Abstract
Climate differences across latitude can result in seasonal constraints and selection on life-history characters. Because Aedes albopictus (Skuse) invaded North America in the mid-1980s, it has spread across a range of ≈14° latitude and populations in the north experience complete adult mortality because of cold winter temperatures that are absent in the south. Life-table experiments were conducted to test for differences in the adult survival and reproductive schedules of Ae. albopictus females from three populations from the northern (Salem, NJ; Springfield, IL; Eureka, MO; ≈39° N) and southern (Palm Beach, Palmetto, Tampa, FL; ≈27–28° N) extremes of the species distribution in North America. There were consistent differences between northern and southern populations in incidence of photoperiodically-induced egg diapause. Under short daylength, diapause eggs constituted twice the proportion of total viable eggs from northern females (81.9–92.1%) than southern females (35.9–42.7%). There were no consistent differences between northern and southern populations in resource allocation between reproduction and maintenance, reproduction over time, and reproductive investment among offspring, and no apparent trade-offs between diapause incidence with reproduction or longevity. Our results suggest that the main response of North American Ae. albopictus to unfavorable winter climates is via the life history strategy of producing diapausing eggs, rather than quantitative variation in reproduction, and that there are no detectable costs to adult survival. Inherent geographic variation in the expression of diapause, consistent with the latitudinal extremes of A. albopictus, indicates evolutionary loss of diapause response in southern populations because of the invasion of A. albopictus in North America.
Keywords: diapause, life history evolution, survival, fecundity, reproductive investment
Geographic differences in environmental conditions can influence the establishment of nonindigenous species, with favorable conditions increasing the probability that a nonindigenous species successfully will invade and spread (Juliano and Lounibos 2005). Of fundamental importance to the invasion and spread of an organism are the temporal patterns of reproduction and survival that make up its life-history (Juliano and Lounibos 2005). In most instances energy available to organisms is limiting and trade-offs occur among life-history traits when energy is partitioned among growth, maintenance, and reproduction. Trade-offs usually manifest as costs of reproduction, such as decreases in parental longevity or reduced ability to reproduce later in life with early reproduction (Bell 1980). Trade-offs also are likely to occur within the reproductive budget, including negative relationships between fecundity and investment per offspring. Because of these trade-offs among fitness-related traits, natural selection favors individuals with trait combinations that attain greatest fitness under local regimes of mortality and reproductive phenology (Michod 1979, Bell 1980, Roff 2002).
For a species that inhabits a range of latitudinal climates, individuals from unfavorable or harsh environments may be selected to commit a high proportion of available resources (e.g., time, energy) to reproduction to ensure that at least some offspring reach maturity despite high expected mortality, resulting in relatively high lifetime reproductive output. Early reproduction also may increase the fitness of individuals in regions subject to seasonal time constraints, such as onset of an unfavorable harsh climate (Rowe and Ludwig 1991). Unfavorable environments also may select for production of higher-investment (i.e., larger) offspring because greater offspring size often increases offspring probably of surviving to adulthood, particularly under harsh conditions (Fox and Czesak 2000). Insects often respond to unfavorable climatic conditions by entering facultative diapause (Denlinger 2002). Individuals usually enter facultative diapause when cued by predictable seasonal changes, such as photoperiod or temperature, and individuals from different populations may become locally adapted via selection for varying sensitivity to these cues (Denlinger 2002).
Diapause is a physiological state of dormancy, but it still has energetic demands. Fats typically are used during diapause and these are dependent on the energy reserves sequestered prior to the entry into diapause (Hahn and Delinger 2007). In addition, the energy reserves expended during diapause can have profound effects on post-diapause fitness (Hahn and Delinger 2007). Energetic demands of diapause likely incur costs on longevity and reproductive output; thus trade-offs may occur between diapause, reproduction, and longevity. Individuals from populations at different latitudes (with different climatic constraints) are thus expected to vary in the incidence of diapause expression, survival, current reproduction, and future reproductive investment among offspring.
The Asian tiger mosquito Aedes albopictus (Skuse) invaded continental United States through a port in Houston, Texas in the mid-1980s (Sprenger and Wuithiranyagool 1986). The presence of photoperiodically-induced egg diapause in all early United States populations suggested that the founding population arrived from temperate Japan (Hawley et al. 1987). Aedes albopictus is medically important because it is a vector of arboviruses, including Chikungunya, dengue, LA Crosse encephalitis, and West Nile virus (Ibañez-Berñal et al. 1997, Gerhardt et al. 2001, Turell et al. 2005). In the last 30 yr, Ae. albopictus has spread rapidly eastward and northward in North America, and now is distributed over a range of ≈14° latitude.
Aedes albopictus has shown differentiation of life history traits subsequent to its invasion of North America (Black et al. 1989, Lounibos et al. 2003, Armbruster and Conn 2006, Leisnham et al. 2008). This evolution of life history differences suggests that many North American populations are sufficiently isolated to allow local adaptation if appropriate selection exists. However, lifetable experiments by Leisnham et al. (2008) yielded no consistent differences among two northern versus two southern populations of Ae. albopictus in resource allocation between reproduction and maintenance, reproduction over time, and reproductive investment among offspring, suggesting that latitudinal variation in climate may not be the main selective factor impinging on adult longevity and reproduction. These experiments were conducted under conditions mimicking summer field conditions and included a 16-h day, and it thus remains unknown how seasonal environments affect life history in this species.
As in most insects, the primary response of North American Ae. albopictus to unfavorable climate conditions appears to be production of diapausing eggs. If females from northern populations lay diapausing eggs in response to shorter daylength and unfavorable seasonal climate, there may be limited selection for other life history responses, such as greater total or early reproductive investment and larger eggs. Diapause itself is likely under strong selection. Incidence of photoperiodically-induced diapause among nine populations was positively correlated with latitude, from East St. Louis, IL, to the Caribbean (Lounibos et al. 2003), indicating that the spread of Ae. albopictus into subtropical climates from more temperate regions was associated with the loss of diapause. No one has evaluated interpopulation variation in adult life histories of Ae. albopictus under long and short day lengths. In this study we conduct laboratory experiments to compare longevity, reproductive allocation, and diapause incidence of northern and southern populations of North American Ae. albopictus under long and short daylengths to test if latitudinal differences in diapause documented by Lounibos et al. (2003) are associated with different survival and reproductive schedules.
Life History Predictions for A. albopictus
The northern range limit of Ae. albopictus corresponds approximately to daily mean January temperatures of −5°C (Nawrocki and Hawley 1987; Hanson and Craig, Jr. 1995), indicating severe winter temperatures. In contrast, the southern limit of the species in North America includes in the southernmost counties of Florida (O’Meara et al. 1995), which have a subtropical climate. In temperate zones, Ae. albopictus experiences high larval and almost complete adult mortality during winter and almost exclusively survives adverse winter temperatures as diapausing eggs (Sota and Mogi 1992, Swanson et al. 2000), whereas in subtropical areas, larvae and adults are often active during the winter (Rios and Maruniak 2004, Lounibos and Escher 2008). Hence, high larval and, more importantly, almost complete adult mortality because of cold winter temperatures in the north are absent in the south, where Ae. albopictus is likely limited by factors impinging solely on immature survival, such as interspecific competition or predation at the larval stage and the desiccation and thermal tolerance of eggs (Juliano et al. 2002, Kesavaraju et al. 2008).
