Abstract
Bioassay-guided fractionation of a solid tumor selective extract of the leaves and twigs of Antheroporum pierrei acquired from the U.S. National Cancer Institute extract repository afforded four new pyranoisoflavones, pierreiones A–D (1 – 4), together with rotenone (5), 12a-hydroxyrotenone (6), and tephrosin (7). The structures of all new compounds were determined on the basis of their spectroscopic data, and the absolute configuration of 1 was assigned with the help of 1H NMR analysis of its Mosher's ester derivatives. Compounds 1, and 5–7 accounted for the majority of the biological activity in terms of either cytotoxicity and/or selective toxicity to solid tumor cell lines. Pierreiones A (1) and B (2) demonstrated solid tumor selectivity with minimal cytotoxicity while pierreione C (3) exhibited no activity.
A major problem with present day cancer chemotherapy is the serious deficiency of drugs to treat solid tumors and the concurrent metastatic disease.1 The majority of cell-based antitumor drug discovery efforts that have led to useful discovery of standard chemotherapeutic agents have relied frequently on their potency rather than selectivity. To address this deficiency we have developed a high-throughput, cost-efficient, simple end-point disk diffusion soft agar assay based on differential clonogenic cytotoxicity between solid tumor cells and either normal or leukemia cells.2 Solid tumor selectivity criteria in this approach incorporate both the cellular and molecular targets associated with the cancer phenotype. In continuing our efforts to uncover potential anticancer agents from plants3, we investigated an extract of Antheroporum pierrei Gagnep. (Fabaceae) acquired from the natural products repository of U.S. National Cancer Institute (NCI.) which exhibited solid tumor selectivity in our assay. The genus Antheroporum Gagnep. contains five species; namely A. banaense, A. glaucum, A. harmandii, A. vidalli, and A. pierrei.4 None of these species has been subjected to any detailed biological and/or chemical investigations, except for a report of the occurrence of the non-protein amino acids arginine and homoarginine in seeds of A. pierrei,5 a perennial tree native to China, Thailand, and Vietnam. Consequently, we investigated the solid tumor selective extract of A. pierrei and herein we report the isolation and characterization of four new isoflavones, pierreiones A–D (1–4), and three known compounds, rotenone (5),6 12a-hydroxyrotenone (6),6,7 and tephrosin (7),8 and in vitro evaluation of their potential anticancer activity.
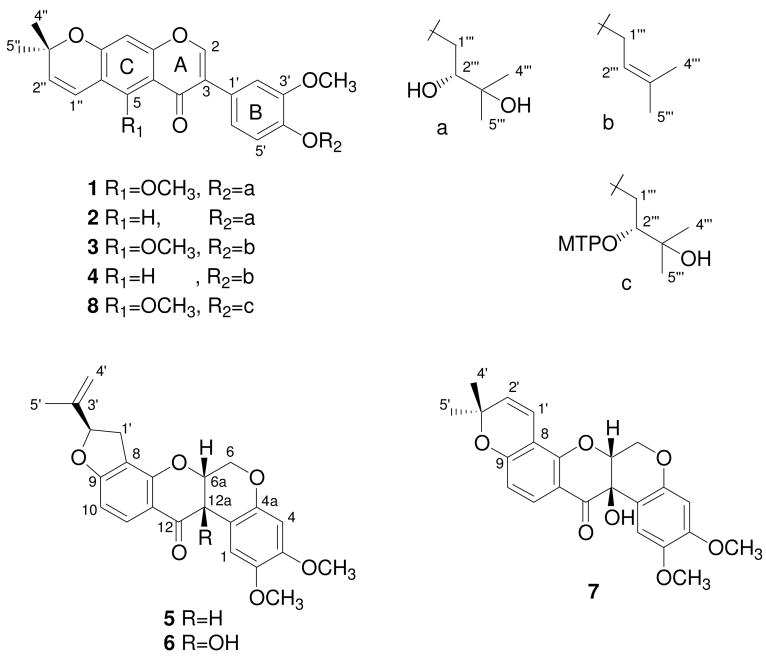
Bioactivity-guided fractionation of the solid tumor selective CH2Cl2-MeOH (1:1) extract of leaves and twigs of A. pierrei afforded compounds 1–7. Pierreione A (1) was obtained as a colorless solid. Its molecular formula was established as C27H30O8 from HRESIMS and 13C NMR data, and indicated thirteen degrees of unsaturation. The IR spectrum of 1 showed absorption bands for OH (3433 cm−1) and conjugated CO (1639 cm−1) functionalities. Its UV spectrum was typical of an isoflavone,9,10 and this was confirmed by the