Abstract
Understanding the neural basis of visual perception is a longstanding fundamental goal of neuroscience. Historically, most vision studies were carried out on humans, macaque monkeys and cats. Over the last five years, however, a growing number of researchers have begun using mice to parse the mechanisms underlying visual processing- the rationale is that despite having relatively poor acuity, mice are unmatched in terms of the variety and sophistication of tools available to label, monitor and manipulate specific cell types and circuits. In this review, we discuss recent advances in understanding the mouse visual system at the anatomical, receptive field and perceptual level, focusing on the opportunities and constraints those features provide toward the goal of understanding how vision works.
Studying vision in mice: The blind leading the blind?
Understanding how the brain gives rise to the experience of sight is an important and fundamental question that has garnered much attention over the years [1]. The longstanding emphasis stems from the fact that many mammals, including humans, rely on vision as their primary sense to evaluate their surroundings and guide their behavior. For more than a century, animal studies of visual system structure and function relied primarily on cats and non-human primates, such as macaque monkeys. The rationale for using those species is clear: they have large eyes, high visual acuity, and their central visual pathways display many of the same features found in humans, such as well segregated parallel pathways in the visual thalamus, and ocular dominance and orientation columns in the visual cortex[2]. Macaques also have a fovea and three cone photopigments, so they can be used to probe the neural basis of high acuity trichromatic color vision[3]. Moreover, macaques can be trained to engage in psychophysical tasks where they “report” to the researcher what they observe[4].
Given the clear advantages of the macaque model, why would anyone opt to study vision in the mouse? After all, mice view the world at extremely low resolution [5], with the equivalent of 20/2000 vision. Moreover, the eyes and visual pathways of mice are one to two orders of magnitude smaller than in cats or primates[6], which creates substantial challenges for performing targeted recordings, and for tracing and lesioning specific neural pathways using conventional approaches.
The obvious, often-stated reason for using mice is ‘genetics’. More precisely, mice allow unparalleled opportunities to study defined categories of cells and circuits. As discussed in Box 1, modern genetic tools provide the opportunity to see the structure of a defined visual cell type, map its connections, record its activity in response to visual stimulation and then selectively silence or activate that cell type in a reversible manner[7, 8]. In theory, this arsenal of tools should allow one to causally link defined categories of visually driven cells with different aspects of vision and in doing so, unify anatomy, physiology and perception.
Box 1. Genetic tools for circuit analysis in the mouse visual system.
Mice allow unparalleled opportunities to label, monitor and manipulate defined cell types and neural circuits. Combinatorial systems for gene expression, such as Cre/lox and the tetO/tTA systems, form the basis of many of the genetic tools thus far developed. In the Cre/lox system, Cre recombinase is expressed in the cells of interest, generally through a transgenic line with a cell-type specific promoter. Second, reporter/effector genes can be turned on specifically in the cells that express Cre, using appropriate regulatory sequences based on lox recombination sites. These reporter/effector genes can include a) fluorescent proteins to visualize specific neuron classes[94], b) trans-synaptic tracers [95, 96] to label connected cells, and c) optogenetic[97] or pharmacogenetic[98] tools to manipulate neuronal activity. Reporter/effector genes can be expressed either in transgenic lines or via local injection of appropriate viruses into brain regions of interest. The modular Cre system means that once a mouse line expressing Cre in a cell-type of interest is available, then anatomical identification, targeted recording, circuit tracing, and disruption of neuronal activity can all be achieved by either crossing to the appropriate effector line or injecting the appropriate virus. Furthermore, because the Cre line clearly defines a cell population, rare neuronal subtypes that would otherwise be difficult to target can be systematically studied. Moreover, such results can be directly compared between labs without the ambiguity of traditional neuronal classification schemes. Although it remains to be seen how well such genetically defined populations align with physiological functional classes in the thalamus and cortex, such an approach has already proven to be effective in identifying specific subpopulations of DSGCs in the retina (eg. [33, 36, 37]) (Figure 1). Genetic ablation of melanopsin ipRGCs in the mouse has also recently been performed [99] and has proven valuable in addressing their specific contributions to visual processing.
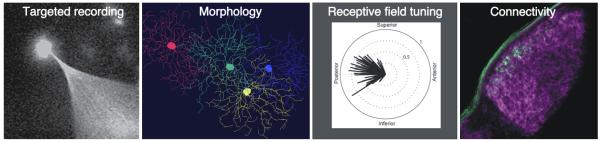
Box 1 Figure I. Cellular and physiological characterization of genetically identified RGC subtypes in mice.
Populations of On-Off DSGCs can be genetically identified using transgenic mouse lines that express fluorescent markers [such as green fluorescent protein (GFP)] under the control of specific promoters. The GFP expressing cells can be targeted for electrophysiological recordings and/or filling with dye. When one or several of the GFP-expressing cells are filled with dye, their morphology and geometric relationship to one another can then be assessed. Their receptive field tuning to specific light stimuli [in this case stimuli moving along different axes of the retina: superior (up), inferior (down), anterior (toward nasal retina) or posterior (toward temporal retina)] can also be determined. The cells shown here are all tuned for posterior motion. These parameters can then be matched to the axonal connectivity of the GFP expressing cells. The example shown here depicts GFP expressing axons from posterior tuned On-Off DSGCs within the visual thalamus. All RGC axons are labeled in magenta. Image of targeted recording provided by Mihai Manu and Steven Baccus and remaining panels reproduced, with permission, from [36].
There are practical reasons for using mice as well. Firstly, the last five years has seen a shift toward the widespread availability of transgenic mice such as Cre expressor lines, as well as molecular tools for in vivo circuit labeling and manipulation (e.g. viral transfection agents). Large-scale mouse consortiums and well-organized online catalogs currently enable any lab in the world to use molecular genetics to address important questions about brain circuitry and function. A second reason is that the smaller overall size of the mouse nervous system can be used to gather data over a large spatial scale–for instance, the total area of mouse primary and extrastriate visual cortex spans only several millimeters across the cortical surface[9], potentially allowing the entire system to be visualized simultaneously. A third, and less obvious (but nonetheless influential) reason why many labs are now using mice to study vision is that mice are safer, simpler, and less expensive to maintain than cats or primates.
Despite all of these advantages, the goal is to understand how vision works, not to make life easy. Therefore, it is essential to know what the mouse visual system consists of and is capable of, and to carefully consider how that shapes the questions one can expect to answer about visual processing using this species.
The hardware and software of the mouse visual system
The last five years have brought a surge in the number of studies aimed at parsing the basic structure and function of mouse central visual pathways. Although that effort is far from complete, these studies generally support the argument that the mouse visual system is much more sophisticated than previously thought. Furthermore, it is proving to be a valuable model for addressing a number of fundamental questions about visual processing. In the following sections, we review what is currently known about the architecture of mouse visual circuits and the types of information processing they perform, using the longstanding categories of retina, retino-geniculo-striate and retino-collicular pathways as a general framework for thinking about the mechanisms that shape visual perception and behavior. While there is a longstanding tradition of using the mouse visual system for studies of neural development and plasticity[10, 11], here we focus on the mouse’s potential as a model of visual processing per se.