Materials and Methods
Collection and Maintenance of Mosquitoes
Aedes albopictus were collected as larvae, pupae, or eggs, from three northern (Salem, NJ [SAL]: 39.6° N, 75.5° W; Springfield, IL [SPR]: 39.8° N, 89.7° W; Eureka, MO [TYS]: 38.5° N, 90.6° W) and three southern (Palm Beach, FL [PB]: 26.7° N, 80.0° W; Palmetto, FL [PAL]: 27.5° N, 82.6° W; Tampa, FL [TAM]: 27.9° N, 881.5° W) populations chosen to represent replicate populations near the extremes of the distribution of Ae. albopictus in North America. Field collection methods varied among populations. For all northern sites, mosquitoes were field collected as larvae from water-filled containers (e.g., discarded tires). For Tampa and Palmetto, FL, mosquitoes were collected as larvae from cemetery vases and as larvae and eggs from black 250-ml ovitraps, lined with an oviposition substrate and half-filled with water, set in the field, and retrieved after 7 d. For Palm Beach County, mosquitoes were collected as eggs and larvae from black ovitraps. Sample sizes of field collections ranged from 100 to 500 individuals for each sites. Populations exposed to experimental treatments were to first to third generation progeny of field collected individuals.
Experiments
Experiments were run in two identical Percival incubators at 21 ± 0.5°C, at which temperature the photoperiodic responses of this species are optimally expressed (Pumpuni et al. 1992), and within 2°C of average mean daily temperatures females would experience at the beginning of autumn, at 38–40° latitude. We used day lengths of 15- or 12-h light, which are within 30 min of the day lengths (sunrise-sunset) naturally observed on the longest day of summer (20 June) and the beginning of autumn (22 September; astronomical reckoning of seasons [http://en.wikipedia.org/wiki/Season#Polar_day_and_night]), respectively, at 38–40° latitudes (http://aa.usno.navy.mil/data/docs/RS_OneYear.php). Illumination was provided by 8-W fluorescent bulbs located within 0.3 m of individuals. Every 20 d, the photoperiod and associated mosquitoes were swapped between incubators minimize incubator effects. Proportion adult time in each incubator also was scored for each mosquito and included in statistical models to control for incubator effects on adult mosquito survival and reproduction.
At each photoperiod, experimental mosquitoes were reared as larvae at low densities (20 larvae per 200 ml) in 250-ml cups provisioned with 30 mg of bovine liver powder. Each day, we collected pupae and put them in individual vials until adult eclosion. Our low-density larval rearing environment yielded relatively large, evenly sized adult females, minimizing heterogeneity of vigor and initial condition, so that we compare morphologically and physiologically similar individuals across populations, giving us a greater chance to detect trade-offs among survivorship, reproduction, and reproductive allocation among populations.
Adult females were isolated into a 600-ml plastic container with a screen top. Within each container were two newly hatched males and an oviposition cup (50-ml black plastic beaker containing ≈30 ml of tap water and lined with a paper towel) and continuous access to 20% sugar solution. Females were allowed five days to mate, after which they were offered a bloodmeal every 4 d, by laying an anesthetized mouse on the screen top and extending its tail through the screen and into the cup for 10 min. We recorded when females ingested blood from the mouse (i.e., hereon called a “bloodmeal”). We determined that a female had completed blood feeding once she had removed her proboscis from the mouse. Mosquitoes were observed (P.T.L., personal observations) mating frequently, and it is highly likely that all females were mated. Sample sizes for Salem, NJ; Springfield, IL; Eureka, MO; Palm Beach, FL; Palmetto, FL; and Tampa, FL were 19, 16, 15, 17, 13, and 14 females, respectively (95 total), which are similar to other studies of adult survival and reproduction of Ae. albopictus (e.g., Braks et al. 2006, Leisnham et al. 2008).
Containers were checked daily for eggs and female survivorship. Any eggs were counted and individual egg papers were stored separately in the incubator they came from for 7–10 d to allow full embryonic development. After storage, 10 eggs from on each egg paper were haphazardly selected (all eggs selected if <10) to have their length (L) and width (W) measured to the nearest 0.01 mm using an ocular micrometer mounted on a microscope at a magnification of 45 ×. The size of each egg then was calculated as the volume (V) of a prolate spheroid (Armbruster and Hutchinson 2002, Leisnham et al. 2008). Eggs in each clutch then were hatched by submerging the paper in a nutrient broth of 0.40 g/liter DI water for 48 h. Hatched larvae from each clutch were counted and scored as developed. Unhatched eggs were bleached and examined under a dissecting microscope for embryos. Unembyronated eggs were scored as infertile and eggs containing fully-formed embryos (criteria of Shroyer and Craig (1983) were scored as diapausing eggs. At death, females were dissected and numbers of mature oocytes (stages 4 and 5, Detinova 1962) counted and scored as retained eggs. Wings were removed from 44 females, dried (24 h at 50°C), and measured. Wings of 51 females were damaged and could not be measured accurately. The experiment continued until all females had died.
Statistical Analyses
For each female we measured the following: longevity; number of bloodmeals; fecundity measures (i.e., numbers of developed, diapause, infertile, and retained eggs); egg volume; age of first oviposition; and daily fecundity. Longevity was measured as the number of days from eclosion to death. Daily fecundity was calculated as the number of total eggs divided by longevity. Incidence diapause was calculated as the total numbers of diapause eggs/developed + diapause eggs (i.e., viable eggs). Average egg size for each female was calculated as the mean of the mean egg sizes from all her clutches. Proportion of time in each incubator was calculated as the number of days in one of the incubators/longevity.
Estimated age-specific hazard rate was compared among populations by using Cox Proportional Hazard model (SAS PROC PHREG, SAS Institute 2003). To test for a physiological cost of reproduction in our laboratory environment, cumulative number of bloodmeals, cumulative number of developed eggs, cumulative number of diapause eggs, and cumulative number of infertile eggs were included in the model as time-dependent covariates. Population, date of initial bloodmeal, and incubator were included in the model as time-independent variables.
Overall associations across individual females between longevity, bloodmeal number, fecundity measures, incidence diapause, and average egg size were examined using partial correlations, after controlling for date and proportion time in each incubator by using SAS PROC CORR. Associations of female oviposition with region (northern versus southern) and photoperiod were tested using Fisher Exact tests. Patterns of life history traits were compared among populations by MANCOVAs and ANCOVAs, using SAS PROC GLM. In all models, photoperiod and population were class variables and date of initial bloodmeal was a covariate.