presence of characteristic NMR signals at δH 7.76 (s, H-2) and δC 150.6 (C-2).10-12 The 1H NMR spectrum also showed four singlets due to methyl groups [δ 1.24 (3H), 1.29 (3H), and 1.46 (6H)], two OCH3 groups [δ 3.86 and 3.87], and an aromatic proton (δ 6.59). In addition, it had signals due to an AB spin system consisting of two doublets (J = 10.0 Hz) at δ 6.72 and 5.71. This together with the presence of a signal due to two methyl groups at δ 1.46 (6H, s), suggested that 1 contained a 2″,2″-dimethylpyran substituent.11 The presence of this substituent was supported further by its 13C NMR signals at δC 130.8, 116.0, 77.7, and 28.3.11,13,14 Long-range correlations observed in the HMBC spectrum of 1 (see Figure S17 in the Supporting Information) allowed placement of the aromatic proton (δ 6.59) at C-8 of ring A and one of the OCH3 groups (δ 3.86) at C-5. In the NOE experiment, irradiation of the signal due to this OCH3 caused enhancement of the olefinic proton at δ 6.72 suggesting that the dimethylpyran moiety is fused to ring A in a linear manner. In the aromatic region of the 1H NMR spectrum, the presence of signals due an ABX spin system [δ 7.14 (1H, d, J = 2.0 Hz), 6.97 (1H, dd, J = 8.0 and 2.0 Hz), and 6.93 (1H, d, J = 8.0 Hz)] suggested that ring B of this isoflavone was 1′,3′,4′-trisubstituted.10 The signal at δH 7.14 was assigned to H-2′ due to its NOE with H-2 (see Figure S17 in the Supporting Information). The chemical shifts of H-6′ (δ 6.97) and H-5′ (δ 6.93) signals were in agreement with those reported for isoflavones.10 The NOE correlation observed between H-2′ (δ 7.14) and the OCH3 at δ 3.87 suggested that the latter group is attached to C-3′. The remaining signals in the 1H NMR spectrum indicated the presence of an oxygenated methylene group [δ 4.29 (1H, dd, J = 9.5 and 2.5 Hz), 4.08 (1H, dd, J = 9.5 and 6.5 Hz)], an oxygenated methine group [δ 3.69 (1H, brs)], and two methyl groups [δ 1.29 (s) and 1.24 (s)] attached to an oxygenated carbon. These signals were assigned to a OCH2CH(OH)C(CH3)2O spin system by comparison with the data reported for similar systems.15,16 Although no correlations were observed between C-1‴ protons (δ 4.08 and 4.29) with the signal due to C-4′ (δ 147.8) in the HMBC spectrum, this moiety was placed at C-4′ based on its NOE data (Figure 1). The configuration at C-2‴ was determined to be R by application of the modified Mosher's ester method according to the reported procedure (see Figure S18 in the Supporting Information).17,18 The structure of pierreione A was thus established as 3′,5-dimethoxy-4′-(2R,3-dihydroxy-3-methylbutoxyl)-3″,3″-dimethylpyrano-(6,7)-isoflavone (1).
The molecular formula of pierreione B (2) was determined as C26H28O7 from its HRESIMS. The 1H and 13C NMR spectra of 2 closely resembled those of 1, except that the OCH3 group at C-5 was absent in 2; instead it showed an additional aromatic proton (δ 7.85). The optical rotation of 2 was close to that of 1 suggesting R configuration at C-2‴. Thus, the structure of pierreione B was elucidated as 3′-methoxy-4′-(2R,3-dihydroxy-3-methylbutoxyl)- 3″,3″-dimethylpyrano-(6,7)-isoflavone (2).
