Abstract
Introduction
Reactive oxygen species (ROS) participate in cellular apoptosis and are involved in pathophysiological etiology of degenerative diseases. However, recent studies suggest that ROS at low levels may play a pivotal role as second messengers and activate normal cellular processes. Intracellular ROS increase the proliferation, migration, and regenerative potential of ASCs. On the contrary, manipulations that diminish intracellular ROS levels interfere with normal ASC function. ROS generation therefore acts like a double-edged sword.
Areas covered
This review discusses the following research questions: 1) Do ROS stimulate or suppress ASCs? 2) How are ROS generated from ASCs? 3) Which function(s) is/are regulated by intracellular ROS generation? In addition, the antioxidant/antiapoptotic effect of ASCs is briefly introduced.
Expert opinion
Whether ROS is harmful or beneficial is primarily a question of dosage. Low or moderate ROS generation increase the proliferation, migration, and regenerative potential of ASCs. Therefore, it is beneficial to expose ASCs to moderate oxidative stress during manipulation. The addition of a ROS donor in culture can reduce the cost for the expansion of ASCs, and a ROS preconditioning can enhance the regenerative potential of ASCs.
Keywords: adipose-derived stem cells, reactive oxygen species, stimulation, apoptosis, hypoxia
1. Introduction
Oxidative stress, caused by the cellular accumulation of reactive oxygen species (ROS), induces cellular apoptosis or death [1-3]. Although ROS exhibit negative effects on cellular homeostasis, there is conflicting evidence that ROS at low and nontoxic levels can actually promote cell proliferation and survival [4-6]. In addition, ROS are known to serve as a second messenger in the intracellular signal transduction pathway for a variety of cellular processes, including inflammation, cell cycle progression, apoptosis, aging, and cancer [7-9]. Therefore, it becomes necessary to understand the complex role of redox balance in the cellular biology and to investigate the role of ROS in cellular homeostasis (Figure 1). A growing body of literature demonstrates that functional characteristics of stem and progenitor cells are under redox control, and physiological levels of intracellular ROS are required to activate the DNA repair pathway for maintaining genomic stability in stem cells and to increase the proliferation/migration of stem cells [10-13]. In addition, stem cells efficiently manage oxidative stress and exhibit a high resistance to ROS-induced death, which correlates with constitutive expression of antioxidant enzymes [14, 15].
Figure 1.

A positive or negative aspect of ROS generation
Adipose-derived stem cells (ASCs) are regarded as an abundant and reliable source of stem cells for tissue regeneration [16-18]. In addition to their differentiation potential, ASCs secrete growth factors that stimulate recovery of damaged tissue [19-22]. Recently, it has been reported that culturing under hypoxia significantly increases the proliferation and migration in vitro [11, 23]. In addition, the secretion of ASCs and the regenerative potential of ASCs were also significantly up-regulated under hypoxia [23, 24]. In the mechanistic explorations, our group found that hypoxia increased intracellular ROS level that in turn triggered a survival signal cascade of ASCs [11]. Platelet-derived growth factor receptor-beta (PDGFR-β) undergoes primary phosphorylateion, which is followed by the phosphorylation of Akt and ERK pathways. As described earlier, ROS serve as a second messenger in the intracellular signal transduction pathway of ASCs; however, most studies dealing with the physiological role of ROS focus on its negative aspects, such as apoptosis induction (Table 1). To better address this issue, this review discusses the following fundamental questions: 1) Do ROS stimulate or suppress ASCs? 2) How are ROS generated from ASCs? 3) Which function(s) is/are regulated by ROS generation? In addition, the antioxidant/antiapoptotic effect of ASCs is briefly introduced. Whether ROS is harmful or beneficial is primarily a question of dosage. Under certain conditions, ROS formation appears to exceed protective levels and even becomes harmful. However, this review highlights the positive effects of ROS generation in ASC physiology. Maintenance of ROS at physiological levels increases the proliferation, migration and ASC secretion of growth factor, which may enhance the regenerative potential of ASCs in clinical settings.
Table 1.
