hole in the center of the spectrum is introduced, which is then refilled due to nonlinear interactions in the sample. In a homodyne approach, the phase of a small spectral portion in the hole is rotated and serves as a local oscillator (LO, Fig. 1(a)) that interferes with and amplifies the small nonlinear signal. Nonlinear absorption and nonlinear refraction modulate the LO spectrum with different phases and can be separated using a lock-in amplifier. However, in this scheme, the same spectral region that is phase modulated is also used for detection, and due to the limited resolution of the pulse shaping apparatus, a residual amplitude modulation is observed. This creates a linear background in the SPM measurements, and while this background can be taken into account in relative measurements, the associated noise cannot be removed and will degrade measurements in inhomogeneous samples.
Fig. 1.
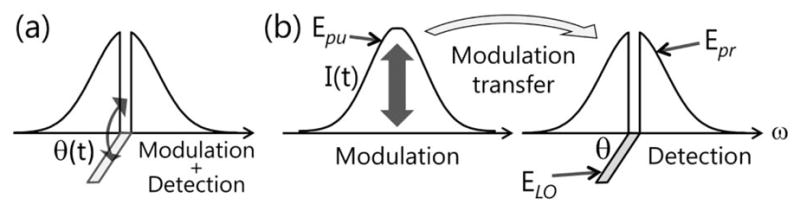
(a) SPM measurements utilizing shaped pulses with a rotating local oscillator (LO). (b) Separation of modulation and detection in the XPM measurements. The pump pulses are amplitude-modulated, and a static phase shift is imposed onto the center of the probe spectrum. Modulation is transferred from pump to probe during nonlinear interaction.
Here we report a technique that combines the spectral reshaping technique with a sensitive dual-color modulation transfer method that has been shown to substantially enhance sensitivity in nonlinear microscopy applications measuring ground and excited state dynamics [8,9]. In our pump-probe configuration, we measure cross-phase modulation (XPM), the dual-color analogue of SPM. XPM causes spectral changes in one pulse through modulation of the index of refraction by a second pulse of a different wavelength. This effect is described by the coupled pulse propagation equations in the slowly varying amplitude approximation for two copropagating plane waves, neglecting linear absorption and dispersion [10]:
| (1) |
where {m, n} = {pu, pr} or {pr, pu}, for the pump and probe pulses. Here, E(z, τ) is the electric field envelope as a function of time, τ = t − z/υ, measured in the reference frame moving with group velocity υ. η = n2ω/c and α2 are the nonlinear refraction and absorption coefficients, respectively. Assuming frequency-independent nonlinear coefficients, Eq. (1) can be Fourier transformed to approximate the changes in the probe pulse spectrum as
| (2) |
where the tilde denotes the Fourier transform. A small part in the center of the probe pulse spectrum is phase shifted by an angle θ to create a static phase reference as shown in Fig. 1(b). This phase reference does not generate any significant nonlinear component but interferes with the pump-probe induced nonlinear polarization:
| (3) |
In this implementation, we impose an amplitude modulation at frequency fo on the pump pulse train, which modulates the nonlinear signal generated by the pump-probe interaction in the medium. All the spectral components outside the phase reference position are rejected using a band-pass filter (BPF). A lock-in amplifier is used to analyze the detected intensity at the reference frequency fo:
| (4) |
Choosing the reference phase as θ = π/2 accesses the nonlinear refractive component, while a reference phase of θ = 0 accesses the absorptive component.