The first MANCOVA was on numbers of developed, diapause, retained, and infertile eggs and was used to examine effects on overall fecundity. A follow-up analysis of covariance (ANCOVA) on incidence diapause of viable eggs was used to test the overall effect of photoperiod on diapause response among populations. A second MANCOVA on total number of eggs, number of bloodmeals, and longevity was used to test for differences in allocation among reproduction and survival. We used total eggs as a measure of reproduction because this represented total reproductive investment. Analysis by using only viable eggs did not yield different conclusions, hence we report only analysis by using total eggs. Follow-up ANCOVA on daily fecundity was done to test the effect of population on reproductive rates. A third MANCOVA on early fecundity, residual fecundity, and time to first oviposition was used to test allocation of reproduction across time. A fourth MANCOVA on fecundity and average egg size tested reproductive allocation among offspring. Partial correlation tested the association of incidence diapause with mean egg size, after controlling for date and proportion time in each incubator using SAS PROC CORR. For all MANCOVAs, we used F statistics derived from Pillai’s Trace (SAS Institute 2003). We interpreted the contributions of individual dependent variables to significant multivariate effects using standardized canonical coefficients (SCCs; Scheiner 2001). Statistical significance was evaluated at α = 0.05.
Populations in this study were not a randomly selected sample of all possible populations in the north or south. Therefore, population was treated as a fixed effect and statistical inferences only confined to the populations selected. This strategy follows that of previous studies on geographic variation of life history traits (e.g., Reznick et al. 2001, Leisnham et al. 2009, Leisnham and Juliano 2010) and is in keeping with a strict interpretation of random effects in linear models. For all analyses, we tested for significant differences among populations using pairwise contrasts (Scheiner 2001), with sequential Bonferroni correction for all possible comparisons within each analysis. We tested for a region effect using the a priori contrast comparing mean values of these three northern versus these three southern sites. To meet assumptions of normality and homogeneity of variances, numbers of developed, diapause, retained, infertile, and total viable eggs, residual fecundity, number of bloodmeals, and time to first oviposition all were log-transformed.
Preliminary tests indicated no significant interaction of wing length with population in any analyses but we still report tests for its main effect. Because wing length could not be measured for 51 of 95 females, it was dropped from models that tested the effects of other variables. Interactions of population with both initial bloodmeal size and date could not be tested because not all populations were represented at each bloodmeal size or date of initial bloodmeal. For hazard analyses, preliminary tests indicated no significant differences among populations for slopes relative to cumulative bloodmeals, cumulative developed eggs, cumulative diapause eggs, or cumulative infertile eggs (results not presented).
Results
Oviposition Occurrence
Of the 95 females that took a bloodmeal and were included in the experiment, 16 (16.8%) did not oviposit and one only laid infertile eggs. Eight of the 17 females (47.1%) that failed to oviposit viable eggs also died without any retained eggs and thus had no investment in offspring despite taking a blood meal. Over three-quarters (13/16, 81.3%) of females that failed to oviposit and almost all (seven-eighths, 87.5%) females that had no offspring investment came from short daylength, and associations of photoperiod with oviposition (two-tailed Fisher exact test, P = 0.061) and offspring investment (two-tailed Fisher exact test, P = 0.068) were both close to the 5% level of significance. TYS and SPR had the greatest proportion of females that did not oviposit with 26.7% (4/15) and 25.0% (4/16) of blood-fed females dying without laying eggs, respectively (TAM: 21.4%, 3/14; PB: 17.7%, 3/17; SAL: 10.5%, 2/19; and PAL 7.1%, 1/13), but the association of oviposition with region was nonsignificant (pooled across populations, two-tailed Fisher exact test, P = 0.60), likely because of low sample sizes. Females that had no offspring investment came from five different populations.
Population Differences and Physiological Costs of Reproduction
Cumulative number of developed eggs, but not cumulative numbers of diapause or infertile eggs or cumulative number of blood meals, affected the age-specific hazard rate of females (Table 1). Overall, mean hazard rate decreased with the production of developed eggs at a rate of 2.2% with every one egg (Table 1). Population and wing length also did not affect hazard rate (Table 1).
Table 1.
Results for survival analysis using multivariate Cox proportional hazard model
| Parameter | χ2 | df | P | Hazard ratio |
|---|---|---|---|---|
| Population | 6.22 | 5,95 | 0.2856 | |
| Cumulative no. developed eggsb | 4.14 | 1,95 | 0.0419 | 0.978 |
| Cumulative no. diapause eggs | 0.04 | 1,95 | 0.8489 | 0.998 |
| Cumulative no. infertile eggs | 0.45 | 1,95 | 0.5020 | 1.009 |
| Date | 6.75 | 1,95 | 0.0094 | 1.009 |
| Incubator | 1.71 | 1,95 | 0.1905 | 0.441 |
| Wing length | 0.44 | 1,42 | 0.5503 | 0.546 |
Significant effects are indicated in bold and are reported in the main text. Pairwise contrasts between populations are not shown for brevity. For parameters corresponding to continuous variables, the hazard ratios are the ratio of hazard rates for an increase of 1 U of the variable.