ROS-induced functional alteration of ASCs.
| ROS donor/scavenger | Cell type | Functional alteration | Reference |
|---|---|---|---|
| Hypoxia | Human ASC | Hypoxia generates ROS, which increases the proliferation, migration and growth factor secretion of hASCs | [11] |
| Antimycin/Rotenone | Human ASC | Preconditioning by antimycin or rotenone (mitochondrial ROS generation) improves the pro-angiogenic potential of ASC-based therapy | [27] |
| Antimycin/Rotenone | 3T3 preadipocyte | Mitochondrial ROS control CHOP-10/GADD153 expression, and trigger hypoxia-dependent inhibition of adipocyte differentiation. | [52] |
| Rotenone/Oligomycin | 3T3 preadipocyte | Inhibition of preadipocyte proliferation by mitochondrial ROS | [44] |
| Ceramide | Human ASC | Ceramide induces cell death through both caspase-dependent and caspase-independent mechanisms mediated by ROS generation in hASCs | [41] |
| High glucose level | Human ASC | ASCs isolated from diabetic rats and exposed at high concentration of glucose have an impaired pro-angiogenic action and the functional impairment is partly due to ROS generated by chronic exposure to high glucose concentrations | [56] |
| NOX4 expression | Human ASC, 3T3 preadipocyte | NOX4 is expressed at high levels both in white and brown preadipocytes and that differentiation into adipocytes results in a decrease in their NOX4 mRNA content. | [53] |
| Hypoxia and 4-(3,4-dihydroxy-phenyl)-derivative (DHP-d) | Human ASC | Hypoxia and a novel anti-oxidant, DHP-d, directly induce hASCs to de-differentiate into more primitive stem cells and increased migration activity. | [8] |
| Selenium | Human ASC | Selenium induces improvement in stem cell behavior of hASCs and increases proliferation, migration and telomerase activity | [49] |
| Mutated inner mitochondrial membrane peptidase 2-like (Immp2l) gene | Mice ASC | Mutant mice showed impaired capability for proliferation, formed significantly few and smaller colonies in colony formation assays, although they retained adipogenic differentiation capability in vitro | [61] |
2. ROS generation
ROS are generated from a numberous sources including NADPH oxidase, the mitochondrial electron-transport system, xanthine oxidase, cytochrome p450, uncoupled nitric oxide synthase, and myeloperoxidase [25, 26, 28]. Among the different subcellular sources, mitochondria are considered to be the main source of ROS, and the respiratory chain reaction in mitochondrial wall contributes superoxide anion [27]. During respiration, a small but significant proportion of O2 molecules are converted to superoxide anion radicals (O2·-) by complex I and complex III of the respiratory chain [28]. The flux of superoxide anion is related to the concentration of potential electron donors and the local oxygen concentration. Chemicals such as antimycin and rotenone inhibit complex I and III and generate ROS by the respiratory chain reaction, which has diverse effects on the proliferation and angiogenic potential of ASCs [27, 29, 44].
In addition to the passive production by the mitochondria, intracellular ROS can be directly generated by the NADPH oxidase enzyme (Nox) [11, 30, 32]. This enzyme was originally characterized in the phagocytes, which utilize a Nox-generated burst of superoxide anion as a mechanism of defend against pathogens. Moreover, generation of ROS through Nox activation was reported in some nonphagocytotic cells, which is an essential component of cellular signaling and survival [31-34]. In particular, Nox is reportedly one of the major sources of ROS in stem/progenitor cells and is activated by growth factors and hypoxia [11, 32, 35]. ROS generated from Nox play an important role in redox signaling involved in angiogenesis, as well as stem cell mobilization and proliferation. Therefore, the Nox-induced ROS activates cell survival pathways such as MAPK and PI3K/Akt pathways [34]. Nox isoforms have been identified in a number of different tissues. Several homologues of gp91phox (also known as Nox2), Nox1, Nox3, Nox4, and Nox5, as well as the dual oxidases (Duox) 1 and 2, have been identified [36-38]. Nox1, Nox2, Nox4, and Nox5 are reportedly expressed in endothelial cells; Nox2 and Nox4 are also found expressed in mesenchymal stem cells [12, 39, 40]. Our preliminary study found that the expression of Nox4 and Nox5 in ASCs was relatively high, while that of Nox1Nox2 and Nox3 was not. Our group first demonstrated the Nox family-mediated ROS generation in ASCs by showing that ROS level was reduced by diphenyleneiodonium, a potent NADPH oxidase inhibitor [11]. Although free radicals generated under hypoxic conditions have still not been identified, the expression of Nox4 and its involvement in ROS generation suggests that H2O2 is the most likely generated free radical under hypoxia.