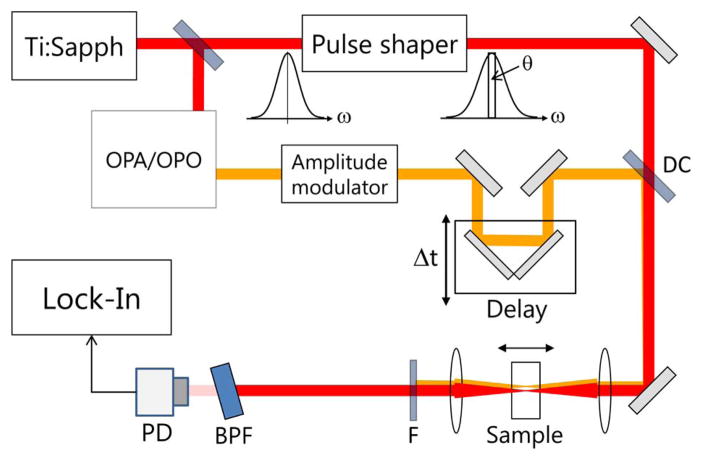
To demonstrate the background suppression achieved by using XPM, we compared it to SPM in a glass slide. The general experimental layout is shown in Fig. 2. For these comparison experiments, we used a regenerative amplifier (RegA, Coherent) seeded by a mode-locked Ti:Sapphire oscillator (Vitesse, Coherent) with a center wavelength of 804 nm and a pulse repetition rate of 20 kHz. Part of the 804 nm beam was used to pump an optical parametric amplifier (Coherent, OPA 9450) to generate a 740 nm pump beam. The remainder of the beam served as the probe and was shaped using an acousto-optic 4-f pulse shaper [11]. For SPM measurements, a rotating LO of about 3 nm width was imparted centered on the 804 nm beam, and the 740 nm pump beam was blocked. For the XPM measurements, the same 3 nm wide spectral component was statically phase shifted by π/2 (Fig. 1(b)) and the pump beam was amplitude-modulated at 5 kHz. The two beams were then combined and focused into the sample. The pump and the wings of the probe spectrum were rejected using a 1 nm BPF. The transmitted spectrum was analyzed using a photodiode (PD) and a lock-in amplifier with an integration time of 10 ms.
Fig. 2.
Experimental setup for SPM/XPM measurements using the spectral reshaping technique: DC, dichroic mirror; F, pump filter; BPF, band-pass filter; PD, photodiode.
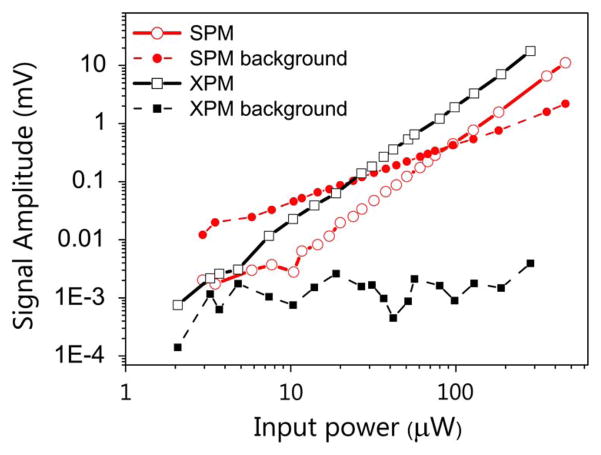
Figure 3 shows measurements of SPM and XPM in glass as a function of input laser power. The nonlinear signals plotted here are background-subtracted, where “background” is the signal measured when the beam focus is outside the sample (in air). SPM exhibits a large linear background, whereas the background for XPM is power independent, suggesting an electrical noise floor of the detection system that could be further reduced.
Fig. 3.
Power scaling of XPM, SPM, and their respective background signals in glass. The power used for SPM measurements is equal to the sum of powers of both the beams used in XPM measurements.
The acousto-optic pulse shaper in the above setup limits the pulse repetition rate to a few hundred kHz. To make XPM measurements applicable to microscopy, we developed a dual-color analogue to the spectral pulse shaper used for measuring SPM with high repetition rate sources [5]. Because for XPM measurements only a static phase profile is required, we employed a liquid crystal modulator based static pulse shaper. The probe source in this setup was a Ti:sapphire oscillator (Tsunami, Spectra Physics) operating at a center wavelength of 800 nm. The tunable 737 nm pump beam from an optical parametric oscillator (Mira OPO, Coherent) was amplitude-modulated at 2 MHz using an acousto-optic modulator.
Figure 4 shows XPM measurements in a glass cuvette filled with a 30 mM Rhodamine 6G (R6G) solution in methanol. When the phase of the LO in the probe pulse was set at π/2 to access the nonlinear refractive component, both glass and the R6G solution showed XPM signals. As Eq. (4) indicates, adjusting the phase to zero, a sum-frequency absorption trace was obtained, localized to the dye-filled region of the cuvette. This shows that our dual-color spectral reshaping technique can quickly switch between refractive and absorptive measurements by selecting the phase of the LO.
Fig. 4.