Egg Status
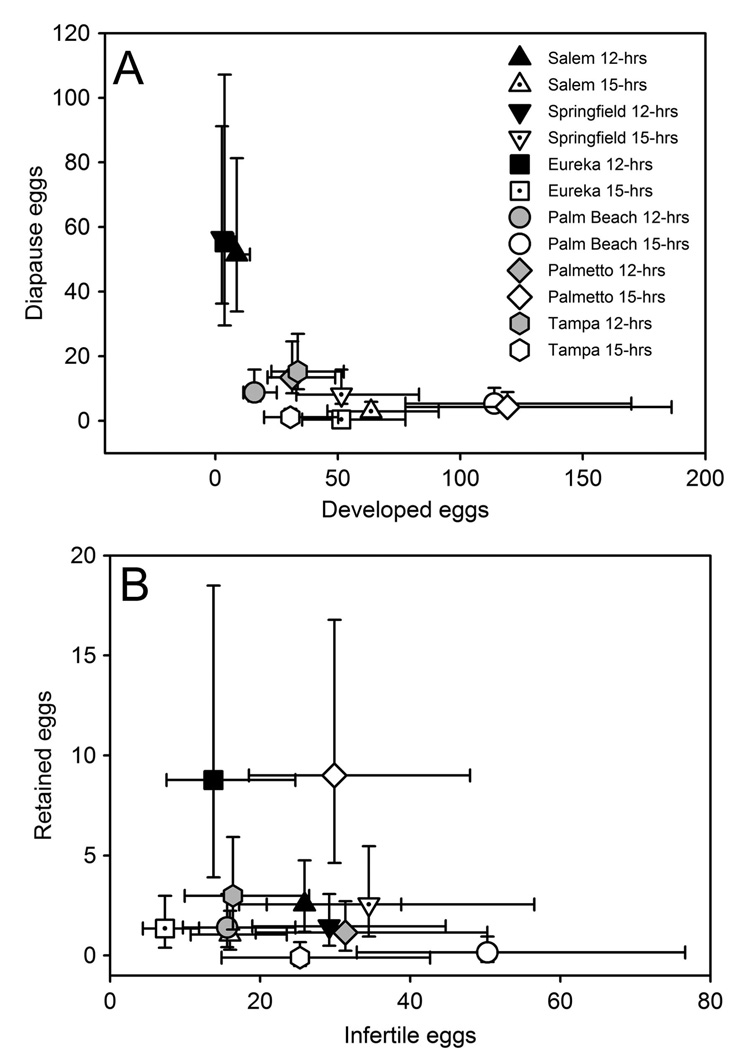
Numbers of developed eggs (r = 0.28; P = 0.0152) and of diapause eggs (r = 0.39; P = 0.0005) were positively associated with number of infertile eggs, and negatively related with each other (r = −0.26; P = 0.0230). MANCOVA on the status of eggs showed a significant interaction between population and photoperiod, which most strongly affected the oviposition of developed eggs and moderately affected the oviposition of diapause eggs (Table 2; Fig. 1). Multivariate pairwise contrasts revealed a significant difference between these northern and southern populations under short daylength but not long daylength (Table 2). Under short daylength, individuals from these northern populations oviposited more diapause eggs and fewer developed eggs than did southern populations (Table 2; Fig. 1). Pairwise contrasts also revealed that under short daylength, SPR was different from TAM (P = 0.0003) and PAL (P = 0.0010), primarily by ovipositing fewer developed (SCCs: TA, 0.88; PAL, 0.89) and more diapause eggs (SCCs: TA, 0.67; PAL, 0.72) (Fig. 1). There was no effect of wing length on egg status (Table 2).
Table 2.
MANCOVA results and standardized canonical coefficients for egg status. Significant effects are indicated in bold
| Source of variation |
Pillai’s Trace (F) |
Df | P | Canonical variates | Standardized canonical coefficients | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variate no. |
Percent variation |
P | Developed eggs |
Diapause eggs |
Infertile eggs |
Retained eggs |
||||
| Population | 1.93 | 20, 256 | 0.0112 | 1 | 66.5 | 0.0082 | 1.21 | −0.95 | 0.17 | −0.06 |
| 2 | 21.1 | 0.2971 | −0.31 | 0.08 | 0.91 | −0.72 | ||||
| 3 | 9.8 | 0.5039 | 0.29 | 0.49 | 0.37 | 0.64 | ||||
| 4 | 2.5 | 0.5723 | −0.76 | −1.08 | 0.81 | 0.37 | ||||
| Photoperiod | 22.24 | 4, 61 | <0.0001 | 1 | 100 | <0.0001 | 1.08 | −1.13 | 0.15 | −0.05 |
| Population × photoperiod | 1.95 | 20, 256 | 0.0098 | 1 | 51.3 | 0.0089 | −1.24 | 0.78 | 0.46 | −0.07 |
| 2 | 35.4 | 0.0859 | 0.43 | 0.75 | −0.46 | 0.81 | ||||
| 3 | 12.7 | 0.4817 | 0.70 | 0.48 | 0.40 | −0.46 | ||||
| 4 | 0.6 | 0.8670 | −0.10 | −0.95 | 1.03 | 0.44 | ||||
| North vs south at 12-h | 10.29 | 4, 61 | <0.0001 | 1 | 100 | <0.0001 | −1.21 | 0.96 | 0.02 | 0.10 |
| North vs south at 15-h | 1.05 | 4, 61 | 0.3883 | 1 | 100 | 0.3883 | −0.82 | 0.78 | −0.10 | 0.23 |
| Date | 6.63 | 4, 61 | 0.0002 | 1 | 100 | 0.0002 | 0.25 | −1.22 | 0.98 | 0.18 |
| Incubator | 0.21 | 4, 61 | 0.9334 | 1 | 100 | 0.9334 | 0.44 | −1.09 | 0.89 | 0.31 |
| Wing length | 0.69 | 4, 18 | 0.6103 | 1 | 100 | 0.6103 | 0.04 | 0.03 | 1.13 | 0.13 |
Significant effects and pairwise contrasts are indicated in bold and are reported in the main text.
Only pairwise contrasts between regions for the population × photoperiod interaction are shown for brevity.
Fig. 1.
Bivariate plots of egg status. Data were statistically tested using MANCOVA. Least squares means have been adjusted for the variables in the analysis and all variables have been back transformed. Error bars are ± SE.
Incidence diapause consistently was greater under short daylength for all populations and greater for the three northern populations compared with the three southern populations (Table 3). Follow-up ANCOVA on incidence diapause showed a significant interaction between population and photoperiod (F5, 77 = 4.62, P = 0.0012), with pairwise contrasts revealing that these northern populations had on average greater incidence of diapause than did these southern populations under short daylength (F1, 77 = 47.78, P < 0.0001) but not long daylength (F1, 77 = 0.29, P < 0.5916). Pairwise contrasts also revealed that each northern population had greater diapause incidence than did each southern population under short daylength (Table 3; P values <0.0008).
Table 3.
Incidence diapause of viable eggs for northern and southern populations at short (12-h) and long (15-h) daylengths
| Population | Region | Photoperiodic response (mean % diapause eggs ± SE) |
|
|---|---|---|---|
| 12-h daylength | 15-h daylength | ||
| Eureka, MO | North | 92.8 ± 10.1a | 2.0 ± 7.8 |
| Springfield, IL | North | 88.5 ± 7.4a | 22.0 ± 8.9 |
| Salem, NJ | North | 81.9 ± 7.0a | 7.6 ± 6.8 |
| Palm Beach, FL | South | 42.7 ± 7.7b | 9.2 ± 7.7 |
| Palmetto, FL | South | 36.6 ± 8.2b | 7.6 ± 6.8 |
| Tampa, FL | South | 35.9 ± 8.3b | 5.1 ± 8.3 |
Different subscripts denote significant differences among populations.
