Abstract
Background
Excess visceral adiposity induces chronic subclinical inflammation resulting in the metabolic syndrome. Whether excess visceral adiposity impacts posttraumatic inflammatory profiles more is unknown. We hypothesized that obese patients (body mass index >30 kg/m2) with higher visceral to subcutaneous adipose tissue distribution would have increased inflammatory outcomes.
Methods
A secondary analysis of a prospective cohort of adult trauma patients requiring >48 hours of intensive care unit care over a 55-month period was analyzed. Body fat distribution was determined by radiologist review of computed tomography scans at L1. Concentric freeform regions were defined manually, and area was calculated. Visceral adiposity was defined as subcutaneous fat area: visceral area >1.35 (the median), whereas subcutaneous adiposity was defined as a ratio <1.35. Primary outcomes were proinflammatory biomarkers known to be associated with chronic visceral obesity (white blood cell count, interleukin 1, 2, 4, 6, 8, 10, and tumor necrosis factor α). Secondary outcomes were all-cause in-hospital mortality, adult respiratory distress syndrome, and nosocomial infections.
Results
Two hundred eighty-one (19%) obese patients with available computed tomography scans from 1,510 trauma patients were included. Visceral adiposity included 140 patients, subcutaneous adiposity included 141 patients. The two groups were similar in regards to age, Trauma Injury Severity Score, and Acute Physiology and Chronic Health Evaluation II score. There was no difference (p > 0.05) in proinflammatory biomarkers. Patients with visceral adiposity had similar clinical outcomes including mortality (p = 0.56), adult respiratory distress syndrome (p = 0.69), and infection (0.43).
Conclusions
Visceral body fat distribution in obese patients is not associated with increased inflammatory profiles or clinical outcomes after trauma. The impact of injury severity on acute inflammation likely overwhelms the metabolic disturbances and subclinical inflammation associated with visceral obesity in the chronic setting.
Keywords: Obesity, Trauma, Visceral adiposity, Visceral obesity
Obesity, particularly central or visceral obesity, has been shown to be linked to chronic low-grade inflammation and comorbidities such as hypertension, diabetes, and cerebrovascular disease.1–7 Although controversy exists as to whether general adiposity, body mass index (BMI), or waist to hip ratio measurements most accurately assess the risk of obesity-related complications, a growing body of evidence suggests the distribution of adipose tissue is important concerning chronic health issues.6,8–13 In the acutely injured patient, the relationship between obesity and negative outcomes has been controversial.14–17 Mixed results have likely stemmed from multiple but poorly understood proposed mechanisms (increased size vs. increased chronic proinflammatory state), which describe why the obese trauma population might experience worse outcomes.18–20 Increased weight and size may be a mechanical disadvantage and predispose to increased pelvic and rib fractures.21 However, decreased head and abdominal injuries have been noted.21,22 Despite the increased propensity for thoracic trauma, obesity has not been shown to predict intubation difficulties, respiratory complications, or mortality.18,23 Obese patients are more likely to suffer chronic inflammation related to the metabolic syndrome, however, increased BMI has not been shown to predict mortality once adjusted for hyperglycemia.24 The acute inflammatory state of adult respiratory distress syndrome (ARDS) has been show to be less likely in the severely obese (BMI >40 kg/m2) trauma patient.18
It is unknown whether or not body fat distribution, as opposed to BMI, impacts inflammatory markers or clinical outcomes in the critically ill trauma patient. The purpose of this study was to examine the relationship between body fat distribution and acute inflammatory profiles. Our hypothesis states that obese patients with visceral adiposity would have increased inflammation and worse clinical outcomes than those obese patients with subcutaneous obesity.
METHODS
This is a secondary analysis from data collected from a multi-institutional study that was supported by funding from the National Institutes of Health: RO1 AI49989-01 (Clinical Trials.gov identifier NCT00170560).25,26 The aim of the original analysis was to determine the predictive ability of estradiol concentrations at 48 hours after critical illness.25,26 In this cohort, all patients, aged 18 years or older, who were admitted to the trauma intensive care unit (ICU) at Vanderbilt University Medical Center (VUMC) for at least 48 hours were eligible for enrollment. The entry criteria of a 48-hour ICU stay was used to select a patient population at risk of death and ICU complications. To be eligible for this study, patients also had to have an admission computed tomography (CT) of the abdomen and pelvis regions that could be reevaluated by the radiology department (described below). Only obese patients, defined as a BMI ≥30 kg/m2, were included in this study population.