Low oxygen tension (2-8% O2 concentration) is an important characteristic of the stem cell niche and hypoxia provides a conducive environment for the maintenance of stem cell properties [58, 59]. In addition, mesenchymal stem cell culture under hypoxic conditions in vitro, enhance their proliferative and self-renewal capacities [60]. Suga et al. [59], established surgically induced ischemia models by disrupting blood supply, which induced cell death including adipocytes, vascular endothelial cells, and blood-derived cells. However, hypoxia induced the proliferation of ASCs, which indicates that hypoxia also act as stimulator in vivo. Despite the fact that hypoxia is considered to be beneficial, ASCs are routinely cultured in normoxia (20% O2 concentration).
Hypoxic culturing (2% O2 concentration) induces ROS generation by the Nox-family in cytosolic membrane and mitochondria (Figure 2). Acute increase in cellular ROS levels activates receptor-type or non-receptor-type tyrosine kinases in ASCs. Of them, PDGFR-β is first to get phosphorylated, followed by the phosphorylation of PI3K/Akt/mammalian targets of the rapamycin (mTOR) and ERK1/2 signaling pathways [11]. Then, activation of these signaling pathways inhibits the degradation of hypoxia-inducible factor-1 alpha (HIF-1α) by propyl-hydroxylation of the von Hippel Lindau tumor suppressor protein and increases cytosolic HIF-1α levels in ASCs [23]. The accumulated HIF-1α translocates to the nucleus where it modulates the transcription of its target genes after binding to the hypoxia-responsive element (HRE) in the nucleus. Among these target genes, expression of the VEGF gene is significantly up-regulated resulting in increased VEGF secretion [23, 42-43].
Figure 2.

Signaling events induced by ROS generation under hypoxia
3. ASC stimulation by ROS
Despite the accumulating evidence that regards intracellular ROS as the signaling molecules of ASC stimulation, there are only few reports demonstrating the stimulatory effect of ROS [11, 27]. However, recent observations of our group indicate that low or intermediate levels of ROS generation exhibit positive effects on ASCs.
3.1 Proliferation
The effects of ROS generation on the proliferation of ASCs were previously examined in 3T3 preadipocytes and human ASCs, but the results are quite different [8, 11, 23, 44, 45]. Carriere et al. [44] investigated the effects of ROS on the proliferation of 3T3 preadipocytes and found that rotenone and oligomycin, inhibitors of complex I and of ATP synthase respectively, increased mitochondrial ROS levels and inhibited cell growth in preadipocytes. Jee et al. reported that hypoxia and the anti-oxidant 4-(3,4-dihydroxy-phenyl)-derivative could also induce ASC proliferation [8]. Recently, Song et al. modulated Thioredoxin1 (TRX1) and Thioredoxin2 (TRX2) expressions and found that the overexpression of TRX1 and TRX2 increased the proliferation of ASCs by decreasing ROS production, and it inhibited oxidant-induced apoptosis [45]. However, our group investigated the proliferative effect of hypoxia (2% O2 concentration) in human ASCs [14, 15], and found that ROS generation by hypoxia significantly increased the proliferation and survival of human ASCs [11, 23]. In that study, Akt and ERK1/2 signaling pathways were phosphorylated by ROS generation. Our group also found that antimycin treatment (0.01–10 μM concentration) significantly increased the proliferation of ASCs (Figure 3A). These opposite results can be explained by the difference in intracellular ROS concentration of the ASCs. Therefore, a precise control of intracellular ROS levels is a key determining factor for ASC proliferation.
Figure 3.