Life Time Allocation of Resources to Maintenance and Reproduction
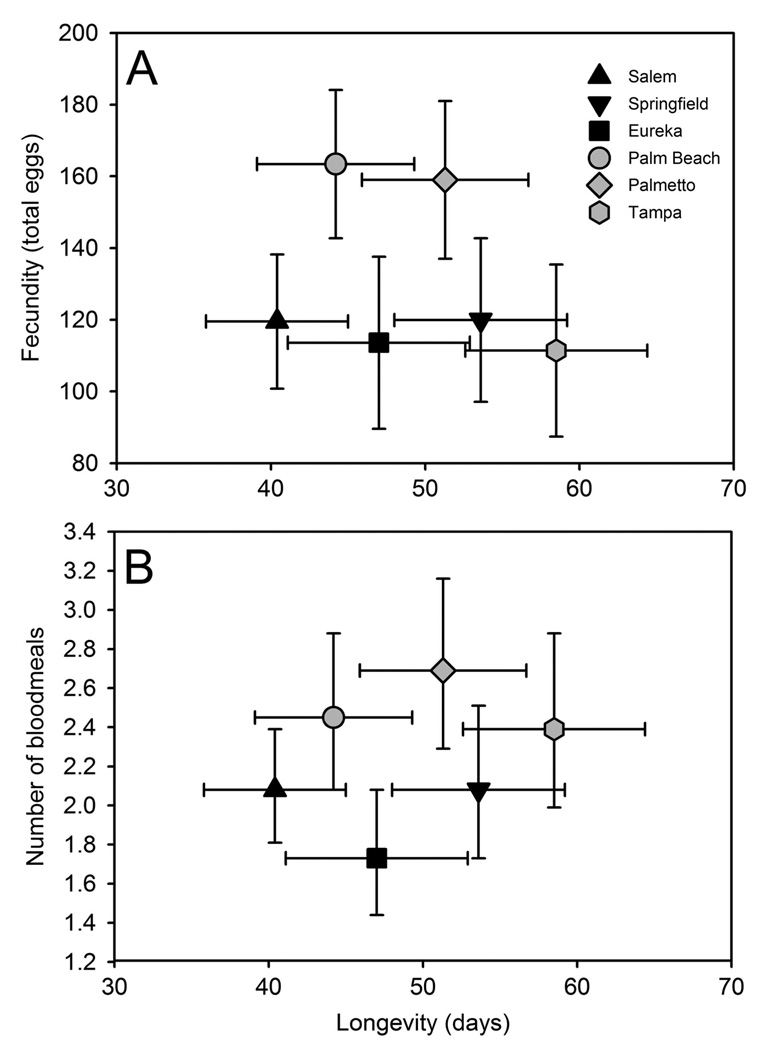
Blood meal number (r = 0.52; P < 0.0001) and total fecundity (r = 0.50; P < 0.0001) were positively correlated with longevity and with each other (r = 0.59; P < 0.0001). MANCOVA on the lifetime allocation of resources to maintenance and reproduction showed differences among populations, with no effect of photoperiod or population-photoperiod interaction (Table 4). Population differences resulted mainly from differences in longevity and fecundity (Table 4; Fig. 2A). Pairwise contrasts revealed no average differences in allocation of resources to maintenance and reproduction between northern and southern populations (Table 4), but that TAM differed from PB (P = 0.002) with moderately greater longevity (SCC: −0.43) and only slightly lower fecundity (SCC: 0.39) (Fig. 2). Follow-up ANCOVA on daily fecundity revealed a higher reproductive rate for PB (3.47 ± 0.40 eggs/d) than TAM (1.80 ± 0.40 eggs/per) (P = 0.002). The effect of wing length on allocation of resources to maintenance and reproduction was close to the 5% level of significance, mainly due to variation in numbers of bloodmeals and longevity, with larger females appearing to take fewer bloodmeals but living longer (Table 4).
Table 4.
MANCOVA results and standardized canonical coefficients for the lifetime allocation of resources
| Source of variation | Pillai’s Trace (F) |
df | P | Canonical variates | Standardized canonical coefficients |
||||
|---|---|---|---|---|---|---|---|---|---|
| Variate no. |
Percent variation |
P | Longevity | No. bloodmeals |
Fecundity | ||||
| Population | 1.99 | 15, 192 | 0.0178 | 1 | 83.7 | 0.0109 | −1.40 | −0.12 | 1.43 |
| 2 | 11.0 | 0.6806 | 0.21 | 0.81 | 0.09 | ||||
| 3 | 5.4 | 0.5950 | 0.52 | −1.07 | 0.94 | ||||
| North vs south | 1.06 | 3, 62 | 0.3744 | 1 | 100 | 0.3744 | −0.22 | 0.78 | 0.47 |
| Photoperiod | 1.46 | 3, 62 | 0.2329 | 1 | 100 | 0.2329 | 1.10 | 0.40 | −0.47 |
| Population × photoperiod | 0.98 | 15, 192 | 0.4776 | 1 | 72.3 | 0.4787 | −0.93 | −0.20 | 1.61 |
| 2 | 18.2 | 0.8343 | 1.14 | 0.03 | −0.17 | ||||
| 3 | 9.5 | 0.6896 | −0.32 | 1.33 | −0.58 | ||||
| Date | 1.76 | 3, 62 | 0.1646 | 1 | 100 | 0.1646 | 0.59 | 0.41 | 0.18 |
| Incubator | 0.80 | 3, 62 | 0.4993 | 1 | 100 | 0.4993 | −1.44 | −0.24 | 1.33 |
| Wing length | 3.01 | 3, 19 | 0.0558 | 1 | 100 | 0.0558 | 0.09 | −1.79 | 1.90 |
Significant effects and pairwise contrasts are indicated in bold and are reported in the main text.
Only pairwise contrasts between regions for the population main effect are shown for brevity.
Fig. 2.
Bivariate plots of allocation of reproduction. Data were statistically tested using MANCOVA. Least squares means have been adjusted for the variables in the analysis. Numbers of bloodmeals have been back-transformed. Error bars are ± SE.