Retina
The photoreceptor mosaic places a fundamental limit on visual information
Just as the charged-coupled device (CCD) in a digital camera converts incoming photons into electrical signals, the retinal photoreceptor mosaic generates neural signals that represent the image reaching the eye. Thus, while the attributes of the pixels in a digital camera (ie. the number of pixels, color coding properties, etc.) determine the quality of the photos a camera takes, the attributes of retinal photoreceptors constrain every aspect of what the brain learns about the visual scene.
The mouse retina is rod-dominated[12] and thereby specialized for vision under low light (scotopic) conditions. Approximately 3% of mouse photoreceptors are cones and those cones express one or a combination of two photopigments: one sensitive to short wavelengths of light (centered at 360nm - ultraviolet), and one sensitive to medium wavelengths of light (centered at 511nm - green) [13]. These are distinct from the third photopigment melanopsin [14], which is expressed by a subset of retinal ganglion cells (discussed below). Because mice lack the long wavelength “red” cone photopigment found in many primates, mice (like cats) are dichromats and cannot discriminate red from green hues. The distribution of rods across the mouse retina is relatively uniform, whereas the cones exhibit marked regional variation; UV-dominant cones, some of which co-express M-opsins, are enriched in the ventral retina.[15, 16]
Owing to the overall difference in the size of their eyes, the total number of photoreceptors (rods and cones) in mice retina dwarfs in comparison to primates. Per unit area of retina, however, the mouse retina actually has more photoreceptors than the macaque [12, 17, 18] . Why then is primate vision so much sharper than mouse vision? First, because a mouse’s eye is much smaller than a primate’s eye, the higher photoreceptor density in the mouse retina corresponds to fewer photoreceptors per area spanning the visual scene. Second, in primates, 99% of cones reside in the fovea- a specialized region that occupies ~1% of the retinal area [19]. The fovea is used for high contrast, high acuity tasks such as reading or detecting small objects at a distance. On the other hand, peripheral vision is low acuity and allows one to either detect large objects at a distance or small objects at closer range, especially objects that are moving. The mouse does not have a fovea, and therefore its entire retina is similar to the peripheral retina of primate; indeed cone density in the mouse retina (per mm2) is similar to that found in the primate eye at ~3mm away from the fovea[12]. Thus, an understanding of the mouse photoreceptor mosaic demonstrates that it is in fact highly efficient at sampling the visual scene, given the constraints of a small eye. Furthermore, it indicates that the mouse may be a promising model for studies of low-acuity, peripheral vision, in addition to studies of rod-dependent night vision.
In theory, mice could be used to study certain aspects of trichromatic foveal vision but that would require, at a minimum, transgenic manipulations of the mouse photoreceptor mosaic. Interestingly, a third long wavelength sensitive photopigment (i.e. a red cone photopigment) was recently genetically inserted into mice[20]. Remarkably, that single manipulation proved sufficient to enable red-green colors to be discriminated by the transgenic mice. Such a finding underscores the extent to which the photoreceptors are the major bottleneck for visual perception. Moreover, it indicates that the rest of the mouse visual system is equipped to process sophisticated combinations of visual information, even when that information is not normally extracted from the world.
Retinal ganglion cells encode diverse and often sophisticated features of the visual world
After photoreceptors convert light into electrical impulses, that information is filtered by the three major classes of retinal interneurons: the horizontal, bipolar and amacrine cells, each of which includes 1-40 types (1-2 horizontal; 10-15 bipolar; 40 amacrine cell types)[21]. All of the major retinal interneuron types found in primates are also present in the mouse. These interneurons filter and transmit visual information to the retinal ganglion cells (RGCs), which in turn, project to a number of central brain regions. Thus, all the information about the visual world that the brain receives is encoded in the spiking activity of RGCs.
The classic textbook description of RGCs consists of three major types, all with center-surround organization [1] In carnivores, these subtypes are commonly known as X (or beta), Y (or alpha),and W (gamma) RGCs; and in monkeys they are called midget, parasol and koniocellular RGCs, respectively [2, 22]. In reality, the number of RGC types (often referred to as subtypes) is far more complex, with more than 20 different subtypes of RGCs having been described across species [23]. Also, the information encoded by an individual RGC subtype is often much more sophisticated than just center-surround – for example, salamander and rabbit RGCs have been shown to code for specific directions of motion, edges, and object versus background motion[24-26]. In addition, a surprising discovery in the last decade is the existence of RGCs which express the photopigment melanopsin. This enables them to transduce photons directly, independently of photoreceptors [14, 27]. These intrinsically photosensitive RGCs (ipRGCs) have been found in many species including mice, macaques and humans[27-29].
Recently, several groups used sophisticated anatomical and genetic techniques to label and record from defined subtypes of mouse RGCs.. The main conclusion of these studies is that mouse RGCs are highly diverse, encompassing at least 22 anatomically distinct subtypes [30] (Figure 1). In addition to having three subtypes of alpha Y-like RGCs[31] and 4-5 subtypes of melanopsin ipRGCs [39], the mouse retina harbors eight or more different subtypes of direction selective RGCs (DSGCs)[33-37]. DSGCs are particularly numerous in mouse retina- current estimates indicate they comprise at least half of the total number of RGCs [12, 33]. Furthermore, one study observed that some mouse RGCs are sensitive to approaching or ‘looming’ stimuli [38], suggesting that other complex feature-detecting RGCs are likely to be found in the near future.
Figure 1. Basic architecture of mouse retinal ganglion cells (RGCs) and visual pathways.
(Left panel) Mouse RGCs include 22 anatomically distinct subtypes, anatomically termed “G1-G22” [30]. Representative examples of reconstructions of RGCs in each category (based on dye-injections, are shown. Dark lines depict the somas and dendritic arbors of each cell. Lighter gray lines indicate dendritic arbors in deeper layers of the inner retina. Each patch of retina includes some, or all, of these 22 subtypes and all of these extend axons into the brain. Reproduced, with permission, from [30]
(Right panel) Schematic diagram of the mouse visual pathways described here, showing direct retinal projections (solid arrows) to the dorsal lateral geniculate nucleus (dLGN) and to the superior colliculus (SC) as well as geniculo-cortical pathways from dLGN to visual cortex (dashed arrows). Note: for simplicity, most of the 20 plus subcortical visual targets are not shown here. Shaded portions of the retinas indicate the location of RGCs whose axons do not cross at the optic chiasm and instead project ipsilaterally. Ovals in the dLGN correspond to the termination zones of the ipsilateral projecting RGC axons. The binocular (“B”) and monocular (“M”) fields in the V1 area of the cortex are shown. The lighter lines in the dLGN and SC represent the approximate boundaries where axons of different functional categories of RGCs terminate [31, 33, 36].