Independent radiologists evaluated the admission CT. For attenuation analysis, concentric freeform regions of interest were defined manually, and an area in square centimeters was calculated via picture archiving and communication system. The regions of interest were defined on axial slices at the level of the L1 vertebral body endplate in all patients. This level has been shown to influence the magnitude of the association with metabolic syndrome definitions.9,27 Two separate regions of interest were created. The most external region of interest (subcutaneous fat) included all subcutaneous structures deep to skin. The inner region of interest (visceral fat region) included all structures deep to the abdominal wall musculature. Visceral adiposity was defined as those patients who had a subcutaneous fat area to visceral fat area ratio that was greater than the median value (1.39); whereas, subcutaneous adiposity was defined as those patients who had a subcutaneous fat area to visceral fat area ration that was less than the median value (1.39; Fig. 1).
Figure 1.
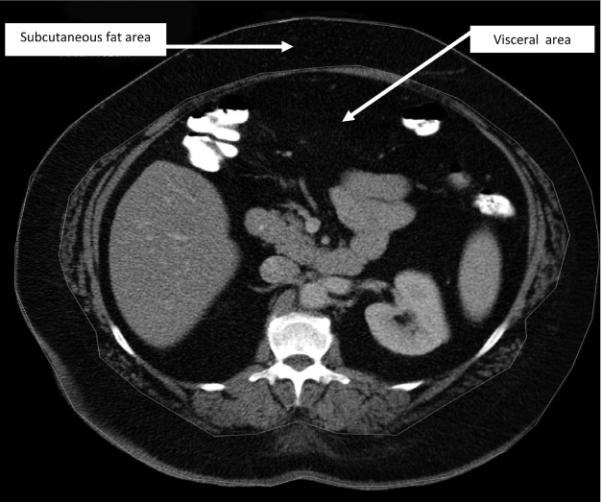
For analysis, concentric freeform regions of interest (subcutaneous fat area and visceral fat region) were defined manually and an area in square centimeters was calculated via picture archiving and communication system.
Gender, age, BMI, Acute Physiology and Chronic Health Evaluation II score at ICU admission date of hospital, Trauma Injury Severity Score, Glasgow Coma Score, Multiple Organ Dysfunction Score, presence of ICU infection or ARDS, and mortality were collected. Patient care was at the discretion of the attending physician according to established trauma and critical care protocols.
One 10-mL blood sample was collected 48 hours after ICU admission (at the time of study entry) for analysis of cytokine levels. Plasma was separated from whole blood and stored at −70°C until analysis. Samples were assayed for interleukin 1, 2, 4, 6, 8, 10, and tumor necrosis factor α. This was accomplished by using a LINCOplex custom kit, following the manufacturer's instructions (Linco Research, St. Charles, MO). Twenty-five microliters of human serum from each collection time point on all patients was run in duplicates on a Luminex100 System (Miraibio, Alameda, CA) to determine the concentration of the cytokines of interest. The Luminex system is an ELISA-based technology allowing multianalyte detection in a single well. Data reduction were performed with STATLia (Brendan Scientific) and subsequently incorporated into the database.
Categorical variables were compared using the Pearson's χ2 test for independence or Fisher's exact test. Normally distributed continuous variables were summarized by reporting the mean and SD and compared using Student's t test. Nonnormally distributed continuous variables were presented by reporting the median and interquartile ranges and compared using the Wilcoxon Rank Sum test. Stata version 10.0 (Stata, College Station, TX) was used for the analysis. Tests for statistical significance were two sided with a level of significance of 0.05.
The study was approved by the institutional review board (IRB) of VUMC. A wavier of consent was obtained from both the IRB for the blood draw at 48 hours and study inclusion, because the study posed minimal risk to subjects. Families were assented during the critical illness phase, and patients were consented after their critical illness resolved when possible. Patients who died or were discharged before consent being obtained remained in the study, with IRB approval. Patients who were able to consent, but refused could either withdraw completely or refuse subsequent participation. All data are maintained in a secure, password protected database that is Health Insurance Portability and Accountability Act-compliant, and all patient information is de-identified before analysis and reporting.