Reproductive Allocation Across Time
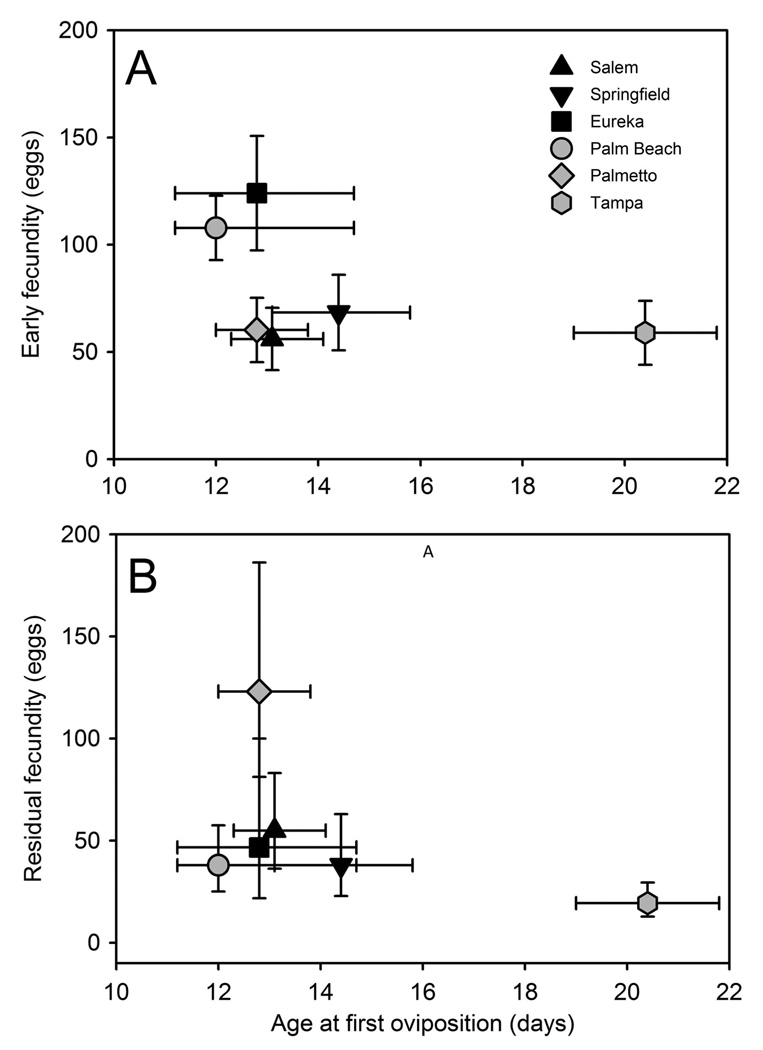
In total, 59 females oviposited after a second bloodmeal and thus had residual reproduction, and these were included in an analysis of reproductive allocation across time. Correlations indicated that age at first oviposition was negatively associated with residual fecundity (r = −0.39; P < 0.0025) but that there were weak correlations between age at first oviposition and early fecundity (r = −0.17; P < 0.2080) and between early fecundity and residual fecundity (r = −0.17; P < 0.1940). MANCOVA on allocation of reproduction over time showed differences among populations, which primarily resulted from age at first oviposition and moderately from early fecundity (Table 5; Fig. 3). Effects of photoperiod and population-photoperiod interaction were not significant (Table 5). Pairwise contrasts revealed no average differences in allocation of resources to maintenance and reproduction between northern and southern populations or associated with wing length (Table 5), but that TAM differed from PB (P = 0.0007) and PAL (P = 0.0037) primarily by greater age at first oviposition (SCCs: −0.91 and −0.93), moderately lower early fecundity compared with PB (SCC: −0.54), lower residual fecundity with compared with PAL (SCC: −0.74) (Fig. 3). Date and incubator also had significant effects (Table 5).
Table 5.
MANCOVA results and standardized canonical coefficients for reproductive allocation over time
| Source of variation | Pillai’s Trace (F) |
df | P | Canonical variates | Standardized canonical coefficients |
||||
|---|---|---|---|---|---|---|---|---|---|
| Variate no. |
Percent variation |
P | Age at first oviposition |
Early fecundity |
Residual fecundity |
||||
| Population | 2.20 | 15, 135 | 0.0092 | 1 | 68.2 | 0.0079 | 1.00 | −0.48 | −0.09 |
| 2 | 26.0 | 0.1949 | 0.28 | 0.77 | 0.30 | ||||
| 3 | 5.9 | 0.5358 | −0.37 | −0.76 | 0.59 | ||||
| North vs south | 0.37 | 3, 43 | 0.7734 | 1 | 100 | 0.7734 | 0.75 | 0.49 | −0.67 |
| Photoperiod | 0.45 | 3, 43 | 0.7209 | 1 | 100 | 0.7209 | 1.02 | −0.48 | 0.07 |
| Population × photoperiod | 1.35 | 15, 135 | 0.1840 | 1 | 54.8 | 0.2020 | −0.01 | 0.80 | −0.72 |
| 2 | 28.9 | 0.3420 | −0.07 | 0.55 | −0.19 | ||||
| 3 | 16.3 | 0.3462 | −0.72 | 0.59 | −0.44 | ||||
| Date | 4.36 | 3, 43 | 0.0091 | 1 | 100 | 0.0091 | −0.01 | 0.49 | −0.93 |
| Incubator | 4.17 | 3, 43 | 0.0112 | 1 | 100 | 0.0112 | 0.49 | 0.20 | −0.81 |
| Wing length | 2.52 | 3, 8 | 0.1315 | 1 | 100 | 0.1315 | 0.41 | 1.26 | 1.25 |
Significant effects and pairwise contrasts are indicated in bold and are reported in the main text.
Only pairwise contrasts between regions for the population main effect are shown for brevity.
Fig. 3.
Bi-variate plot of the allocation of reproduction over time. Data were statistically tested using MANCOVA. Least squares means have been adjusted for the variables used in the analysis. Age at first bloodmeal and residual reproduction have been back-transformed. Error bars are ± SE.
Reproductive Allocation Among Offspring
In total, 63 females yielded data on egg size and were included in these analyses. Mean egg size and total fecundity were not significantly correlated (r = −0.15; P = 0.2031). MANCOVA on allocation of reproduction among offspring only showed a significant effect of date (Table 6). Correlation between mean egg size and incidence of diapause was close to the 5% level of significance (r = 0.20; P = 0.0773).
Table 6.
MANCOVA results and standardized canonical coefficients for the allocation of reproduction across offspring
| Source of variation | Pillai’s Trace (F) |
df | P | Canonical variates | Standardized canonical coefficients |
||||
|---|---|---|---|---|---|---|---|---|---|
| Variate no. |
Percent variation |
P | Egg size | Fecundity | |||||
| Population | 1.17 | 10, 128 | 0.3187 | 1 | 56.0 | 0.3310 | −0.76 | 0.71 | |
| 2 | 44.0 | 0.2852 | 0.79 | 0.77 | |||||
| North vs south | 1.31 | 2, 63 | 0.2778 | 1 | 100 | 0.2778 | 0.91 | −0.47 | |
| Photoperiod | 2.54 | 2, 63 | 0.0871 | 1 | 100 | 0.0871 | 0.92 | −0.52 | |
| Population × photoperiod | 1.08 | 10, 128 | 0.3803 | 1 | 70.7 | 0.3879 | 0.21 | 0.98 | |
| 2 | 29.3 | 0.5274 | 0.99 | −0.12 | |||||
| Date | 10.32 | 2, 63 | 0.0001 | 1 | 100 | 0.0001 | 0.98 | 0.02 | |
| Incubator | 1.73 | 2, 63 | 0.1853 | 1 | 100 | 0.1853 | 1.09 | 0.15 | |
| Wing length | 0.79 | 2, 20 | 0.4693 | 1 | 100 | 0.4693 | 1.02 | 0.17 | |
Significant effects are indicated in bold and are reported in the main text.
No contrasts between regions for the population main effect are shown for brevity.
Discussion
Our data show consistent differences between northern verses southern populations in photoperiodically-induced egg diapause (Tables 2 and 3), a result consistent with previous work on diapause in North American Ae. albopictus (Lounibos et al. 2003). Under short daylength, diapause eggs constituted twice the proportion of total viable eggs produced by northern females (81.9–92.1%) compared with southern females (35.9–42.7%). There were no consistent differences between northern versus southern populations in resource allocation between reproduction and maintenance (Table 4), reproduction over time (Table 5), and reproductive investment among offspring (Table 6). The results of this study suggest that the main response of North American Ae. albopictus to unfavorable winter conditions is via the life history strategy of producing diapausing eggs rather than quantitative variation in the allocation to reproductive output.