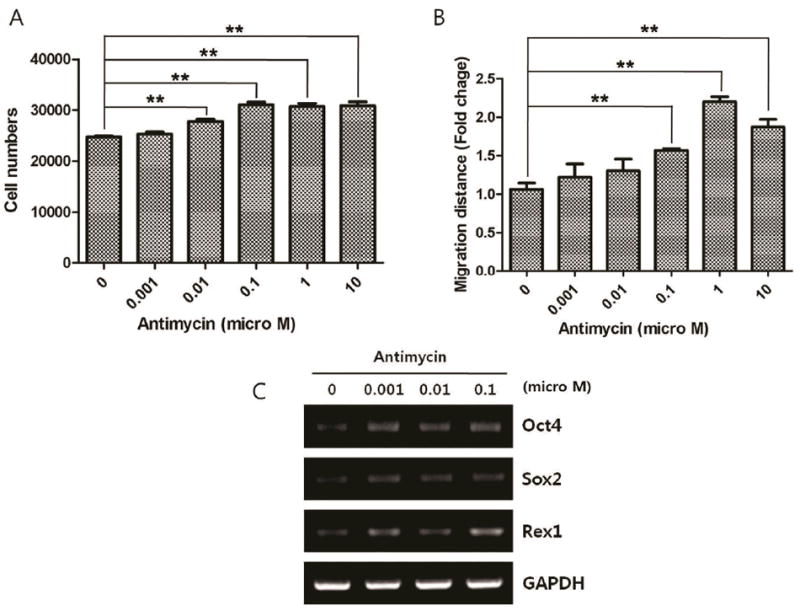
Stimulatory effect of antimycin on ASCs. Note that antimycin significantly increased the proliferation (A) and migration (B) of ASCs (n=3, **P<0.01). In addition, mRNA expression of Oct4, Sox2 and Rex1 (C) was increased with antimycin treatment.
3.2 Expression of early undifferentiation markers
Recently, 4 transcription factors (Oct4, Sox2, Klf4, and c-Myc) have been shown to reprogram fibroblasts and generate induced-pluripotent stem cells [46-48]. Although they are not as abundant as embryonic stem cells, ASCs express early undifferentiation markers such as Oct4, Sox2, Nanog, Rex1, and c-Myc [8, 49, 50]. ASCs may undergo an increase in developmental potential and multipotency via the up-regulation of these transcription factors. Like proliferation, the intracellular ROS level has a different effect on the early undifferentiation gene expression of ASCs and plays a pivotal role in the maintenance of ASC multipotency. Jee et al. reported that a combination of low oxygen tension and 4-(3,4-dihydroxy-phenyl)-derivative induced ASCs to de-differentiate into more primitive stem cells and significantly increased the mRNA expression of Rex1 and Oct4 [8]. They also reported that selenium improved Rex1, Nanog and Oct4 expression in ASCs and induced DNA demethylation on the promoter regions of these genes via the inhibition of ROS generation [49]. Recently, George et al., generated a mouse mutant with a mutated inner mitochondrial membrane peptidase 2-like gene and examined the effects of excessive mitochondrial ROS generation on stem cell self-renewal. ASCs from mutant mice showed impaired ability to proliferate, and formed significantly few and smaller colonies in colony formation assays, although they retained adipogenic differentiation capability in vitro [61]. However, an experiment by our group showed that ROS generation under hypoxia (2% O2 concentration) increased the mRNA expression of Oct4 and Rex1 in ASCs, and a low concentration of antimycin (1-100 nM) increased mRNA expression of Oct4, Sox2, and Rex1 in ASCs (Figure 3-C). Although it depends on concentration, low-level ROS generation increases the expression of early undifferentiation markers.