Pierreione C (3) was obtained as a white amorphous solid that analyzed for C27H28O6 by HRESIMS. Its IR spectrum showed the presence of OH (3433 cm−1) and conjugated CO (1640 cm− 1) groups. Compound 3 was also suspected to be an isoflavone based on its UV spectrum and the 1H NMR signal due to H-2 at δ 7.76.9-12 In the 1H NMR spectrum of 3, the OCH3 group at C-5 appeared at δ 3.87, while the signals of the 2″,2″-dimethylpyran substituent on ring A were at δ 6.72 (1H, d, J = 10.2 Hz), 5.70 (1H, d, J = 10.2 Hz), and 1.45 (6H, s). Signals due to an ABX system at [δ 7.11 (1H, d, J = 1.8 Hz), 6.90 (1H, d, J = 7.8 Hz), and 6.97 (1H, dd, J = 7.8 and 1.8 Hz)] suggested that C-3′ and C-4′ of 3 were oxygenated.9,14 The 1H NMR signal at δ 3.88 was assigned to an OCH3 group (at C-3′). Based on literature precedence, the signals at δ 4.59 (2H, br d, J = 6.6 Hz), 5.51 (1H, t, J = 7.0 Hz), 1.75 (3H, s), and 1.72 (3H, s) were assigned to a 3‴-methyl-2‴-butenyl moiety linked to C-4′ through an oxygen atom.19,20 The foregoing suggested that the structure of pierreione C should be identical to the dimethoxy derivative of the previously known pyranoisoflavone, isoauriculasin.20 Thus, pierreione C was identified as 3′,5-dimethoxy-4′-O-(3-methyl-2-butenyl)-3″,3″-dimethylpyrano-(6,7)-isoflavone (3).
Pierreione D (4), obtained as a white amorphous solid, had molecular formula C26H26O5 based on its HRESIMS data. The 1H NMR spectrum of 4 showed very close resemblance to that of 3, except for the presence of an aromatic proton signal at δ 7.86 in 4 instead of the signal due to the OCH3 (δ 3.87) in 3. The foregoing evidence identified pierreione D as 3′-methoxy-4′-O-(3-methyl-2-butenyl)-3″,3″-dimethylpyrano-(6,7)-isoflavone (4). Comparison of the spectroscopic and optical rotation data of compounds 5–7 with those reported in the literature allowed these to be identified as rotenone,6 12a-hydroxyrotenone,6,7 and tephrosin,8 respectively.
A disk diffusion soft agar assay, using a panel of two solid tumor cell lines [C38 (colon adenocarcinoma) and HCT-116 (colon cancer)] and one leukemia cell line [L1210 (lymphocytic leukemia)] was used to evaluate the differential cytotoxicity (solid tumor selectivity) of the extract, fractions, and pure compounds.2 IC50 data for compounds 1–7 in the human colon cancer cell line HCT-116 were also determined. Taken together, these data (Table 2) suggested that pierreione A (1), rotenone (5), 12a-hydroxyrotenone (6), and tephrosin (7) accounted for the majority of the biological activity in terms of either cytotoxicity and/or selective toxicity to solid tumor cell lines, and pierreiones A (1) and B (2) demonstrated solid tumor selectivity with minimal cytotoxicity while pierreione C (3) exhibited no activity. As the only difference between the structures of pierreione A (1) and pierreione C (3) is the presence of a 2,3-dihydroxyisopentane side-chain in ring C of 1 instead of an isopentenyl moiety in 3, it is likely that the presence of the former is required for the cytotoxic activity of 1. It is noteworthy that cytotoxic activities for rotenone (5),21-25 12a-hydroxyrotenone (6),23 and tephrosin (7)23,24 against several cancer cell lines have been reported previously.