In this experiment, phenotypic measurements on females were made in a common environment so that observed differences in population mean phenotypes probably reflect underlying genetic differences that imply local life history differentiation. Our short photoperiod of 12:12 (L:D) h resembles the autumnal daylength Ae. albopictus females experience at northern latitudes, before winter conditions lower adult and larval survival. Our results show that northern females respond to this photoperiod by laying mostly diapausing eggs and thus may avoid strong selection for greater total and early reproduction and larger eggs. These results are consistent with a prior laboratory experiment comparing a two northern and two southern populations under a summer photoperiod of 16:8 (L:D) h (Leisnham et al. 2008). Three of the populations in this prior experiment (Bloomington, IN, Manassas, VA, and Ft. Myers, FL), originated from sites not represented in the present experiment. The fourth population used by Leisnham et al. (2008) originated in Tampa, FL, from a different cemetery (Rose Hill) than the Tampa population used in the present experiment (Oaklawn Cemetery). Together, our current findings and those of Leisnham et al. (2008) suggest little latitudinal selection for differential quantitative allocation of reproductive effort in Ae. albopictus.
Our findings also are consistent with prior work that has showed increased incidence of egg diapause among North American Ae. albopictus populations with increasing latitude, from East St. Louis, IL, to the Caribbean (Lounibos et al. 2003). Latitudinally comparable populations studied by Lounibos et al. (2003) consistently showed greater incidence of diapause (Vero Beach, FL [27.6°]: 85.6–86.9%; E. St Louis, IL [38°8]: 99.4%) than populations in the current study (southern, 35.9–42.7%; northern, 81.9–92.8%). Greater incidence of diapause observed by Lounibos et al. (2003) likely is a result of using a substantially shorter photoperiod (10:14 [L:D] h) than that used in our study (12:12 [L:D] h). Aedes albopictus has demonstrated considerable variation in diapause response across even small changes in daylength (Focks et al. 1994). However, we cannot rule out continued evolutionary loss of diapause response in Florida populations over the 8 yr since the experiment by Lounibos et al. (2003) and this study.
To test whether northern A. albopictus females would have different reproduction in response to the onset of winter conditions compared with southern A. albopictus, and that latitudinal differences in diapause affect survival and reproductive schedules, a daylength mimicking conditions in the field just before the onset of winter conditions is required. Thus, comparing diapause incidence between populations at the latitudinal extremes of A. albopictus’ range under 12-h daylength is appropriate and more realistic of field conditions compared with those in Lounibos et al. (2003), who used short daylengths that ovipositing females would never encounter at its northern extremes.
We found a marginal correlation between incidence diapause and mean egg size among females. Diapause eggs are likely to have higher metabolic reserves (lipids, carbohydrates, and amino acids) (Hahn and Delinger 2007). Aedes albopictus diapause eggs have one-third more surface lipids and one-half the water loss rate compared with non-diapausing eggs (Urbanski et al. 2010). Increased mean size of eggs, however, was not negatively correlated with numbers of eggs per female. These results suggest that the production of diapause eggs, which have higher metabolic reserves, does not impact survival and longevity.
Regardless of photoperiod, TAM appeared to have a different suite of life history characters. TAM females were older at first reproduction than those from PB and PAL, had lower residual fecundity than PAL, and lower early fecundity and reproductive rate than PB. Interpopulation differentiation in life history traits may arise via nonadaptive evolution, including founder effects or genetic drift. The close geographic proximity of all these Florida populations suggests that these differences in reproductive tactics may be nonadaptive. Unique mitochondrial haplotypes commonly occur in some United States populations, indicating that the breeding structure of Ae. albopictus is characterized by drift in local populations (Birungi and Munstermann 2002).
Although temperature and humidity in our experiment represented favorable conditions, females sometimes oviposited high numbers of infertile eggs. We did not include these infertile eggs in calculations of incidence diapause but they still represent a reproductive investment; albeit an unproductive one. Some of these infertile eggs had noticeably incomplete shells (P.T.L., personal observations). Among the many reasons females may lay infertile eggs are failure of insemination, mating with incompatible Wolbachia-bearing males (Dobson et al. 2001), and abnormal oogenesis. In this experiment, we determined egg status (via tests of hatchability and bleaching) within 10 d, which means that it is unlikely that eggs died in storage. If females regularly lay incompletely formed or infertile eggs, then realized fecundity will be overestimated from egg counts.
This study has shown interpopulation differentiation in the survival and photoperiodic diapause response of North American Ae. albopictus that are strongly associated with the latitudinal extremes of the species, but no evidence for latitudinal differences in other life history characteristics. These findings support the hypothesis that diapause is the main way Ae. albopictus has adapted to unfavorable winter environments. We conclude that rapid evolutionary changes in diapause has made possible the spread of A. albopictus over a wide geographic range with variable local conditions by increasing survival of its developing stages.
Acknowledgments
We thank Deborah O’Donnell, Peter Armbruster, Michelle Tseng, Courtney Janiec, and Ebony Murrell for field collections or help maintaining the experiment, and Kavitha Damal, Phil Lounibos, and Sabine Loew for useful discussion. Anesthetization of mice was done under IACUC Protocol #02-2007. This experiment was funded subcontract to SAJ from NIAID grant R01-(AI)-44793.