3.3 Migration
The effects of ROS on the migration of stem cells have been studied extensively in hematopoietic stem cells and endothelial progenitor cells. These stem cells circulate in the vascular system and are involved in the regeneration of a variety of tissues, and their migration can be enhanced by ROS [13, 51]. ASCs localize in subcutaneous and visceral regions, and regenerate skin and adipose tissue through migration and self-renewal mechanisms. When local inflammation or injury occurs, ROS and chemokines are secreted from damaged cells and induce ASC migration. The effect of ROS on ASC migration was directly measured in vitro, which showed that hypoxia significantly increased the migration of human ASCs, whereas neutralizing ROS generation by NAC or DPI treatment decreased ASC migration [11]. We further investigated the mechanism of action and found that activities of matrix metalloproteinases 2 and 9 were increased by ROS generation in a preliminary study. In addition, low-dose treatment with antimycin (0.1–10 μM) significantly increased the migration of ASCs (Figure 3B). It appears reasonable to believe that local hypoxia or inflammation induces the migration of ASCs through generation of ROS.
3.4 Differentiation
ASCs have multi-differentiation potentials, which is major mechanism of action underlying tissue regeneration by ASCs. Adipogenic, angiogenic, osteogenic and chodrogenic differentiation has been largely reported [17, 27, 52-54]. Differentiation of ASCs is regulated by a multitude of factors, and ROS are shown to regulate ASC differentiation.
Carriere et al. (2004) tested the implication of mitochondrial ROS in adipogenic differentiation of ASCs [52]. Mitochondrial ROS generation demonstrated a very strong negative correlation with adipogenic differentiation of 3T3 preadipocytes. Moreover, mitochondrial ROS positively controlled the expression of the adipogenic repressor CHOP-10/GADD153. However, there are contradicting reports that ROS generation induces adipogenesis. Kanda et al., recently reported the ROS involvement in adipocyte differentiation of mesenchymal stem cells (MSCs) and found that the increase in the intracellular ROS level via Nox4 mediates adipocyte differentiation in MSCs [62]. Mouche et al., showed that Nox4 is expressed at high levels both in white and brown preadipocytes and that differentiation into adipocytes results in a decrease in their Nox4 mRNA content [53]. These were confirmed in vivo by demonstration of localization of Nox4 expressing cells chiefly within the preadipocyte-containing stromal or vascular fraction, rather than in the intact adipose tissue with mature adipocytes.
Although controversial, ASCs are reported to differentiate into endothelial cells as well [63, 64]. Carriere et al. investigated the pro-angiogenic differentiation of human ASCs by mitochondrial ROS generation [27]. Transient stimulation of mitochondrial ROS generation in ASCs did not affect their ability to differentiate into endothelial cells in vitro, but strongly improved revascularization and the number of ASC-derived CD31-positive cells in vivo. Moreover, ASC preconditioning by mitochondrial ROS effectively protected ASCs against apoptosis. Recently, the age- and ROS-dependent pro-angiogenic potential of ASCs was examined by Effimenco et al. (2011) who found that aged ASCs exhibited impaired angiogenic stimulation which was mitigated by hypoxia preconditioning [54]. In general, ROS generation stimulates angiogenic differentiation of ASCs.
Instead of the direct effect of ROS, there are many articles that investigated the effect of hypoxia on the chondrogenic and osteogenic differentiation. Regulation of chondrogenic and osteogenic differentiation by hypoxia is quite contrastive. While it induces chondrogenic differentiation in one hand, hypoxia inhibits the osteogenic lineage [65-67]. Therefore, preconditioning with hypoxia is recommended for promoting chondrogenic differentiation of ASCs.
3.5 Paracrine effect
The transplanted ASCs act as “building blocks” in the human body. In addition, ASCs exhibit a paracrine effect through the secretion of growth factors. The functional improvement and attenuation of tissue injury following ASC transplantation can be reproduced in part by treatment with a cell-free conditioned medium of ASCs, which supports the paracrine mechanism of ASCs [19, 21, 22]. Secretion of these paracrine factors is reportedly regulated by ROS generation [11, 23, 27, 43]. Rehman et al. first demonstrated an increased secretion of vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) under hypoxia, that have been shown to be responsible for the enhanced regenerative potential of ASCs in ischemia models [43]. Direct evidence for the effect of ROS on the secretion of ASCs was reported by Carriere et al. (2009) who found that mitochondrial ROS generated by antimycin and rotenone increased HGF and VEGF production in human ASCs [27]. Our group also found that hypoxia-induced ROS generation increased the VEGF and bFGF expression in ASCs, which was associated with an accelerated wound-healing in animal models [23].