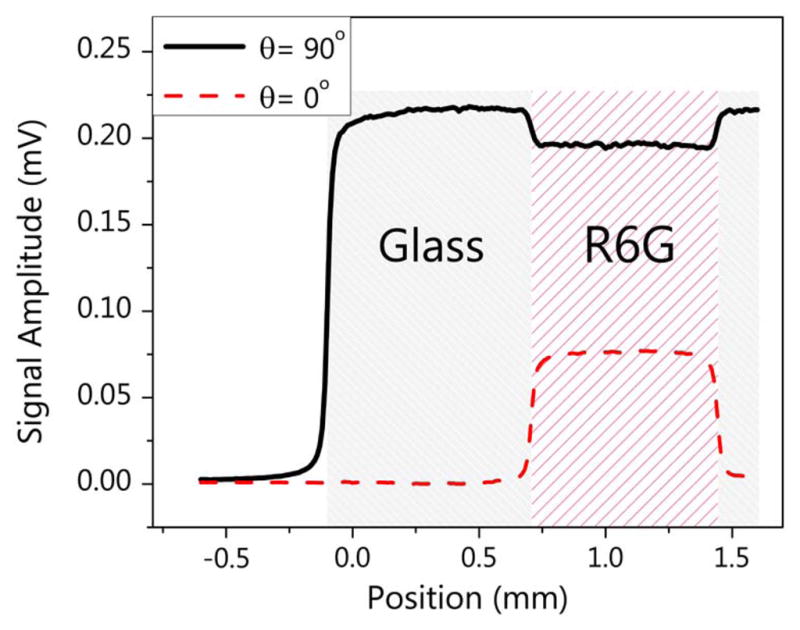
XPM measurements of Rhodamine 6G solution in a glass cuvette. θ is the static phase shift of the LO. The total input power was about 22 mW.
To verify that we can extract XPM signal even in the presence of strong nonlinear absorption, we performed pump-probe delay scans of a 30-mM R6G solution in methanol and compared them to those of pure methanol. The shape of the delay trace in Fig. 5 originates from the Fourier transform of the probe spectrum (a Gaussian shape with a central hole). The comparison of both traces shows that in the pulse overlap region, the dispersive signal component measuring XPM is essentially unaffected by the strong two-photon absorption of R6G.
Fig. 5.
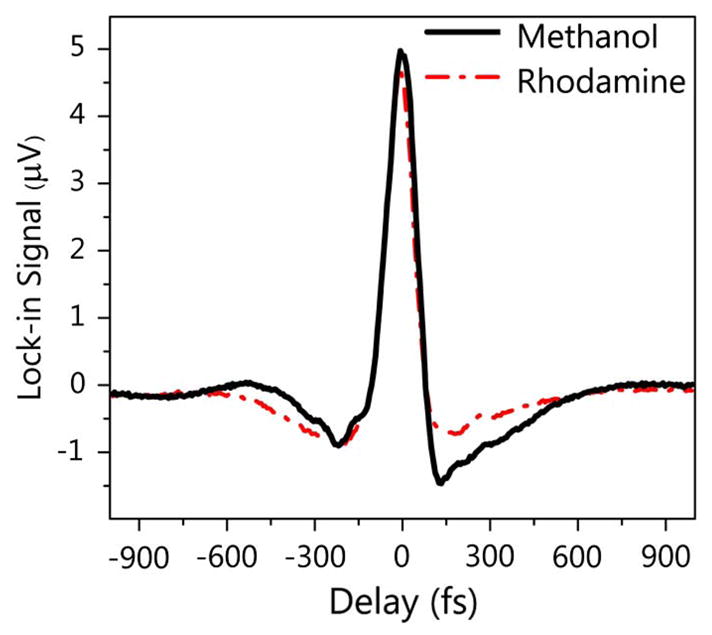
Pump-probe delay scans with the spectral reshaping technique in pure methanol and R6G in methanol. The pump and probe wavelengths were 672 nm and 802 nm, respectively, with a total power of about 25 mW.
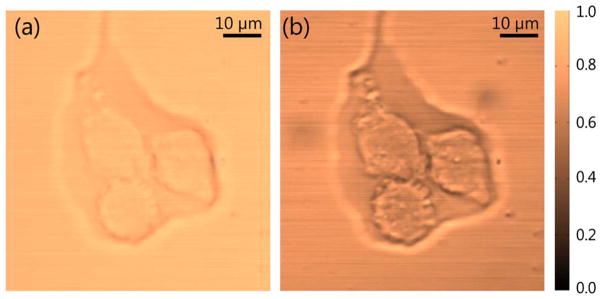
To demonstrate the imaging capability of our spectral reshaping technique, we incorporated the dual-color source and the detection setup into a laser scanning microscopy system. Figure 6 has a linear transmission image (a) and an XPM image (b) of three fixed breast cancer cells in transmission mode. We can see that XPM provides detailed structural contrast. While the linear transmission image in Fig. 6(a) shows very little contrast, the XPM image exhibits a substantially higher dynamic range and highlights different subcellular structures. The contrast obtained with our current laser parameters was mostly nonresonant electronic in origin (and hence wavelength-independent), but further studies utilizing other XPM contributions are currently underway.