References Cited
- Armbruster P, Conn JE. Geographic variation of larval growth in North American Aedes albopictus (Diptera: Culicidae) Ann. Entomol. Soc. Am. 2006;99:1234–1243. [Google Scholar]
- Armbruster P, Hutchinson RA. Pupal mass and wing length as indicators of fecundity in Aedes albopictus and Aedes geniculatus (Diptera: Culicidae) J. Med. Entomol. 2002;39:699–704. doi: 10.1603/0022-2585-39.4.699. [DOI] [PubMed] [Google Scholar]
- Bell G. The costs of reproduction and their consequences. Am. Nat. 1980;116:45–76. [Google Scholar]
- Birungi J, Munstermann LE. Genetic structure of Aedes albopictus (Diptera: Culicidae) populations based on mitochondrial ND5 sequences: evidence for an independent invasion into Brazil and United States. Ann. Entomol. Soc. Am. 2002;95:125–132. [Google Scholar]
- Blackmore MS, Lord CC. The relationship between size and fecundity in Aedes albopictus. J. Vector Ecol. 2000;35:212–217. [PubMed] [Google Scholar]
- Braks MAH, Juliano SA, Lounibos LP. Superior reproductive success on human blood without sugar is not limited to highly anthrophilic mosquito species. Med. Vet. Entomol. 2006;20:53–59. doi: 10.1111/j.1365-2915.2006.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger DL. Regulation of diapause. Annu. Rev. Entomol. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- Detinova TS. Age-grouping methods in Diptera of medical importance. Geneva, Switzerland: World Health Organization; 1962. [PubMed] [Google Scholar]
- Dobson SL, Marsland EJ, Rattanadechakul W. Wolbachia-induced cytoplasmic incompatibility in single-and superinfected Aedes albopictus (Diptera: Culicidae) J. Med. Entomol. 2001;38:382–387. doi: 10.1603/0022-2585-38.3.382. [DOI] [PubMed] [Google Scholar]
- Focks DA, Linda SB, Craig GB, Hawley WA, Pumpuni CB. Aedes albopictus (Diptera: Culicidae): a statistical model of the role of temperature, photoperiod geography in the induction of egg diapause. J. Med. Entomol. 1994;31:278–286. doi: 10.1093/jmedent/31.2.278. [DOI] [PubMed] [Google Scholar]
- Fox CW, Czesak ME. Evolutionary ecology of progeny size in arthropods. Annu. Rev. Entomol. 2000;45:341–369. doi: 10.1146/annurev.ento.45.1.341. [DOI] [PubMed] [Google Scholar]
- Gerhardt RR, Gottfried KL, Apperson CS, Davis BS, Erwin PC, Smith AB, Panella NA, Powell EE, Nasci RS. The first isolation of La Crosse virus from naturally occurring infected Aedes albopictus. Emerging Infect. Dis. 2001;7:807–811. doi: 10.3201/eid0705.017506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn DA, Delinger DL. Meeting the energetic demands of insect diapause: nutrient storage and utilization. J. Insect Physiol. 2007;53:760–773. doi: 10.1016/j.jinsphys.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Hanson SM, Craig GB., Jr Aedes albopictus (Diptera: Culicidae) eggs: field survivorship during northern Indiana winters. J. Med. Entomol. 1995;32:599–604. doi: 10.1093/jmedent/32.5.599. [DOI] [PubMed] [Google Scholar]
- Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB. Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- Ibañez-Berñal SB, Briseño JP, Mutebi EA, Rodriguez G. First record in America of Aedes albopictus naturally infected with dengue virus during the 1995 outbreak at Reynosa, Mexico. Med Vet. Entomol. 1997;11:305–309. doi: 10.1111/j.1365-2915.1997.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol. Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, O’Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130:458–469. doi: 10.1007/s004420100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Damal K, Juliano SA. Do natural container habitats impede invader dominance? Predator-mediated coexistence of invasive and native container-dwelling mosquitoes. Oecologia. 2008;155:631–639. doi: 10.1007/s00442-007-0935-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, Juliano SA. Interpopulation differences in competitive effect and response of the mosquito Aedes aegypti and resistance to invasion by a superior competitor. Oecologia. 2010;164:221–230. doi: 10.1007/s00442-010-1624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, Sala LM, Juliano SA. Geographic variation in adult survival and reproductive tactics of the mosquito Aedes albopictus. J. Med. Entomol. 2008;45:210–221. doi: 10.1603/0022-2585(2008)45[210:gviasa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, Lounibos LP, O’Meara GF, Juliano SA. Interpopulation divergence in competitive interactions of the mosquito Aedes albopictus. Ecology. 2009;90:2405–2413. doi: 10.1890/08-1569.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP, Escher RL. Sex ratios of mosquitoes from long-term censuses of Florida tree holes. J. Am. Mosq. Control Assoc. 2008;24:11–15. doi: 10.2987/5656.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP, Escher RL, Lourenco-de-Oliveria R. Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae) Ann. Entomol. Soc. Am. 2003;96:512–518. [Google Scholar]
- Michod RE. Evolution of life histories in response to age-specific mortality factors. Am. Nat. 1979;113:531–550. [Google Scholar]
- Nawrocki SJ, Hawley WA. Estimation of the northern limits of distribution of Aedes albopictus in North America. J. Am. Mosq. Control Assoc. 1987;3:314–317. [PubMed] [Google Scholar]
- O’Meara GF, Evans LF, Gettman AD, Cuda J. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J. Med. Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Pumpuni CB, Knepler J, Craig GB. Influence of temperature of temperature and larval nutrition on the diapause inducing photoperiod of Aedes albopictus. J. Am. Mosq. Control Assoc. 1992;8:223–227. [PubMed] [Google Scholar]
- Reznick D, Butler MJ, IV, Rodd H. Life history evolution in guppies. VII. The comparative ecology of high- and low- environments. Am. Nat. 2001;157:12–26. doi: 10.1086/318627. [DOI] [PubMed] [Google Scholar]
- Rios L, Maruniak JE. Asian Tiger Mosquito, Aedes albopictus (Skuse) (Insecta: Diptera: Culicidae) [April 2004];Publication EENY-319, Featured Creatures series, Entomology and Nematology Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. 2004 ( http://creatures.ifas.ufl.edu)
- Roff DA. Life history evolution. Sunderland, MA: Sinauer; 2002. [Google Scholar]
- Rowe L, Ludwig D. Size and time of metamorphosis in complex life cycles: time constraints and variation. Ecology. 1991;72:413–427. [Google Scholar]
- SAS Institute. Cary, NC: SAS Institute; 2003. SAS user’s guide: statistics, version 9.1. [Google Scholar]
- Scheiner SM. MANOVA: multiple response variables and multispecies interactions. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2001. pp. 99–115. [Google Scholar]
- Shroyer DA, Craig GB. Egg diapause in Aedes triseriatus (Diptera, Culicidae): geographic variation in photoperiodic response and factors influencing diapause termination. J. Med. Entomol. 1983;20:601–607. doi: 10.1093/jmedent/20.6.601. [DOI] [PubMed] [Google Scholar]
- Sota T, Mogi M. Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquito eggs is correlated with habitat and egg size. Oecologia. 1992;90:353–358. doi: 10.1007/BF00317691. [DOI] [PubMed] [Google Scholar]
- Sprenger D, Wuithiranyagool T. The discovery and distribution of Aedes albopictus in Harris County, Texas. J. Am. Mosq. Control Assoc. 1986;2:217–219. [PubMed] [Google Scholar]
- Swanson J, Lancaster M, Anderson J, Crandell M, Haramis L, Grimstad P, Kitron U. Overwintering and establishment of Aedes albopictus (Diptera: Culicidae) in an urban La Crosse virus enzootic site in Illinois. J. Med. Entomol. 2000;37:454–460. doi: 10.1093/jmedent/37.3.454. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, O’Guinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Urbanski JM, Benoit JB, Michaud MR, Denlinger DL, Armbruster P. The molecular physiology of increased egg desiccation resistance during diapause in the invasive mosquito, Aedes albopictus. Proc. R. Soc. B-Biol. Sci. 2010;277:2683–2692. doi: 10.1098/rspb.2010.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]