RESULTS
Originally, 1,510 patients from the cohort were recruited from VUMC. From the 1,510 critically ill patients at VUMC, 1,020 (68%) were trauma patients and 976 (65%) had CT scans of the abdomen and pelvis. The majority of patients were not obese (695 patients; 71%) and were not included in analysis. Two hundred eighty-one obese patients (19%) met criteria for analysis: a BMI greater than or equal to 30 kg/m2 and a trauma admission CT (Fig. 2). The 281 obese patients represented 29% of those who met criteria at VUMC. The median ratio for subcutaneous fat area to visceral fat area for all of these 281 patients was 1.39. Patients with a lower ratio than the median were defined as visceral adiposity (N = 140). These obese patients had a higher amount of fat within the abdominal cavity contributing to their elevated BMI. Patients with an equal or higher ratio than the median 1.39 were defined as subcutaneous adiposity (N = 141). These obese patients had a higher amount of fat within the subcutaneous tissue contributing to their elevated BMI. The mean between the groups demonstrated a statistical difference (1.17 ± 0.21 vs. 1.60 ± 0.34; p < 0.001).
Figure 2.
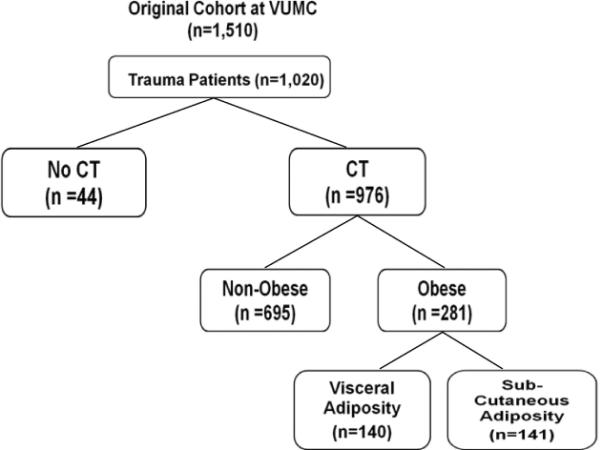
Patient inclusion into the study groups.
The visceral and subcutaneous adiposity groups were similar in age, Acute Physiology and Chronic Health Evaluation II scores, Trauma Injury Severity Score, and Injury Severity Score. The visceral adiposity group were more likely to be men, have a lower BMI, and a worse head injury measured by the admission Glasgow Coma Scale (Table 1).
TABLE 1.
Clinical Characteristics and Outcomes by Body Fat Distribution in Obese Trauma Patients
| Visceral Fat (n = 140) | Subcutaneous Fat (n = 141) | p | |
|---|---|---|---|
| Age (yr)* | 51 | 48 | 0.19 |
| Males (%)† | 89 | 45 | <0.01 |
| BMI (kg/m2)‡ | 33.5 | 36.7 | <0.01 |
| APACHE II* | 17 | 17 | 0.96 |
| ISS‡ | 29 | 29 | 0.71 |
| TRISS | 0.887 | 0.889 | 0.78 |
| GCS | 8 | 10 | 0.02 |
| MODS | 8.6 | 8.1 | 0.13 |
| Any ICU infection | 56 | 51 | 0.43 |
| Pneumonia | 38 | 33 | 0.36 |
| ARDS | 31 | 30 | 0.69 |
| Mortality (%) | 15 | 14 | 0.89 |
APACHE, acute physiology and chronic health evaluation; ISS, Injury Severity Score; TRISS, Trauma Injury Severity Score; GCS, Glasgow Coma Scale; MODS, Multiple Organ Dysfunction Score.
Mean (95% CI); compared using two-sample t test.
Proportion (95% CI); compared using χ2 test.
Median (interquartile range); compared using Wilcoxon Rank Sum test.
Table 2 illustrates median cytokine blood levels drawn 48 hours after admission. There was no significant difference between the two adiposity groups when comparing interleukin 1, 2, 4, 6, 8, 10, 12, or tumor necrosis factor. The white blood cell count was higher in those patients in the subcutaneous adiposity group.
TABLE 2.