Fig. 6.
(a) Linear transmission and (b) cross-phase modulation images of three fixed breast cancer cells. The pump and probe wavelengths used are 712 nm and 804 nm, respectively, with a total power of about 21 mW.
In conclusion, we have demonstrated that dual-color XPM measurements can dramatically reduce the background associated with single-color SPM measurements, making nonlinear phase modulation a viable contrast mechanism for optical microscopy. Furthermore, the demonstrated spectral reshaping technique has the ability to quickly switch between nonlinear refractive and nonlinear absorptive measurements. Similar to the SPM measurement method [7], our dual-color spectral reshaping technique encodes the nonlinear signal in the spectral domain and preserves this information even in highly scattering samples, such as biological tissue. The localized nature of the nonlinear interaction provides inherent sectioning capability, and in principle the XPM signal can be extracted from back-scattered (epidetected) light, making this technique applicable to thick, nontransmissive samples. XPM measurement techniques would allow the extension of widely employed phase microscopy methods (phase contrast, differential interference contrast) to the nonlinear regime, providing an intrinsic and ubiquitous nonlinear contrast for biological imaging. Specifically, nonlinear phase contrast imaging could provide a structural context for the resonant chemical contrast detected with pump-probe imaging [12].
Acknowledgments
This work was supported by the National Institutes of Health (1RC1CA145105) and funding from Duke University.
References
- 1.Sheik-Bahae M, Said AA, Van Stryland EW. Opt Lett. 1989;14:955. doi: 10.1364/ol.14.000955. [DOI] [PubMed] [Google Scholar]
- 2.Chapple PB, Staromlynska J, Hermann JA, McKay TJ, McDuff RG. J Nonlinear Opt Phys. 1997;6:251. [Google Scholar]
- 3.Fischer MC, Ye T, Yurtsever G, Miller A, Ciocca M, Wagner W, Warren WS. Opt Lett. 2005;30:1551. doi: 10.1364/ol.30.001551. [DOI] [PubMed] [Google Scholar]
- 4.Fischer MC, Liu HC, Piletic IR, Warren WS. Opt Express. 2008;16:4192. doi: 10.1364/oe.16.004192. [DOI] [PubMed] [Google Scholar]
- 5.Piletic IR, Fischer MC, Samineni P, Yurtsever G, Warren WS. Opt Lett. 2008;33:1482. doi: 10.1364/ol.33.001482. [DOI] [PubMed] [Google Scholar]
- 6.Fischer MC, Liu HC, Piletic IR, Escobedo-Lozoya Y, Yasuda R, Warren WS. Opt Lett. 2008;33:219. doi: 10.1364/ol.33.000219. [DOI] [PubMed] [Google Scholar]
- 7.Samineni P, Perret Z, Warren WS, Fischer MC. Opt Express. 2010;18:12727. doi: 10.1364/OE.18.012727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu D, Ye T, Matthews TE, Yurtsever G, Warren WS. J Biomed Opt. 2007;12:054004. doi: 10.1117/1.2780173. [DOI] [PubMed] [Google Scholar]
- 9.Fu D, Ye T, Matthews TE, Grichnik J, Hong L, Simon JD, Warren WS. J Biomed Opt. 2008;13:054036. doi: 10.1117/1.2976424. [DOI] [PubMed] [Google Scholar]
- 10.Spencer PS, Shore KA. J Opt Soc Am B. 1995;12:67. [Google Scholar]
- 11.Hillegas CW, Tull JX, Goswami D, Strickland D, Warren WS. Opt Lett. 1994;19:737. doi: 10.1364/ol.19.000737. [DOI] [PubMed] [Google Scholar]
- 12.Matthews TE, Piletic IR, Selim MA, Simpson MJ, Warren WS. Sci Transl Med. 2011;3:71. doi: 10.1126/scitranslmed.3001604. [DOI] [PMC free article] [PubMed] [Google Scholar]