Table 2. Zone Unit Differentials in the Disk Diffusion Soft Agar Colony Formation Assaya and IC50 values for Compounds 1 – 7.
| murine tumor selectivity | toxicity to human colon cancer cell line HCT-116 | ||
|---|---|---|---|
| compound | conc. (μg/disk) | ZC38– ZL1210 | IC50 (μg/disc) |
| 1 | 68 | 300 | 7.5 |
| 2 | 66 | 300 | 18 |
| 3 | NAb | >46 | |
| 4 | 1.9 | 600 | 30 |
| 5 | 0.23 | 500 | 0.045 |
| 6 | 0.26 | 450 | 0.055 |
| 7 | 0.24 | 550 | 0.040 |
Measured in zone units: 200 zone units = 6 mm. Murine cell lines: C38 (colon adenocarcinoma), L1210 (lymphocytic leukemia).
NA = Not active.
Experimental Section
General Experimental Procedures
Optical rotations were measured with a Jasco Dip-370 polarimeter using CHCl3 as solvent. IR spectra using KBr disks were recorded on a Shimadzu FTIR-8300 spectrometer. 1D and 2D NMR spectra were recorded in CDCl3 with a Bruker DRX-500 and a Bruker DRX-600 instrument at 500 MHz or 600 MHz for 1H NMR and 125 MHz for 13C NMR using residual CHCl3 as the internal standard. Chemical shift values (δ) are given in parts per million (ppm) and the coupling constants are in Hz. Low-resolution and high-resolution MS were recorded on Shimadzu LCMS-DQ8000α and JEOL HX110A spectrometers, respectively. HPLC was carried out on a Hitachi instrument consisting of a DAD and an ELSD detector. Semi-preparative C-8 column (250 × 10 mm, 5 μm) used was from Phenomenex Inc.
Plant Material
Leaves and twigs of Antheroporum pierrei were collected in Thailand in Aug 1987 for the U.S. National Cancer Institute (NCI) program on anticancer drug discovery and were identified by Prof. J. S. Burley. The NCI Open Repository Sample number N001507 was assigned to the sample.
Extraction and Isolation
Dried plant material was extracted with CH2Cl2-MeOH (1:1), residual solvents were removed under vacuum, and the extract (N001507) was stored at −20 °C in the NCI repository at the Frederick Cancer Research and Development Center (Frederick, MD). The crude extract (5.0 g sample from an NCI stock supply) was partitioned between hexanes and 80 % aq. MeOH. The bioactive aq. MeOH fraction was diluted to 50% aq. MeOH by the addition of water and extracted with CHCl3. Evaporation of the CHCl3 fraction yielded a dark green residue (474 mg) which was subjected to size-exclusion chromatography over Sephadex LH-20 (15.0 g) made up in hexanes/CH2Cl2 (1:4) and eluted with 250 mL each of hexanes/CH2Cl2 (1:4), CH2Cl2/acetone (3:2), CH2Cl2/acetone (1:4), and finally with MeOH (500 mL). The bioactive hexanes/CH2Cl2 (1:4) fraction (248 mg) was subjected to column chromatography over Lichroprep diol (60 g) made up in hexanes/EtOAc (5:1) and eluted with hexanes/EtOAc (5:1, 500 mL), hexanes/EtOAc (3:1, 500 mL), hexanes/EtOAc (1:1, 500 mL), EtOAc (250 mL), EtOAc/MeOH (1:1, 250 mL), and MeOH (500 mL). The resulting fractions were combined based on their TLC profiles to afford twelve fractions (F1-F12). Of these, fraction F10 (78.8 mg) which was found to be active, was subjected to size-exclusion chromatography on Sephadex LH-20 (150 g) and eluted with CH2Cl2/MeOH (3:2) to afford three sub-fractions (F10a-c). The sub-fraction F10a (34.1 mg) was further fractionated on Sephadex LH-20 (150 g) and eluted with MeOH to obtain four sub-fractions of which only the third fraction was found to be active. Compounds 1 (5.3 mg) and 2 (2.2 mg) were obtained from this active fraction by HPLC on a C-8 semi-preparative column. Further fractionation of the active fraction F4 (4.3 mg) by HPLC under the same conditions afforded compounds 3 (2.3 mg) and 4 (0.9 mg). The active fraction F5 (29.2 mg) on Sephadex LH-20 (150 g) size-exclusion chromatography and elution with CH2Cl2/MeOH (3:2) yielded two sub-fractions F5a and F5b. Further fractionation of the active sub-fraction F5b by HPLC as above afforded compounds 5 (1.4 mg), 6 (3.0 mg), and 7 (1.1 mg).