In considering whether the mouse is a generalizable model for studying vision, an important issue to resolve is how the 22 RGC subtypes found in the mouse correspond to the 20 subtypes in the primate. The recent identification of RGC subtype-specific genes and immunohistochemical markers in the mouse [33-37], will allow this question to be further addressed in the monkey and human. Indeed, studies of mouse ipRGCs helped generate tools that were applied to probe for and confirm the existence of the same cells in primate retina [29, 32, 39]. It will be interesting to see if such tools also help reveal the presence of DSGCs in primate. In the meantime, it remains to be determined if the preponderance of these non-classical RGC response types in the mouse retina makes it more attractive, or less ideal, as a model of primate vision.
Mouse visual thalamus is positioned to relay complex information to the cortex
Mammalian RGCs project to more than 20 subcortical targets[40], including the dorsal lateral geniculate nucleus (dLGN) of the thalamus, which sends information to the cortex, and a number of other regions that mediate behaviors ranging from reflexive eye movements to pupil dilation. Of the two dozen subcortical visual areas, by far the most experimental attention has focused on the dLGN, because of its role in processing and relaying visual information to the cortex for conscious visual perception (Figure 1). However, to date there has been only one dedicated quantitative survey of dLGN receptive fields in the mouse[41]. This study confirmed the presence of neurons with classical On and Off center-surround receptive fields, transient and sustained responses, and sizes similar to those measured in mouse RGCs. Neurons in the mouse dLGN also have both burst and tonic modes of firing[42, 43]. Thus, aside from an apparent lack of ‘frequency doubling’ Y-like cells, the physiological accounts of mouse dLGN neurons indicate they have the same basic properties as the dLGN of cats or primates.
Recent genetic-anatomical tracing studies revealed that the mouse dLGN may be even more sophisticated than previously thought. These studies demonstrated that both alpha (Y-like) RGCs as well as different subtypes of On-Off DSGCs project to the dLGN[31, 33]. Moreover, the different functional categories of RGCs connect to dLGN neurons in a series of parallel spatially distinct layers, and appear to include dedicated ‘direction selective’ territories [31, 33]. Given the known role of the dLGN in relaying visual information to the cortex (Figure 1), an intriguing question is how the specialized tuning of RGCs and dLGN neurons influences receptive fields in the cortex.
With the existence of DSGCs, and perhaps other complex feature-encoding RGCs projecting to the dLGN, why thus far, have only linear center-surround dLGN neurons have been reported in the mouse [41]? One possibility is that mouse dLGN receptive fields were assessed using white-noise reverse correlation based mapping; one third of the dLGN cells recorded in this study could not be analyzed with this procedure, and thus, could not characterized [41]. Another possible reason is that many On-Off DSGCs have relatively thin, slow conductive axons and they project to a narrow (~200 μm wide) layer adjacent to the optic tract[33]. It is unlikely that dLGN cells restricted to that layer would be recorded unless one was specifically looking for them. In light of the recent findings of DSGC and other complex inputs projecting to the mouse dLGN, it will be important to revisit the issue of response properties and cell types in the mouse dLGN, and to relate them to the laminar location of recording.
It is worth pointing out that the notion of dLGN neurons tuned for visual features more sophisticated than center-surround is not just an oddity of the mouse. Direction-selective receptive fields have been described in the rabbit dLGN[44], and both orientation and direction selective neurons are present in the cat dLGN[45]. Interestingly, many of the 20 plus RGC subtypes described in the primate retina appear to project directly to the dLGN[46], suggesting that primate dLGN neurons also receive- and may be tuned for - complex features of the visual world, and not just center-surround.
Primary visual cortex: a canonical circuit for extracting image features?
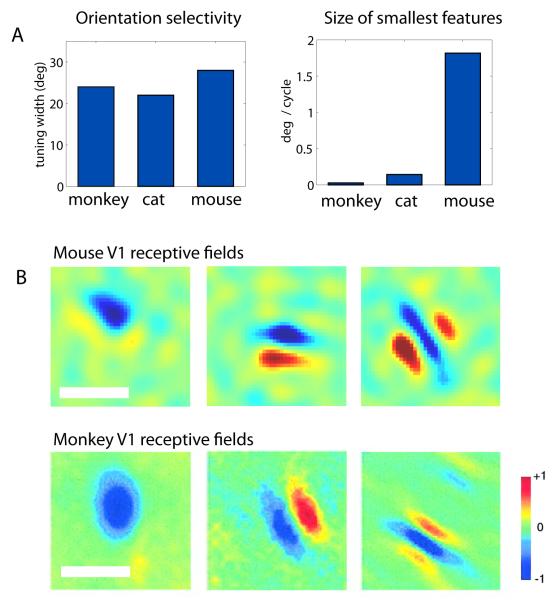
The visual cortex of the mouse bears many similarities to that of other species, with the typical 6-layered structure, retinotopic organization, and a variety of excitatory and inhibitory neuronal subtypes. The classical hallmark of primary visual cortex (V1), as described by Hubel and Wiesel[47], is that cells respond most strongly to edges or bars of light at a particular orientation, a property known as ‘orientation selectivity’. Consistent with the conserved aspects of visual representation in the retina and LGN, the mouse cortex demonstrates orientation selectivity[48-50]. Indeed, the degree of orientation selectivity in mouse, in terms of the range of orientations a given cell will respond to [50], is quite similar to that seen in the cat or monkey, despite the fact that visual acuity varies by almost two orders of magnitude across these species[51] (Figure 2A). The fact that the degree of orientation selectivity is relatively invariant from mouse to primate suggests this may be a fundamental aspect of the cortical computation[51].
Figure 2. Cortical response properties are similar across species despite differences in spatial resolution.
A) The degree of orientation selectivity is comparable across species (left), despite the fact that their behavioral acuity - the smallest feature they can detect - varies by nearly two orders of magnitude (right). Mouse data reproduced from [50, 92], others from [51].
B) Three example simple cell receptive fields in V1, from mouse (upper row) and monkey (lower row), showing a similar range of spatial structure. Note that the scale bar for mouse is 20 degrees, while that for monkey is approximately 1 degree. Red and blue correspond to On and Off subregions, respectively. The similarity in structures demonstrates that cortical neurons in both species respond to similar visual features, but of different size. Mouse data modified, with permission, from [50]; monkey data reproduced, with permission, from [93]. In both studies, receptive fields were measured by reverse correlation methods in anesthetized animals.
A recent quantitative electrophysiological survey of response properties across the layers of mouse V1 confirmed that nearly all of the hallmarks of cortical visual processing are present[50], including spatial frequency tuning, direction selectivity, linear and nonlinear responses (corresponding to Hubel and Wiesel’s simple and complex cells), as well as contrast gain control and contrast-invariant tuning. Indeed, even the spatial sub-structure of individual receptive fields is similar from mouse to monkey (Figure 2B), once the relative size of those receptive fields has been compensated, as quantitative scale-free analysis has shown[50]. Together, these findings suggest that the mouse cortex is indeed performing similar computations as in other species, just at lower spatial resolution.