Inflammatory Profiles by Body Fat Distribution in Obese Trauma Patients
| Visceral Adiposity (n = 140) | Subcutaneous Adiposity (n = 141) | p | |
|---|---|---|---|
| IL-1 (ng/mL) | 2.7 | 2.7 | 0.44 |
| IL-2 (ng/mL) | 8.3 | 2.7 | 0.71 |
| IL-4 (ng/mL) | 35 | 36 | 0.77 |
| IL-6 (ng/mL) | 138 | 193 | 0.27 |
| IL-8 (ng/mL) | 16 | 29 | 0.26 |
| IL-10 (ng/mL) | 50 | 52 | 0.96 |
| IL-12 (ng/mL) | 2.9 | 2.8 | 0.77 |
| TNF-α (ng/mL) | 4.8 | 6.9 | 0.06 |
| WBC (K/μL) | 14 | 17 | 0.01 |
IL, interleukin; TNF-α, tumor necrosis factor α; WBC, white blood cell count.
All values reported as medians, compared using Wilcoxon Rank Sum test.
Clinical outcomes also did not differ between the two adiposity groups (Table 1). Multiorgan dysfunction scores, infection rates, and ARDS rates demonstrated no statistical differences. The odds ratio for death for those patients with visceral adiposity was also not significant (0.81 [0.39–1.67]; p = 0.56).
DISCUSSION
Obesity has become an epidemic, especially in the younger population.28–30 As obesity becomes a health issue in younger patients, the comorbidity becomes an issue in the injured patient merely for the fact that trauma affects younger patients. According to many studies, obese trauma patients have a worse outcome.14–18 However, mixed outcome results may appear to stem from the lack of an accepted mechanism driving the potential worse outcome. Larger patient size provides a risk factor; however, the subclinical chronic inflammation may also drive a worse outcome.
In this study, obese patients who demonstrate the body composition of visceral adiposity demonstrated by admission CT scan were compared with obese patients who have less visceral adiposity and more subcutaneous adiposity. This former group, in relation to the metabolic syndrome, should have more baseline inflammation. The hypothesis of this study stated that obese patients with visceral obesity would have not only increased inflammation measured by cytokine blood levels but also worse clinical outcomes, such as infection and ARDS. The results did not support the hypothesis. Although the white blood cell count was greater in the subcutaneous adiposity group, all cytokines tested did not demonstrate a significant difference at 48 hours after admission. In addition, clinical outcomes related to the inflamma-tory process such as ARDS, infection, and mortality were not different between the two obese groups.
If the obese patient is at a disadvantage because of size, different injury patterns would be observed. Choban et al. and Boulanger et al. have reported less head and abdominal trauma.14,21,22 Boulanger et al. noted more rib fractures, pulmonary contusions, and pelvic fractures. Assuming the BMI provides the patient's size and shape, it can often prove that the larger patient does carry inherent risk. However, this size variation would likely be seen in those requiring intubation. However, this preconceived idea was negated as Sifri et al.23 noted that BMI was not an independent risk factor for failed intubations.
The chronic or baseline inflammatory response in the central obese patient is increased. This chronic subclinical inflammation has been linked to hypertension, diabetes, and cerebrovascular disease.1–7 Obese patients suffer hyperglycemia as a complication of the metabolic syndrome. Hyperglycemia in trauma patients, even at admission, is a risk factor for higher infectious complications and mortality.31–34 However, morbid obesity has not been found to be an independent risk factor for mortality in critically ill trauma patients once adjusted for hyperglycemia.24 In addition, inflammation manifested by ARDS was actually observed to be lower in the severely obese compared with normal-weighted individuals.18 When evaluating all obese patients and stratifying them on the basis of fat distribution, this study could not demonstrate a difference in the rate of ARDS or other clincial outcomes such as infection or mortality. However, this analysis was not adequately powered for these outcomes.
This secondary analysis has several important limitations. First, its inherent limitations of reexamining previously reported data, albeit prospectively collected. The only retrospective data collected was the evaluation of the CT. However, this data were objective in nature from independent radiologists. Some patients did not have CT scans available. Although this only represents 44 patients (3%) of the entire population, patients with missing scans (those who cannot fit into the scanner) may represent a selection bias that could affect our desired results. Patients without CT scans because of prohibitive size are the type obese patients who were examined in this study. Classification of body fat distribution by CT scan in the acute trauma setting has not been validated. Our determined ratio of visceral and subcutaneous tissue to distinguish the two obese groups was chosen as above and below the entire group's median. Changing group distinction, at the extremes for example, could demonstrate differences among groups. However, smaller group size would lower the power of the study and, subsequently, make it more difficult to show significant differences. Finally, fat distribution may influence the late, greater than 48 hours, inflammatory profiles. In this study, cytokines were measured at 48 hours after admission.