4. ROS-induced apoptosis
Accumulation of ROS secondary to excessive production has the potential to damage cells and is the underlying basis of aging and disease. Overflow of intracellular ROS lead to excess mitochondrial Ca2+ entry that eventually triggers cellular apoptosis and necrosis. Severe cellular stress induces apoptosis of ASCs via ROS generation [8, 41, 49, 55, 56]. For example, exposure of human ASCs to high glucose concentrations significantly increases ROS production and reduces the viability of ASCs [55]. Ceramide induced ROS generation and disruption of mitochondrial membrane potential, and evoked cellular death through both caspase-dependent and caspase-independent mechanisms [41]. Selenium, a powerful ROS scavenger, improved the early undifferentiation markers and survival of ASC [49]. Song et al. modulated antioxidant gene expressions and found that an overexpression of TRX1 and TRX2 increased the proliferation of ASCs by decreasing ROS production [45]. In high intracellular ROS concentration, ROS might damage ASCs and antioxidants could rescue ASCs from apoptosis.
5. Antioxidant and antiapoptotic effect of ASCs
Stem cells efficiently manage oxidative stress [10, 11, 13, 15]. A set of stress response genes is highly expressed in stem cells and they are a high resistance to oxidative stress [14, 15]. Like other stem cells, ASCs are resistant to oxidative stress and play a key role in the protection of neighboring mature cells from oxidative damage. Our group demonstrated their antiapoptotic and antioxidant effects through dermatological application of ASCs and their soluble factors [17, 19, 22, 57]. A conditioned medium of ASCs (ASC-CM) was used to evaluate the antioxidant and antiapoptotic effects of ASCs because these functions are mediated by soluble factors of ASCs [17, 19]. We analyzed the ASC-CM and detected several antioxidant and antiapoptotic proteins such as insulin-like growth factor binding proteins, granulocyte-colony stimulating factor, platelet-derived growth factor, super oxide dismutase, and HGF [19]. The antioxidant and antiapoptotic effect of ASCs was also investigated in dermal fibroblasts and epidermal keratinocytes after inducing oxidative stress by tert-butyl hydroperoxide or UVB [17, 19, 22-]. A cell survival assay revealed that incubation with ASC-CM protected these cells from free radical attack. In a cell-cycle analysis, ASC-CM treatment was reported to reduce apoptosis as evidenced by reduction in caspase-3 activity. In addition, ASC-CM enhanced the superoxide dismutase and glutathione peroxidase activities in fibroblasts and keratinocytes. Collectively, these results indicate that ASCs exhibit an antioxidant/antiapoptotic function, which plays a key role in protecting the neighboring cells from oxidative stress.
6. Conclusion
While excess ROS are involved in apoptosis, low and moderate intracellular ROS levels act as a signal transducer that activates ASCs. PDGFR-β, Akt and ERK pathways are primarily phosphorylated, and related gene expressions are up-regulated. ROS generation serves as a second messenger in the intracellular signal transduction pathway of ASCs. Therefore, a precise control over ROS generation is essential to keep them at appropriate levels that stimulate ASCs and has a positive effect in ASC physiology.
7. Expert opinion
Since generation of ROS stimulates the proliferation, migration, and regenerative potential of ASCs under well controlled conditions, it could be beneficial to expose ASCs to moderate oxidative stress during cell manipulation. The addition of a ROS donor may reduce the costs for the expansion of ASCs in culture, while ROS preconditioning may potentially enhance the regenerative capacity of ASCs in clinical application. However, future studies are needed to address the efficiency of ROS donors and optimal concentrations for ASC stimulation. In addition, future research should be directed to develop a direct approach for monitoring the intracellular ROS concentration in ASCs and for measuring the critical concentration of ROS that determines the fate of ASCs.
highlights.
ROS at low levels may play a pivotal role as second messengers and activate normal cellular processes of ASCs.
PDGFR-β, Akt and ERK pathways are phosphorylated, and related gene expressions are up-regulated by ROS generation.
Intracellular ROS increase the proliferation, migration, and regenerative potential of ASCs.