On the other hand, although mouse V1 has a clear topographic map of visual space, other types of large-scale organization are absent. In particular, many higher species show an ordered map of orientation[52], which varies across the cortical surface in a stereotypical pattern of “pinwheels.”[53] The lack of such an orientation map may have been one reason that many researchers suspected that mice would have decreased orientation selectivity, since some theoretical models hypothesize that this large-scale orientation map would play a role the tuning of individual cells[54]. However, orientation maps appear to be absent in all rodents, even those rodent species with relatively higher acuity such as rats[55, 56] and squirrels[57], despite equivalent orientation tuning of individual cortical cells (Figure 2B). Thus, orientation maps are dispensable for generating sharp orientation tuning in individual cells. Although such maps may not play a direct role in cortical function[58], they may be important for wiring efficiency[59]. Further studies determining which aspects of visual cortical processing are shared across species, and which are not, can thus help elucidate the essential principles of both the visual system [51] and cortical function generally [60].
Amongst cortical neurons, the most striking morphological diversity lies in the class of inhibitory neurons. A range of intricate morphologies have been observed, leading to terms that include chandelier, basket, and double bouquet cells [61]. The highly specialized structures have led to many proposals for correspondingly specialized function in cortical processing[62]. However, testing such proposals has been challenging, due to the difficulty in matching up morphological, molecular markers, and electrophysiological properties, and then using these properties to identify neurons in an active circuit.
Recently, a combination of in vivo recording techniques together with molecular tools to enable the identification of specific inhibitory subtypes has led to a flurry of studies addressing the difference in visual response properties between inhibitory and excitatory neurons in mouse V1. These studies used a variety of approaches to identify recorded cell types, including two-photon imaging of Cre lines with either Cre-dependent viral expression[63] or fluorescent reporter lines[64], two-photon imaging followed by retrospective immunohistochemistry[65], and two-photon targeted cell-attached recordings[66]. Although there are some discrepancies, the general conclusion is that a large class of inhibitory neurons, the parvalbumin expressing fast-spiking neurons, show much less selectivity than the corresponding excitatory neurons. This significant population of untuned cells is a feature observed in the mouse but that possibly is not present in other species, although recent studies in cat and monkey have shown more diversity in orientation selectivity than previously appreciated[67, 68].
Furthermore, in one of the aforementioned studies [66], a separate subset of inhibitory neurons, which express somatostatin and are generally dendrite-targeting Martinotti cells, was targeted. This class of cells did show orientation selectivity and tended to fire at a delay relative to the parvalbumin subclass of interneurons. Therefore, these two populations could be subserving very different functions; in one case delivering inhibition representing the sum of local activity to the soma (which would be ideal for gain control), and in the other case, delivering tuned - but slightly delayed - inhibition to the dendrites (which would be ideal for gating of excitatory inputs).
Thus, genetic tools are beginning to allow the identification and targeted recording of defined neuronal subtypes in the cortex, leading to much more specific hypotheses about their roles in processing visual information and in governing cortical dynamics. It is now just a short step away to causally test these hypotheses, by using genetic tools to not only label and record from specific cortical cell types, but also to regulate their activity, and monitor the impact that has on cortical function and visual perception.
Mouse extrastriate cortex has multiple tiers for higher level visual processing
The primary visual cortex sends its output to a hierarchical series of extrastriate visual areas[69], which in cat, primate, and human have been show to represent a variety of higher order visual features, including motion, depth perception, image segmentation, and object recognition[70]. An organizing principle of these areas is the notion of a ventral stream carrying information about object identity (the “what” pathway), and a dorsal stream, carrying information about object location (the “where” pathway)[71]. However, beyond this rough flowchart, much less is known about how the particular visual features are computed by the neural circuitry within and between these regions.
Anatomical[9] and other mapping studies[72, 73] have revealed the presence of a number of extrastriate visual areas in mouse cortex, with up to 9 receiving direct input from V1. Furthermore, their patterns of connectivity are consistent with a dorsal and ventral stream as in other species[74]. However, very little is known about the functional properties of these areas in mouse. Several studies using extracellular recording[75] (Gao, E. et al, , 2008: Soc for Neurosci Abstr ) and two-photon imaging (Garrett, M., et al.Soc Neurosci Abstr, 2010) have begun to demonstrate different tuning properties in various regions of mouse visual cortex, however it is not yet clear what the different functional roles of these regions might be. In particular, it would be intriguing to probe these regions with stimuli or behavioral tasks that might be appropriate for assessing roles in the “what” versus “where” pathways, and to use new circuit analysis techniques to determine how these areas perform the image processing that results in high level visual representations.
Superior colliculus: a midbrain center for visually driven behavior
The superior colliculus (SC) is a midbrain structure that receives direct retinal input to its superficial layers (Figure 1), as well as integrating other sensory modalities across its full depth [76]. In non-mammalian vertebrates such as fish, frogs, and birds, the SC is known as the optic tectum, and is the primary center for visual processing. Neurons in the optic tectum include a broad and sophisticated repertoire of visual responses, including direction selectivity and looming detectors[77]. In mammals, neurons in the superficial (visual) layers of the SC primarily respond to small, moving spots, either light or dark, within a relatively broad region of visual space, and direction selective SC neurons have also been described. Activity in the deep layers of the SC results in eye and/or head movements toward the corresponding region of visual space [78].
A recent electrophysiological study of the mouse SC confirms that most receptive fields are similar to other mammals[79], but larger due to the lower resolution of the mouse’s retina. This study also found direction- and even orientation-selective SC cells. Interestingly, orientation selectivity was not dependent on input from the cortex, suggesting that it is either inherited directly from the orientation selective RGCs or computed separately in the SC- for instance, by summing inputs from DSGCs with opposite directions of preferred motion.
The presence of these diverse visual responses in the SC may be due to the intermediate position of the mouse in the evolutionary hierarchy, with SC and cortex playing a shared role in visual processing. Indeed, mice can still perform simple target detection tasks even after lesions of the visual cortex [5], suggesting that some of the functions ascribed to the visual cortex in cats and primates may actually be collicular-mediated in mice. On the other hand, the role of the SC in parsing the visual scene and directing visually-guided behaviors may be under-estimated in higher species, where the majority of focus has been on the SC’s role in saccadic eye movements [80]. As the mouse takes on an ever-more prominent role as a model for visual processing, it will be critical to address whether much of what we assume to be cortically mediated in other species is actually carried out by the SC in mice, and to consider the potentially broader role of the SC across species[80].