Pierreione A (1)
white amorphous solid;[α]D25 –5.4 (c 0.1, CHCl3); UV (MeOH) λmax (log ε) 325 (3.72), 271 (4.48), 228 (4.28), 203 (4.40) nm; IR (KBr) νmax 3433, 2974, 2931, 1639, 1604, 1515, 1465, 1265, 1207, 1126, 1180, 1064 cm−1 ; H NMR data, see Table 1; 13C NMR (125 MHz, CDCl3) δ 174.9 (C-4), 158.6 (C-9), 158.1 (C-7), 155.7 (C-5), 150.6 (C-2), 149.3 (C-3′), 147.8 (C-4′), 130.8 (C-2″), 126.1 (C-1′), 125.5 (C-3), 121.3 (C-6′), 116.0 (C-1″), 114.2 (C-5′), 113.3 (C-6), 113.1 (C-10), 113.0 (C-2′), 100.6 (C-8), 77.7 (C-3″), 74.9 (C-2‴), 71.9 (C-1‴), 71.9 (C-3‴), 62.8 (5-OCH3), 55.9 (3′-OCH3), 28.3 (C-4″), 28.3 (C-5″), 26.7 (C-4‴), 25.2 (C-5‴); HR-ESIMS m/z 483.2004 [M + H]+ (calcd for C27H31O8, 483.2019).
Table 1. 1H NMR Data (500 MHz or 600 MHz, δ, Hz) for Compounds 1–4 in CDCl3.
| position | 1a,b | 2b | 3c | 4c |
|---|---|---|---|---|
| 2 | 7.76 (s) | 7.87 (s) | 7.76 (s) | 7.87 (s) |
| 5 | 7.85 (s) | 7.86 (s) | ||
| 8 | 6.59 (s) | 6.76 (s) | 6.59 (s) | 6.76 (s) |
| 2′ | 7.14 (d, 2.0) | 7.19 (d, 2.0) | 7.11 (d, 1.8) | 7.17 (d, 1.0) |
| 5′ | 6.93 (d, 8.0) | 6.93 (d, 8.0) | 6.90 (d, 7.8) | 6.90 (d, 8.0) |
| 6′ | 6.97 (dd, 8.0, 2.0) | 7.00 (dd, 8.0, 2.0) | 6.97 (dd, 7.8, 1.8) | 7.00 (br d, 8.0) |
| 1″ | 6.72 (d, 10.0) | 6.43 (d, 10.0) | 6.72 (d, 10.2) | 6.43 (d, 10.0) |
| 2″ | 5.71 (d, 10.0) | 5.73 (d, 10.0) | 5.70 (d, 10.2) | 5.72 (d, 10.0) |
| 4″ | 1.46 (s) | 1.47 (s) | 1.45 (s) | 1.47 (s) |
| 5″ | 1.46 (s) | 1.47 (s) | 1.45 (s) | 1.49 (s) |
| 1‴ | 4.29 (dd, 9.5, 2.5) | 4.30 (dd, 10.0, 2.0) | 4.59 (br d, 6.6) | 4.60 (br d, 6.6) |
| 4.08 (dd, 9.5, 6.5) | 4.09 (dd, 10.0, 5.5) | |||
| 2‴ | 3.69 (br s) | 3.70 (br s) | 5.51 (t, 7.0) | 5.51 (t, 7.0) |
| 4‴ | 1.29 (s) | 1.30 (s) | 1.72(s) | 1.72 (s) |
| 5‴ | 1.24 (s) | 1.29 (s) | 1.75(s) | 1.75(s) |
| 5-OCH3 | 3.86 (s) | 3.87(s) | ||
| 3′-OCH3 | 3.87 (s) | 3.88 (s) | 3.88(s) | 3.89 (3H, s) |
Signals were assigned by HMQC, HMBC and 1H–1H COSY experiments.
At 500 MHz.
At 600 MHz.