CONCLUSION
The visceral obese patient suffers a chronic inflamma-tory condition resulting in the metabolic syndrome (diabetes, hyperlipidemia, and cardiovascular disease). Although some studies suggest obese trauma patients have worse outcomes, the mechanism is not clear. This study suggests through a secondary analysis that the impact of acute injury on acute inflammation likely overwhelms the chronic inflammation associated with visceral obesity. Therefore, no difference in various inflammatory markers can be seen between those obese patients with or without visceral adiposity. Further evaluation of this population is warranted to determine whether in fact obese patients have a worse outcome with acute injury. In addition, potential mechanisms should be investigated to develop strategies to overcome this possible detriment.
Acknowledgments
Supported by National Institutes of Health grant RO1 AI49989-01 (Clinical Trials.gov identifier NCT00170560) and an Agency for Healthcare Research Quality grant T32 HS 013833.
REFERENCES
- 1.Calabro P, Yeh ET. Intra-abdominal adiposity, inflammation, and cardiovascular risk: new insight into global cardiometabolic risk. Curr Hypertens Rep. 2008;10:32–38. doi: 10.1007/s11906-008-0008-z. [DOI] [PubMed] [Google Scholar]
- 2.Cottone S, Lorito MC, Riccobene R, et al. Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J Nephrol. 2008;21:175–179. [PubMed] [Google Scholar]
- 3.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14:1225–1230. doi: 10.2174/138161208784246153. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo Y, Hashizume T, Shioji S, Akasaka T. Metabolic syndrome is strongly associated with chronic subclinical inflammation in patients achieving optimal low-density lipoprotein-cholesterol levels in secondary prevention of cardiovascular disease. Circ J. 2008;72:2046–2050. doi: 10.1253/circj.cj-08-0337. [DOI] [PubMed] [Google Scholar]
- 5.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 6.van der PD, Milner KL, Hui J, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449–457. doi: 10.1002/hep.22350. [DOI] [PubMed] [Google Scholar]
- 7.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 2009;33:54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards LA, Bugaresti JM, Buchholz AC. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr. 2008;87:600–607. doi: 10.1093/ajcn/87.3.600. [DOI] [PubMed] [Google Scholar]
- 9.Kuk JL, Church TS, Blair SN, Ross R. Does measurement site for visceral and abdominal subcutaneous adipose tissue alter associations with the metabolic syndrome? Diabetes Care. 2006;29:679–684. doi: 10.2337/diacare.29.03.06.dc05-1500. [DOI] [PubMed] [Google Scholar]
- 10.Mathieu P. Abdominal obesity and the metabolic syndrome: a surgeon's perspective. Can J Cardiol. 2008;24(suppl D):19D–23D. doi: 10.1016/s0828-282x(08)71045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oda E. The metabolic syndrome as a concept of adipose tissue disease. Hypertens Res. 2008;31:1283–1291. doi: 10.1291/hypres.31.1283. [DOI] [PubMed] [Google Scholar]
- 12.Solá E, Jover A, López-Ruiz A, et al. Parameters of inflammation in morbid obesity: lack of effect of moderate weight loss. Obes Surg. 2009;19:571–576. doi: 10.1007/s11695-008-9772-8. [DOI] [PubMed] [Google Scholar]
- 13.Zuliani G, Volpato S, Galvani M, et al. Elevated C-reactive protein levels and metabolic syndrome in the elderly: the role of central obesity Data from the InChianti study. Atherosclerosis. 2009;203:626–632. doi: 10.1016/j.atherosclerosis.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bochicchio GV, Joshi M, Bochicchio K, Nehman S, Tracy JK, Scalea TM. Impact of obesity in the critically ill trauma patient: a prospective study. J Am Coll Surg. 2006;203:533–538. doi: 10.1016/j.jamcollsurg.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Newell MA, Bard MR, Goettler CE, et al. Body mass index and outcomes in critically injured blunt trauma patients: weighing the impact. J Am Coll Surg. 2007;204:1056–1061. doi: 10.1016/j.jamcollsurg.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 16.Porter SE, Graves ML, Qin Z, Russell GV. Operative experience of pelvic fractures in the obese. Obes Surg. 2008;18:702–708. doi: 10.1007/s11695-007-9320-y. [DOI] [PubMed] [Google Scholar]