Visual psychophysics – linking circuits to perception
Perhaps the most promising aspect of studying vision in the mouse is the prospect of linking molecular and cellular aspects of neural circuit function with actual visual perception. In particular, one can begin to address the question of how the various parts of the visual system described above, with their specific cell types and connectivity, confer the ability to detect and identify stimuli in the visual world, as measured quantitatively with psychophysics. Traditionally, it was thought that visual psychophysics could only be performed with primates. However, the increased focus on in the mouse visual system, has led to a growing number of psychophysical and behavioral assays that are allowing an increasingly detailed characterization of mouse visual function. These range from basic reflexive behaviors to relatively complex visual discrimination tasks. The following overview is not meant to be exhaustive, but rather, to illustrate the range of different behavioral assays available, as well as the factors to be taken into consideration in choosing a behavioral task to test a particular visual function in the mouse (see also [81] for an earlier review).
The optomotor response provides a rapid measure of visual function without training
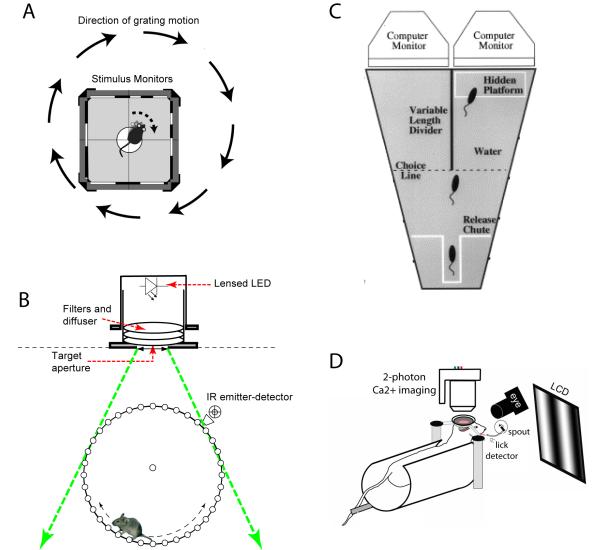
The optomotor response is a reflexive behavior whereby an animal will move its eyes or head to follow a moving visual field. This reflex behavior (also known as optokinetic tracking), which serves to stabilize the image of the visual world on the retina, has been exploited in the mouse as a way to quantitatively assess visual thresholds [82]. A computer-controlled stimulus and head-tracking system provides a semi-automated measure of the range of spatial frequencies and contrast that an animal can track (Figure 3a). This method provides a sensitive measure of visual thresholds without the need for behavioral training, which can be useful as a rapid screen for deficits in the eye. However, this reflex is likely controlled by subcortical mechanisms involving pretectal, collicular and brainstem nuclei and is unlikely to require the cortex for its expression. In fact, the spatial frequency thresholds measured for the optomotor reflex are slightly lower than for cortical-dependent tasks.[5]. The basic neural pathways thought to generate the optomotor response have been described [83], but rigorous tests of the involvement of specific pathways or cell types have not yet been performed to determine the circuit mechanisms that generate and modulate the optomotor response.
Figure 3. Behavioral paradigms for assessing mouse visual function.
A) Optomotor response as a measure for visual thresholds [82]. The mouse reflexively tracks moving gratings that are presented on computer screens surrounding the enclosure. The spatial frequency of the gratings is varied to determine the smallest features that the mouse will track. B) Running task for the measurement of photoreceptor thresholds [84],. A filtered light-emitting diode (LED) is used to provide a defined luminance source. When the luminance changes, the mouse must stop running on the wheel in order to receive a reward (eg. water, food). The animal’s motion is automatically recorded by an infrared (IR) emitter-detector. The defined geometry allows precise measurements of photon fluxes at the eye.
C) Two-alternative forced choice swimming task [92]. The mouse is placed in a water-filled Y-maze, with a hidden platform on one end. A computer-controlled stimulus is presented on the side with the platform, and the mouse must detect the stimulus and swim toward the correct side in order to find the platform and be released from the water.
D) Go/no-go licking task, combined with two-photon imaging[90]. Head-fixed mice are trained to lick only when a grating of the correct orientation is presented on a monitor in front of them. Incorrect licking is punished by a “time-out”. Concurrent two-photon imaging of calcium signals in the cortex allows for the measurement of neural correlates, and a camera detects eye movement. Reproduced, with permission, from (A) [82] , (B) [84], (C) [92], (D) [90].
Trained behavioral tasks reveal visual function: from photoreceptor sensitivity to orientation discrimination
The perceptual correlates of the biophysical properties of photoreceptors have recently been tested in a novel behavioral paradigm[84], wherein a mouse is trained to receive a water reward if it stops running when it detects a luminance stimulus (Figure 3b). By measuring detection thresholds in wildtype mice and two mouse lines lacking rod and cone signaling respectively, this study was able to determine the perceptual sensitivity of both rod and cone pathways [84]. Remarkably, although the mouse retina is rod-dominated, their cone sensitivities were found to be quite similar to that of humans[85]. Furthermore, the rod sensitivity is so powerful that the mice could detect and report as few as ~30 photons arriving at the eye [84]. In addition to providing a direct link between photoreceptor function and a mouse’s visual perception, it should prove possible to adapt such a task, which takes advantage of the natural propensity of mice to run, in order to measure the detection of more complex (e.g. patterned) visual stimuli.
Typical tests of spatial memory and navigation, such as the Morris water maze and Barnes maze, are inherently also tests of visual function, since the landmarks in these tests are generally distal visual cues[81]. However, these visual stimuli are generally not well-defined (in terms of distance, visual angle, etc), thereby precluding true psychophysical measurements. In a recent technical tour de force, head-fixed mice were trained to navigate a virtual environment, wherein images were projected onto a screen while the mouse ran on a spherical treadmill[86]. The fact that mice can perform such tasks, and create spatial representations in the brain based on the visual environment[87], clearly demonstrate that mice use their vision for such navigation. However, quantification of visual function is more difficult in these navigational tasks, since cues can vary with distance and viewing angle, and are not generally manipulated in a parameterized way, although the virtual reality maze may provide a means to do so.
As a more quantitative test of pattern vision, a two-alternative forced choice task[5] has been used, where mice must swim toward an appropriate visual stimulus in order to escape the water (Figure 3c). By using computer-generated stimuli and a succession of trials, it is possible to measure properties such as acuity and contrast sensitivity thresholds, which confirm that mice can behaviorally report the presence of a stimulus with an acuity limited primarily by retinal sampling [5]. This technique has also been to study differences in visual ability across mouse strains [88]. However, due to the time-consuming nature of each trial, and the level of stress induced in the mice, only about 50 trials can be performed per day on such a test, limiting the number of parameters that can be tested. An alternative method, utilizing a touch-screen for the animal to indicate responses, and delivery of a food pellet as a reward, has had success in demonstrating that mice can perform complex shape discrimination [89], although with a similar constraint in the number of trials per day.
In order to take advantage of many of the new tools available, a head-fixed preparation would be preferable, in order to allow concurrent imaging or the delivery of light stimulation for optogenetic studies. Furthermore, it is desirable for the animals to be able to perform a large number of trials in order to obtain reliable characterization across a range of stimulus parameters. A go/no-go task for mice was recently reported [90], where the mice learned to lick only in response to the appropriate stimulus to receive a water reward (Figure 3d). Hundreds of trials of such a task could be performed per day.