Pierreione B (2)
white amorphous solid; [α]D25 –5.8 (c 0.1, CHCl3); UV (MeOH) λmax (log ε) 331 (3.96), 267 (4.52), 228 (4.45), 206 (4.47) nm; IR (KBr) νmax 3425, 2974, 1639, 1621, 1515, 1477, 1269, 1203, 1141, 1110 cm−1 ; 1H NMR data, see Table 1; 13C NMR (125 MHz, CDCl3) δ 175.6 (C-4), 158.0 (C-9), 157.4 (C-7), 152.1 (C-2), 149.4 (C-3′), 147.8 (C-4′), 131.7 (C-2″), 126.1 (C-1′), 124.5 (C-3), 123.7 (C-5), 121.2 (C-6′), 121.0 (C-1″), 114.2 (C-5′), 119.9 (C-6), 118.5 (C-10), 112.8 (C-2′), 103.9 (C-8), 77.9 (C-3″), 74.9 (C-2‴), 71.9 (C-1‴), 71.9 (C-3‴), 55.8 (3′-OCH3), 28.5 (C-4″), 28.5 (C-5″), 26.7 (C-4‴), 25.2 (C-5‴); HR-ESIMS m/z 453.1872 [M + H]+ (calcd for C26H29O7, 453.1913).
Pierreione C (3)
white amorphous powder; UV (MeOH) λmax (log ε) 325 (3.84), 272 (4.60), 228 (4.42), 206 (4.54) nm; IR (KBr) νmax 3433, 2927, 1639, 1640, 1512, 1461, 1265, 1207, 1126 cm−1 ; 1H NMR (600 MHz, CDCl3) data, see Table 1; HR-ESIMS m/z 449.1968 [M + H]+ (calcd for C27H29O6, 449.1964).
Pierreione D (4)
white amorphous powder; UV (MeOH) λmax (log ε) 331 (3.93), 267 (4.49), 228 (4.43), 204 (4.48) nm; IR (KBr) νmax 3461, 2931, 1647, 1612, 1517, 1473 cm−1 ; 1H NMR (600 MHz, CDCl3) data, see Table 1; HR-ESIMS m/z 419.1853 [M + H]+ (calcd. for C26H27O5, 419.1859).
Rotenone (5)
white solid; [α]D25 –173.3 (c 0.03, CHCl3); LR-APCIMS and 1H NMR data were consistent with literature values.6
12a-Hydroxyrotenone (6)
white solid; [α]D25 –90.7 (c 0.07, CHCl3); LR-APCIMS, 1H NMR, and 13C NMR data were consistent with those reported.6,7
Tephrosin (7)
white solid; [α]D25 –100.0 (c 0.09, CHCl3); LR-APCIMS and 1H NMR data were consistent with literature values.8
Preparation of the (R)-and (S)-MTPA Ester Derivatives (8a and 8b) of 1 by a Convenient Mosher Ester Procedure18,19
Compound 1 (1.0 mg) was transferred into a clean NMR tube and was dried under vacuum. Pyridine-d5 (0.5 mL) and (S)-(–)-α-methoxy- α-(trifluoromethyl)-phenylacetyl (MTPA) chloride (5 μL) were added to the NMR tube immediately under a stream of N2 and was shaken carefully to mix the sample and the MTPA chloride. The NMR tube was allowed to stand at room temperature for 2 h to afford the (S)-MTPA ester derivative (8a) of 1. 1H NMR data of 8a (600 MHz, pyridine-d5): δ 8.08 (1H, s, H-2), 7.50 (1H, brs, H-2′), 7.27 (1H, d, J = 8.4 Hz, H-5′), 6.83 (1H, d, J = 10.2 Hz, H-1″), 6.03 (1H, d, J = 9.0 Hz, H-2‴), 5.76 (1H, d, J = 9.6 Hz, H-2″), 4.89 (1H, d, J = 11.4 Hz, H-1‴a), 4.56 (1H, dd, J = 11.4, 9.0 Hz, H-1‴b), 4.01 (3H, s, OCH3, OCH3-5 or OCH3-3′), 3.80 (3H, s), 3.77 (3H, s, OCH3-5 or OCH3-3′), 1.43 (3H, s, H-4‴ or H-5‴), 1.42 (6H, s, H-4″ and 5″), 1.37 (3H, s, H-4‴ or H-5‴). In the manner described for 8a, another portion of 1 (0.9 mg) was reacted in a second NMR tube with (R)-(+)-α-methoxy-α-(trifluoromethyl)-phenylacetyl chloride (5 μL) at room temperature for 2 h in pyridine-d5 (0.5 mL) to afford the (R)-MTPA ester derivative (8b). 