- 17.Ryb GE, Dischinger PC. Injury severity and outcome of overweight and obese patients after vehicular trauma: a Crash Injury Research and Engineering Network (CIREN) study. J Trauma. 2008;64:406–411. doi: 10.1097/TA.0b013e31802beff9. [DOI] [PubMed] [Google Scholar]
- 18.Dossett LA, Heffernan D, Lightfoot M, et al. Obesity and pulmonary complications in critically injured adults. Chest. 2008;134:974–980. doi: 10.1378/chest.08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viano DC, Parenteau CS, Edwards ML. Crash injury risks for obese occupants using a matched-pair analysis. Traffic Inj Prev. 2008;9:59–64. doi: 10.1080/15389580701737645. [DOI] [PubMed] [Google Scholar]
- 20.Zarzaur BL, Marshall SW. Motor vehicle crashes obesity and seat belt use: a deadly combination? J Trauma. 2008;64:412–419. doi: 10.1097/TA.0b013e3180f61c33. [DOI] [PubMed] [Google Scholar]
- 21.Choban PS, Weireter LJ, Jr, Maynes C. Obesity and increased mortality in blunt trauma. J Trauma. 1991;31:1253–1257. doi: 10.1097/00005373-199109000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Boulanger BR, Milzman D, Mitchell K, Rodriquez A. Body habitus as a predictor of injury pattern after blunt trauma. J Trauma. 1992;33:228–232. doi: 10.1097/00005373-199208000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Sifri ZC, Kim H, Lavery R, Mohr A, Livingston DH. The impact of obesity on the outcome of emergency intubation in trauma patients. J Trauma. 2008;65:396–400. doi: 10.1097/TA.0b013e31817f97fd. [DOI] [PubMed] [Google Scholar]
- 24.Diaz JJ, Jr, Norris PR, Collier BR, et al. Morbid obesity is not a risk factor for mortality in critically ill trauma patients. J Trauma. 2009;66:226–231. doi: 10.1097/TA.0b013e31815eb776. [DOI] [PubMed] [Google Scholar]
- 25.Dossett LA, Swenson BR, Evans HL, Bonatti H, Sawyer RG, May AK. Serum estradiol concentration as a predictor of death in critically ill and injured adults. Surg Infect (Larchmt) 2008;9:41–48. doi: 10.1089/sur.2007.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dossett LA, Swenson BR, Heffernan D, et al. High levels of endogenous estrogens are associated with death in the critically injured adult. J Trauma. 2008;64:580–585. doi: 10.1097/TA.0b013e31816543dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen W, Punyanitya M, Chen J, et al. Visceral adipose tissue: relationships between single slice areas at different locations and obesity-related health risks. Int J Obes (Lond) 2007;31:763–769. doi: 10.1038/sj.ijo.0803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 29.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 30.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 31.Bochicchio GV, Salzano L, Joshi M, Bochicchio K, Scalea TM. Admission preoperative glucose is predictive of morbidity and mortality in trauma patients who require immediate operative intervention. Am Surg. 2005;71:171–174. doi: 10.1177/000313480507100215. [DOI] [PubMed] [Google Scholar]
- 32.Bochicchio GV, Joshi M, Bochicchio KM, et al. Early hyperglycemic control is important in critically injured trauma patients. J Trauma. 2007;63:1353–1358. doi: 10.1097/TA.0b013e31815b83c4. [DOI] [PubMed] [Google Scholar]
- 33.Collier B, Diaz J, Jr, Forbes R, et al. The impact of a normoglycemic management protocol on clinical outcomes in the trauma intensive care unit. JPEN J Parenter Enteral Nutr. 2005;29:353–358. doi: 10.1177/0148607105029005353. [DOI] [PubMed] [Google Scholar]
- 34.Toschlog EA, Newton C, Allen N, et al. Morbidity reduction in critically ill trauma patients through use of a computerized insulin infusion protocol: a preliminary study. J Trauma. 2007;62:1370–1375. doi: 10.1097/TA.0b013e318047b7dc. [DOI] [PubMed] [Google Scholar]