The addition of a ROS donor may reduce the costs for the expansion of ASCs in culture, while ROS preconditioning may enhance the regenerative potential of ASCs in clinical use.
Acknowledgments
Declaration of Interest:
This study was supported by a grant of basic Science Research Program through the National Research Foundation of Korea (2011-0019636). Ying Xia was partially supported by NIH (HD-034852, AT-004422) and Vivian L. Smith Neurologic Foundation.
References
- 1.Kizaki M, Xian M, Sagawa M, Ikeda Y. Induction of apoptosis via the modulation of reactive oxygen species (ROS) production in the treatment of myeloid leukemia. Curr Pharm Biotechnol. 2006;7(5):323–329. doi: 10.2174/138920106778521541. [DOI] [PubMed] [Google Scholar]
- 2.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5(5):415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 3.Xia M, Wang D, Wang M, Tashiro S, Onodera S, Minami M, Ikejima T. Dracorhodin perchlorate induces apoptosis via activation of caspases and generation of reactive oxygen species. J pharmacol sci. 2004;95(2):273–283. doi: 10.1254/jphs.fpj03102x. [DOI] [PubMed] [Google Scholar]
- 4.Blanchetot C, Boonstra J. The ROS-NOX connection in cancer and angiogenesis. Crit Rev Eukaryot Gene expr. 2008;18(1):35–45. doi: 10.1615/critreveukargeneexpr.v18.i1.30. [DOI] [PubMed] [Google Scholar]
- 5.Chiarugi P, Fiaschi T. Redox signalling in anchorage-dependent cell growth. Cell Signal. 2007;19(4):672–682. doi: 10.1016/j.cellsig.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Leslie NR. The redox regulation of PI 3-kinase-dependent signaling. Antioxi Redox Signal. 2006;8(9-10):1765–1774. doi: 10.1089/ars.2006.8.1765. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Essner E, Shichi H. Ultrastructural and immunocytochemical studies of smooth muscle cells in iris arterioles of rats with experimental autoimmune uveoretinitis. Exp Mol Pathol. 1994;61(3):153–163. doi: 10.1006/exmp.1994.1033. [DOI] [PubMed] [Google Scholar]
- 8.Jee MK, Kim JH, Han YM, Jung SJ, Kang KS, Kim DW, Kang SK. DHP-derivative and low oxygen tension effectively induces human adipose stromal cell reprogramming. PloS One. 2010;5(2):e9026. doi: 10.1371/journal.pone.0009026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coant N, Ben Mkaddem S, Pedruzzi E, Guichard C, Treton X, Ducroc R, Freund JN, Cazals-Hatem D, Bouhnik Y, Woerther PL, Skurnik D, Grodet A, Fay M, Biard D, Lesuffleur T, Deffert C, Moreau R, Groyer A, Krause KH, Daniel F, Ogier-Denis E. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol Cell Biol. 2010;30(11):2636–2650. doi: 10.1128/MCB.01194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noble M, Proschel C, Mayer-Proschel M. Oxidative-reductionist approaches to stem and progenitor cell function. Cell Stem Cell. 2011;8(1):1–2. doi: 10.1016/j.stem.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Park SH, Park SG, Choi JS, Xia Y, Sung JH. The Pivotal Role of Reactive Oxygen Species Generation in the Hypoxia-Induced Stimulation of Adipose-Derived Stem Cells. Stem Cells Dev. 2011 doi: 10.1089/scd.2010.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroder K, Kohnen A, Aicher A, Liehn EA, Buchse T, Stein S, Weber C, Dimmeler S, Brandes RP. NADPH oxidase Nox2 is required for hypoxia-induced mobilization of endothelial progenitor cells. Circ Res. 2009;105(6):537–544. doi: 10.1161/CIRCRESAHA.109.205138. [DOI] [PubMed] [Google Scholar]
- 13.Ushio-Fukai M, Urao N. Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxid Redox Signal. 2009;11(10):2517–2533. doi: 10.1089/ars.2009.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104(12):3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 15.Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19(12):1885–1893. doi: 10.1089/scd.2010.0093. [DOI] [PubMed] [Google Scholar]
- 16.Park BS, Jang KA, Sung JH, Park JS, Kwon YH, Kim KJ, Kim WS. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg. 2008;34(10):1323–1326. doi: 10.1111/j.1524-4725.2008.34283.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim WS, Park BS, Park SH, Kim HK, Sung JH. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci. 2009;53(2):96–102. doi: 10.1016/j.jdermsci.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, Shao PL, Chang KC, Leu S, Yip HK. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;5(9):51. doi: 10.1186/1479-5876-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, Chung SJ, Kim DD, Sung JH. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci. 2008;49(2):133–142. doi: 10.1016/j.jdermsci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Kim WS, Park BS, Sung JH. Protective role of adipose-derived stem cells and their soluble factors in photoaging. Arch Dermatol Res. 2009;301(5):329–336. doi: 10.1007/s00403-009-0951-9. [DOI] [PubMed] [Google Scholar]