In addition to characterizing learning and visual performance, by measuring psychometric curves and detection thresholds on consecutive days of training, this study demonstrated that simultaneous measurement of neural responses could be performed repeatedly on subsequent days by two-photon imaging of a genetically-encoded calcium indicator. However, it should be noted that a more extensive training period (several weeks) was required compared to reflexive or innate behaviors, and that some animals failed to learn the task. Thus, there is currently a tradeoff between ease of training and the type of information that can be obtained in behavioral paradigms.
A major advantage of these psychophysical assays is that they provide a means to record from cells during a relevant behavioral task, as opposed to during passive viewing. This is an important point, given that visual processing depends on behavioral state [43, 91]. Furthermore, in combination with genetic methods to map and selectively activate/inactivate defined cell types, they allow the possibility of linking cellular and network mechanisms with visually guided behavior and perception.
Conclusion
In summary, the studies presented here provide a compelling argument for the use of the mouse in studying visual processing. Although fueled by the rapid development of genetic tools for circuit analysis (Box 1), this approach is bolstered by the growing evidence that despite the obvious differences in visual behaviors and acuity in the mouse compared to primates, the similarities dramatically outnumber the differences.
Along with the promise of the mouse as a model system, however, come certain potential pitfalls (Box 2). In particular, it is necessary to pay careful attention to potential species differences if one hopes to draw general conclusions about visual system function from mouse studies. Another fundamental challenge will involve combining the powerful molecular-genetic tools with the quantitative measurements of vision science. Although these are very different fields, harnessing the strengths of both should allow unprecedented traction in questions that date back to the earliest investigations of how the visual system works (Box 3).
Box 2. Challenges of studying vision in mouse.
Despite the potential benefits of using the mouse as a model for visual studies, there are a number of caveats that must be kept in mind.
1) Mouse strains can differ dramatically in terms of visual function, and in fact many strains are partially or completely blind due to inherited photoreceptor degeneration and albinism [88].
2) Mice are non-foveal, and as a result they may rely on head movements more than eye movements to view specific portions of visual space.
3) Behavioral assays need to be adapted to tasks mice can accomplish, both in terms of stimulus parameter range and the means of reporting responses.
4) It is unclear to what extent visual perception in mice relies on the cortex, and in general, controls are needed to ensure that a particular task depends on the brain region being studied, even if it has been demonstrated in other species.
5) Although it has been shown that mice can make simple discriminations such as orientation and shape, it remains to be determined what types of higher order visual processing and cognitive decisions they can perform.
6) Laboratory mice are often raised in what effectively are visually and behaviorally deprived environments, which has significant effects on visual system development [100].
7) In the end, some questions may simply not be addressable in mice. Obvious examples include face recognition and the cognitive visual functions underlying reading. However, it may turn out that even fundamental aspects of vision, such as stereopsis or spatially localized attention, are not tractable in the mouse.
Box 3. Important open questions in visual neuroscience.
Although the genetic tools in mouse open up a number of new experimental paradigms, a big challenge is to apply them to broadly significant questions about how vision works. The following are some long-standing questions that may now be amenable to direct experimental investigation.
- What is the full repertoire of retinal response properties, which convey the visual image to the brain?
- How much of classical cortical response properties are in fact computed in the retina (e.g., direction selectivity)?
- Do specific interneuron subtypes regulate distinct aspects of cortical function, such as contrast gain control or attentional modulation?
- How do ensemble responses of visual neurons relate to perception and decision making?
- How are image scenes segmented by the visual system, to identify objects versus background?
-- How does the visual system recognize objects despite changes in their position, size, illumination, etc.?
- How and where are visual memories stored?
- How do various disease processes compromise visual function, and what can be done to remedy the deficits?
Acknowledgements
We are grateful to Drs. Chuck Stevens, Brian Wandell, and Michael Stryker for suggestions and comments on this manuscript and to Dr. Harvey Karten for helpful discussions on comparative anatomy and physiology of the mammalian visual system.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Werner JS, Chalupa LM. The visual neurosciences. MIT Press; Cambridge, Mass.: 2004. [Google Scholar]
- 2.Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nat Rev Neurosci. 2009;10(5):360–72. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs GH. Primate color vision: a comparative perspective. Vis Neurosci. 2008;25(5-6):619–33. doi: 10.1017/S0952523808080760. [DOI] [PubMed] [Google Scholar]
- 4.Britten KH, et al. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992;12(12):4745–65. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prusky GT, Douglas RM. Characterization of mouse cortical spatial vision. Vision Res. 2004;44(28):3411–8. doi: 10.1016/j.visres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Remtulla S, Hallett PE. A schematic eye for the mouse, and comparisons with the rat. Vision Res. 1985;25(1):21–31. doi: 10.1016/0042-6989(85)90076-8. [DOI] [PubMed] [Google Scholar]
- 7.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57(5):634–60. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connor DH, Huber D, Svoboda K. Reverse engineering the mouse brain. Nature. 2009;461(7266):923–9. doi: 10.1038/nature08539. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Burkhalter A. Area map of mouse visual cortex. J Comp Neurol. 2007;502(3):339–57. doi: 10.1002/cne.21286. [DOI] [PubMed] [Google Scholar]
- 10.Guido W. Refinement of the retinogeniculate pathway. J Physiol. 2008;586(Pt 18):4357–62. doi: 10.1113/jphysiol.2008.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hensch TK. Critical period mechanisms in developing visual cortex. Curr Top Dev Biol. 2005;69:215–37. doi: 10.1016/S0070-2153(05)69008-4. [DOI] [PubMed] [Google Scholar]
- 12.Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18(21):8936–46. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderone JB, Jacobs GH. Regional variations in the relative sensitivity to UV light in the mouse retina. Vis Neurosci. 1995;12(3):463–8. doi: 10.1017/s0952523800008361. [DOI] [PubMed] [Google Scholar]
- 14.Provencio I, et al. A novel human opsin in the inner retina. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(2):600–5. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haverkamp S, et al. The primordial, blue-cone color system of the mouse retina. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(22):5438–45. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szel A, et al. Spatial and temporal differences between the expression of short- and middle-wave sensitive cone pigments in the mouse retina: a developmental study. The Journal of comparative neurology. 1993;331(4):564–77. doi: 10.1002/cne.903310411. [DOI] [PubMed] [Google Scholar]