1H NMR data of 8b (600 MHz, pyridine-d5): δ 8.07 (1H, s, H-2), 7.50 (1H, d, J = 1.8 Hz, H-2′), 7.25 (1H, dd, J = 8.4, 1.8 Hz, H-6′), 7.12 (1H, d, J = 8.4 Hz, H-5′), 6.83 (1H, d, J = 10.2 Hz, H-1″), 6.77 (1H, s, H-8), 6.07 (1H, dd, J = 9.0, 1.8 Hz, H-2‴), 5.77 (1H, d, J = 10.2 Hz, H-2″), 4.76 (1H, dd, J = 10.8, 1.8 Hz, H-1‴a), 4.42 (1H, dd, J = 10.8, 9.0 Hz, H-1‴b), 4.00 (3H, s, OCH3-5 or OCH3-3′), 3.79 (3H, s), 3.77 (3H, s), 1.53 (3H, s, H-4‴ or H-5‴), 1.52 (3H, s, H-4‴ or H-5‴), 1.43 (6H, s, H-4″ and 5″).
In vitro Disk Diffusion Assay for Cytotoxicity
The disk diffusion assay was used to define differential cell killing among human and murine normal and malignant cell types as previously reported.2 Since the initial extract (N001507) obtained from NCI was found to demonstrate selectivity between murine Colon 38 and leukemia L1210 cells, these cell lines were used for bioassay-guided fractionation and evaluation of the active compounds. Both the magnitude of the zonal difference as well as the potency was used for prioritization of fractions.
IC50 Determinations
IC50 studies were carried out against human HCT-116 colon cancer cells. These cells were grown in 5 mL culture medium (RPMI-1640 + 15% FBS containing 1% penicillin-streptomycin, and 1% glutamine) at 37 °C and 5% CO2 at a starting concentration of 5 × 104 cells/T25 flask. On day 3, cells were exposed to different concentrations of the drug. Flasks were incubated for 120 h (5 d) in a 5% CO2 incubator at 37 °C and the cells harvested with trypsin, washed once with HBSS, re-suspended in HBSS, and then counted using a hemocytometer. The results are normalized to an untreated control. The IC50 values were determined using Prism 4.0 software.
Supplementary Material
Figure 1.
Acknowledgments
Financial support for this work was provided by Grant R01 CA092143 funded by the National Cancer Institute (NCI). We thank Dr. David Newman of the NCI for providing the crude extract, and Dr. Xiao-Ning Wang for reading the manuscript and suggesting some modifications.
Footnotes
Supporting Information: 1H NMR spectra for compounds 1–7, 8a and 8b; 13C NMR spectra for compounds 1–3, and 6; 1H-1H COSY, HSQC, and HMBC spectra for compound 1; Key HMBC and NOE correlations for 1 (Figure S17); Δδ values obtained for (S)- and (R)-MTPA esters (8a and 8b, respectively) of 1 (Figure S18). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Monga M, Sausville EA. Leukemia. 2002;16:520–526. doi: 10.1038/sj.leu.2402464. [DOI] [PubMed] [Google Scholar]; (b) Jones D. Nat Revs Drug Disc. 2008;7:875–877. doi: 10.1038/nrd2748. [DOI] [PubMed] [Google Scholar]
- 2.Valeriote F, Grieshaber CK, Media J, Pietraszkewicz H, Hoffmann J, Pan M, McLaughlin S. J Exp Ther Oncol. 2002;2:228–236. doi: 10.1046/j.1359-4117.2002.01038.x. [DOI] [PubMed] [Google Scholar]
- 3.Subramanian B, Nakeff A, Tenney K, Crews P, Gunatilaka L, Valeriote F. J Exp Ther Oncol. 2006;5:195–204. [PMC free article] [PubMed] [Google Scholar]