- 17.Wikler KC, Williams RW, Rakic P. Photoreceptor mosaic: number and distribution of rods and cones in the rhesus monkey retina. J Comp Neurol. 1990;297(4):499–508. doi: 10.1002/cne.902970404. [DOI] [PubMed] [Google Scholar]
- 18.Packer O, Hendrickson AE, Curcio CA. Photoreceptor topography of the retina in the adult pigtail macaque (Macaca nemestrina) The Journal of comparative neurology. 1989;288(1):165–83. doi: 10.1002/cne.902880113. [DOI] [PubMed] [Google Scholar]
- 19.Perry VH, Cowey A. The ganglion cell and cone distributions in the monkey’s retina: implications for central magnification factors. Vision research. 1985;25(12):1795–810. doi: 10.1016/0042-6989(85)90004-5. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs GH, et al. Emergence of novel color vision in mice engineered to express a human cone photopigment. Science. 2007;315(5819):1723–5. doi: 10.1126/science.1138838. [DOI] [PubMed] [Google Scholar]
- 21.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4(9):877–86. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 22.Stone C, Pinto LH. Response properties of ganglion cells in the isolated mouse retina. Vis Neurosci. 1993;10(1):31–9. doi: 10.1017/s0952523800003205. [DOI] [PubMed] [Google Scholar]
- 23.Field GD, Chichilnisky EJ. Information processing in the primate retina: circuitry and coding. Annu Rev Neurosci. 2007;30:1–30. doi: 10.1146/annurev.neuro.30.051606.094252. [DOI] [PubMed] [Google Scholar]
- 24.Barlow HB, Hill RM. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science. 1963;139:412–4. doi: 10.1126/science.139.3553.412. [DOI] [PubMed] [Google Scholar]
- 25.Olveczky BP, Baccus SA, Meister M. Segregation of object and background motion in the retina. Nature. 2003;423(6938):401–8. doi: 10.1038/nature01652. [DOI] [PubMed] [Google Scholar]
- 26.van Wyk M, Taylor WR, Vaney DI. Local edge detectors: a substrate for fine spatial vision at low temporal frequencies in rabbit retina. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(51):13250–63. doi: 10.1523/JNEUROSCI.1991-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 28.Rollag MD, Berson DM, Provencio I. Melanopsin, ganglion-cell photoreceptors, and mammalian photoentrainment. Journal of biological rhythms. 2003;18(3):227–34. doi: 10.1177/0748730403018003005. [DOI] [PubMed] [Google Scholar]
- 29.Dacey DM, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433(7027):749–54. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 30.Volgyi B, Chheda S, Bloomfield SA. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol. 2009;512(5):664–87. doi: 10.1002/cne.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huberman AD, et al. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008;59(3):425–38. doi: 10.1016/j.neuron.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hattar S, et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huberman AD, et al. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62(3):327–34. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yonehara K, et al. Identification of retinal ganglion cells and their projections involved in central transmission of information about upward and downward image motion. PLoS One. 2009;4(1):e4320. doi: 10.1371/journal.pone.0004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim IJ, et al. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452(7186):478–82. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- 36.Rivlin-Etzion M, et al. Transgenic mice reveal unexpected diversity of on-off direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(24):8760–9. doi: 10.1523/JNEUROSCI.0564-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kay JN, et al. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(21):7753–62. doi: 10.1523/JNEUROSCI.0907-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munch TA, et al. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci. 2009;12(10):1308–16. doi: 10.1038/nn.2389. [DOI] [PubMed] [Google Scholar]
- 39.Ecker JL, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67(1):49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling C, Schneider GE, Jhaveri S. Target-specific morphology of retinal axon arbors in the adult hamster. Vis Neurosci. 1998;15(3):559–79. doi: 10.1017/s0952523898153178. [DOI] [PubMed] [Google Scholar]
- 41.Grubb MS, Thompson ID. Quantitative characterization of visual response properties in the mouse dorsal lateral geniculate nucleus. J Neurophysiol. 2003;90(6):3594–607. doi: 10.1152/jn.00699.2003. [DOI] [PubMed] [Google Scholar]
- 42.Grubb MS, Thompson ID. Visual response properties of burst and tonic firing in the mouse dorsal lateral geniculate nucleus. J Neurophysiol. 2005;93(6):3224–47. doi: 10.1152/jn.00445.2004. [DOI] [PubMed] [Google Scholar]
- 43.Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65(4):472–9. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levick WR, Oyster CW, Takahashi E. Rabbit lateral geniculate nucleus: sharpener of directional information. Science. 1969;165(894):712–4. doi: 10.1126/science.165.3894.712. [DOI] [PubMed] [Google Scholar]
- 45.Thompson KG, et al. Stimulus dependence of orientation and direction sensitivity of cat LGNd relay cells without cortical inputs: a comparison with area 17 cells. Vis Neurosci. 1994;11(5):939–51. doi: 10.1017/s0952523800003898. [DOI] [PubMed] [Google Scholar]
- 46.Dacey DM, et al. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37(1):15–27. doi: 10.1016/s0896-6273(02)01143-1. [DOI] [PubMed] [Google Scholar]
- 47.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol. 1962;160:106–54. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drager UC. Receptive fields of single cells and topography in mouse visual cortex. J Comp Neurol. 1975;160(3):269–90. doi: 10.1002/cne.901600302. [DOI] [PubMed] [Google Scholar]
- 49.Metin C, Godement P, Imbert M. The primary visual cortex in the mouse: receptive field properties and functional organization. Exp Brain Res. 1988;69(3):594–612. doi: 10.1007/BF00247312. [DOI] [PubMed] [Google Scholar]
- 50.Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci. 2008;28(30):7520–36. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Hooser SD. Similarity and diversity in visual cortex: is there a unifying theory of cortical computation? Neuroscientist. 2007;13(6):639–56. doi: 10.1177/1073858407306597. [DOI] [PubMed] [Google Scholar]
- 52.Hubel DH, Wiesel TN, Stryker MP. Orientation columns in macaque monkey visual cortex demonstrated by the 2-deoxyglucose autoradiographic technique. Nature. 1977;269(5626):328–30. doi: 10.1038/269328a0. [DOI] [PubMed] [Google Scholar]
- 53.Bonhoeffer T, Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature. 1991;353(6343):429–31. doi: 10.1038/353429a0. [DOI] [PubMed] [Google Scholar]
- 54.Sompolinsky H, Shapley R. New perspectives on the mechanisms for orientation selectivity. Curr Opin Neurobiol. 1997;7(4):514–22. doi: 10.1016/s0959-4388(97)80031-1. [DOI] [PubMed] [Google Scholar]
- 55.Ohki K, et al. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433(7026):597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- 56.Girman SV, Sauve Y, Lund RD. Receptive field properties of single neurons in rat primary visual cortex. J Neurophysiol. 1999;82(1):301–11. doi: 10.1152/jn.1999.82.1.301. [DOI] [PubMed] [Google Scholar]
- 57.Van Hooser SD, et al. Orientation selectivity without orientation maps in visual cortex of a highly visual mammal. J Neurosci. 2005;25(1):19–28. doi: 10.1523/JNEUROSCI.4042-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horton JC, Adams DL. The cortical column: a structure without a function. Philos Trans R Soc Lond B Biol Sci. 2005;360(1456):837–62. doi: 10.1098/rstb.2005.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koulakov AA, Chklovskii DB. Orientation preference patterns in mammalian visual cortex: a wire length minimization approach. Neuron. 2001;29(2):519–27. doi: 10.1016/s0896-6273(01)00223-9. [DOI] [PubMed] [Google Scholar]