- 4.Wojciechowski MF, Lavin M, Sanderson MJ. Am J Bot. 2004;91:1846–1862. doi: 10.3732/ajb.91.11.1846. [DOI] [PubMed] [Google Scholar]
- 5.Evans SV, Fellows LE, Bell EA. Biochem Syst Ecol. 1985;13:271–302. [Google Scholar]
- 6.Dagne E, Yenesew A, Waterman PG. Phytochemistry. 1989;28:3207–3210. [Google Scholar]
- 7.Puyvelde LV, De Kimpe N, Mudaheranwa JP, Gasiga A, Schamp N, Declercq JP, Meerssche MV. J Nat Prod. 1987;50:349–356. [Google Scholar]
- 8.Andrei C, Vieira PC, Fernandes JB, Silva MFGF, Fo ER. Phytochemistry. 1997;46:1081–1085. [Google Scholar]
- 9.Veitch NC, Sutton PSE, Kite GC, Ireland HE. J Nat Prod. 2003;66:210–216. doi: 10.1021/np020425u. [DOI] [PubMed] [Google Scholar]
- 10.Nkengfack AE, Azebaze AGB, Waffo AK, Formum ZT, Meyer M, Heerden FR. Phytochemisty. 2001;58:1113–1120. doi: 10.1016/s0031-9422(01)00368-5. [DOI] [PubMed] [Google Scholar]
- 11.Murthy MS, Rao EV. Magn Reson Chem. 1986;24:225–230. [Google Scholar]
- 12.Khalid S, Waterman PG. Phytochemisty. 1983;22:1001–1003. [Google Scholar]
- 13.Mahabusarakam W, Deachathai S, Phongpaichit S, Jansakul C, Taylor WC. Phytochemisty. 2004;65:1185–1191. doi: 10.1016/j.phytochem.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Yin S, Fan CQ, Wang Y, Dong L, Yue JM. Bioorg Med Chem. 2004;12:4387–4392. doi: 10.1016/j.bmc.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 15.He HP, Shen YM, Chen ST, He YN, Hao XJ. Helv Chim Acta. 2006;89:2836–2840. [Google Scholar]
- 16.Debenedetti SL, Palacios PS, Nadinic EL, Coussio JD. J Nat Prod. 1994;57:1539–1542. [Google Scholar]
- 17.Bashyal BP, Wijeratne EMK, Faeth SH, Gunatilaka AAL. J Nat Prod. 2005;68:724–728. doi: 10.1021/np058014b. [DOI] [PubMed] [Google Scholar]
- 18.Su BN, Park EJ, Mbwambo ZH, Santarsiero BD, Mesecar AD, Fong HHS, Pezzuto JM, Kinghorn AD. J Nat Prod. 2002;65:1278–1282. doi: 10.1021/np0202475. [DOI] [PubMed] [Google Scholar]
- 19.Asomaning WA, Amoako C, Oppong IV, Phillips WR, Addae-Mensah I, Osei-twum EY, Waibel R, Achenbach H. Phytochemisty. 1995;39:1215–1218. [Google Scholar]
- 20.Minhaj N, Khan H, Kapoor SK, Zaman A. Tetrahedron. 1976;32:749–751. [Google Scholar]
- 21.Blaskó G, Shieh HL, Pezzuto JM, Cordell GA. J Nat Prod. 1989;52:1363–1366. doi: 10.1021/np50066a035. [DOI] [PubMed] [Google Scholar]
- 22.Deng YT, Huang HC, Lin JK. Mol Carcinog. 2010;49:141–151. doi: 10.1002/mc.20583. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda H, Yoshida K, Miyagawa K, Asao Y, Takayama S, Nakashima S, Xu F, Yoshikawa M. Bioorg Med Chem. 2007;15:1539–1546. doi: 10.1016/j.bmc.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Cao S, Schilling JK, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DGI. J Nat Prod. 2004;67:454–456. doi: 10.1021/np0303815. [DOI] [PubMed] [Google Scholar]
- 25.Fang N, Casida JE. J Agric Food Chem. 1999;47:2130–2136. doi: 10.1021/jf981188x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.