- 60.Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annual review of neuroscience. 2004;27:419–51. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- 61.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 62.Moore CI, et al. Neocortical interneurons: from diversity, strength. Cell. 2010;142(2):189–93. doi: 10.1016/j.cell.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Runyan CA, et al. Response features of parvalbumin-expressing interneurons suggest precise roles for subtypes of inhibition in visual cortex. Neuron. 2010;67(5):847–57. doi: 10.1016/j.neuron.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zariwala HA, et al. Visual tuning properties of genetically identified layer 2/3 neuronal types in the primary visual cortex of cre-transgenic mice. Front Syst Neurosci. 2011;4:162. doi: 10.3389/fnsys.2010.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kerlin AM, et al. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron. 2010;67(5):858–71. doi: 10.1016/j.neuron.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma WP, et al. Visual representations by cortical somatostatin inhibitory neurons--selective but with weak and delayed responses. J Neurosci. 2010;30(43):14371–9. doi: 10.1523/JNEUROSCI.3248-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ringach DL, Shapley RM, Hawken MJ. Orientation selectivity in macaque V1: diversity and laminar dependence. J Neurosci. 2002;22(13):5639–51. doi: 10.1523/JNEUROSCI.22-13-05639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cardin JA, Palmer LA, Contreras D. Stimulus feature selectivity in excitatory and inhibitory neurons in primary visual cortex. J Neurosci. 2007;27(39):10333–44. doi: 10.1523/JNEUROSCI.1692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 70.Orban GA. Higher order visual processing in macaque extrastriate cortex. Physiol Rev. 2008;88(1):59–89. doi: 10.1152/physrev.00008.2007. [DOI] [PubMed] [Google Scholar]
- 71.Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4(2):157–65. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 72.Kalatsky VA, Stryker MP. New paradigm for optical imaging: temporally encoded maps of intrinsic signal. Neuron. 2003;38(4):529–45. doi: 10.1016/s0896-6273(03)00286-1. [DOI] [PubMed] [Google Scholar]
- 73.Wagor E, Mangini NJ, Pearlman AL. Retinotopic organization of striate and extrastriate visual cortex in the mouse. J Comp Neurol. 1980;193(1):187–202. doi: 10.1002/cne.901930113. [DOI] [PubMed] [Google Scholar]
- 74.Wang Q, Gao E, Burkhalter A. Gateways of ventral and dorsal streams in mouse visual cortex. J Neurosci. 2011;31(5):1905–18. doi: 10.1523/JNEUROSCI.3488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van den Bergh G, et al. Receptive-field properties of V1 and V2 neurons in mice and macaque monkeys. J Comp Neurol. 518(11):2051–70. doi: 10.1002/cne.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.May PJ. The mammalian superior colliculus: laminar structure and connections. Progress in brain research. 2006;151:321–78. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- 77.Vanegas H, Centro Latino Americano de Ciencias Biolâogicas . Comparative neurology of the optic tectum. Plenum Press; New York: 1984. p. 850. [Google Scholar]
- 78.Stryker MP, Schiller PH. Eye and head movements evoked by electrical stimulation of monkey superior colliculus. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1975;23(1):103–12. doi: 10.1007/BF00238733. [DOI] [PubMed] [Google Scholar]
- 79.Wang L, et al. Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. J Neurosci. 2010;30(49):16573–84. doi: 10.1523/JNEUROSCI.3305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nature neuroscience. 2010;13(2):261–6. doi: 10.1038/nn.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pinto LH, Enroth-Cugell C. Tests of the mouse visual system. Mamm Genome. 2000;11(7):531–6. doi: 10.1007/s003350010102. [DOI] [PubMed] [Google Scholar]
- 82.Prusky GT, et al. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45(12):4611–6. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 83.Simpson JI. The accessory optic system. Annual review of neuroscience. 1984;7:13–41. doi: 10.1146/annurev.ne.07.030184.000305. [DOI] [PubMed] [Google Scholar]
- 84.Naarendorp F, et al. Dark light, rod saturation, and the absolute and incremental sensitivity of mouse cone vision. J Neurosci. 2010;30(37):12495–507. doi: 10.1523/JNEUROSCI.2186-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stiles WS. Increment thresholds and the mechanisms of colour vision. Documenta ophthalmologica. Advances in ophthalmology. 1949;3:138–65. doi: 10.1007/BF00162601. [DOI] [PubMed] [Google Scholar]
- 86.Harvey CD, et al. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature. 2009;461(7266):941–6. doi: 10.1038/nature08499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muzzio IA, et al. Attention enhances the retrieval and stability of visuospatial and olfactory representations in the dorsal hippocampus. PLoS Biol. 2009;7(6):e1000140. doi: 10.1371/journal.pbio.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong AA, Brown RE. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes, brain, and behavior. 2006;5(5):389–403. doi: 10.1111/j.1601-183X.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 89.Bussey TJ, Saksida LM, Rothblat LA. Discrimination of computer-graphic stimuli by mice: a method for the behavioral characterization of transgenic and gene-knockout models. Behavioral neuroscience. 2001;115(4):957–60. doi: 10.1037//0735-7044.115.4.957. [DOI] [PubMed] [Google Scholar]
- 90.Andermann ML, Kerlin AM, Reid RC. Chronic cellular imaging of mouse visual cortex during operant behavior and passive viewing. Front Cell Neurosci. 2010;4:3. doi: 10.3389/fncel.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Y, et al. Task difficulty modulates the activity of specific neuronal populations in primary visual cortex. Nat Neurosci. 2008;11(8):974–82. doi: 10.1038/nn.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prusky GT, West PW, Douglas RM. Behavioral assessment of visual acuity in mice and rats. Vision Res. 2000;40(16):2201–9. doi: 10.1016/s0042-6989(00)00081-x. [DOI] [PubMed] [Google Scholar]
- 93.Ringach DL. Spatial structure and symmetry of simple-cell receptive fields in macaque primary visual cortex. J Neurophysiol. 2002;88(1):455–63. doi: 10.1152/jn.2002.88.1.455. [DOI] [PubMed] [Google Scholar]
- 94.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–40. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wickersham IR, et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53(5):639–47. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Braz JM, Rico B, Basbaum AI. Transneuronal tracing of diverse CNS circuits by Cre-mediated induction of wheat germ agglutinin in transgenic mice. Proc Natl Acad Sci U S A. 2002;99(23):15148–53. doi: 10.1073/pnas.222546999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang F, et al. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8(8):577–81. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 98.Rogan SC, Roth BL. Remote Control of Neuronal Signaling. Pharmacol Rev. 2011 doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guler AD, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–5. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cancedda L, et al. Acceleration of visual system development by environmental enrichment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(20):4840–8. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]