- 21.Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48(1):15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Kim WS, Park BS, Sung JH. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin Biol Ther. 2009;9(7):879–887. doi: 10.1517/14712590903039684. [DOI] [PubMed] [Google Scholar]
- 23.Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS, JH Sung. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17(4):540–547. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 24.Chung HM, Won CH, Sung JH. Responses of adipose-derived stem cells during hypoxia: enhanced skin-regenerative potential. Expert Opin Biol Ther. 2009;9(12):1499–1508. doi: 10.1517/14712590903307362. [DOI] [PubMed] [Google Scholar]
- 25.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 26.Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1014–1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 27.Carriere A, Ebrahimian TG, Dehez S, Auge N, Joffre C, Andre M, Arnal S, Duriez M, Barreau C, Arnaud E, Fernandez Y, Planat-Benard V, Levy B, Penicaud L, Silvestre JS, Casteilla L. Preconditioning by mitochondrial reactive oxygen species improves the proangiogenic potential of adipose-derived cells-based therapy. Arterioscler Thromb Vasc Biol. 2009;29(7):1093–1099. doi: 10.1161/ATVBAHA.109.188318. [DOI] [PubMed] [Google Scholar]
- 28.Turrens JF. Superoxide production by the mitochondrial respiratory chain. Biosci Rep. 1997;17(1):3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 29.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambeth JD, Krause KH, Clark RA. NOX enzymes as novel targets for drug development. Semin immunopathol. 2008;30(3):339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Lou MF. The regulation of NADPH oxidase and its association with cell proliferation in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2009;50(5):2291–2300. doi: 10.1167/iovs.08-2568. [DOI] [PubMed] [Google Scholar]
- 32.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302(2):148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein BJ, Mahadev K, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54(2):311–321. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270(5234):296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 35.Hu T, Ramachandrarao SP, Siva S, Valancius C, Zhu Y, Mahadev K, Toh I, Goldstein BJ, Woolkalis M, Sharma K. Reactive oxygen species production via NADPH oxidase mediates TGF-beta-induced cytoskeletal alterations in endothelial cells. Am J Physiol Renal Physiol. 2005;289(4):F816–825. doi: 10.1152/ajprenal.00024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71(2):289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4(3):181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 38.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285(2):R277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 39.Wang N, Xie K, Huo S, Zhao J, Zhang S, Miao J. Suppressing phosphatidylcholine-specific phospholipase C and elevating ROS level, NADPH oxidase activity and Rb level induced neuronal differentiation in mesenchymal stem cells. J Cell Biochem. 2007;(100):1548. doi: 10.1002/jcb.21139. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, Jaconi ME. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell. 2006;17(9):3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JY, Kim MJ, Kim YK, Woo JS. Ceramide induces apoptosis via caspase-dependent and caspase-independent pathways in mesenchymal stem cells derived from human adipose tissue. Arch Toxicol. 2011 doi: 10.1007/s00204-011-0645-x. [DOI] [PubMed] [Google Scholar]
- 42.Song SY, Chung HM, Sung JH. The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration. Expert Opin Biol Ther. 2010;10(11):1529–1537. doi: 10.1517/14712598.2010.522987. [DOI] [PubMed] [Google Scholar]
- 43.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 44.Carriere A, Fernandez Y, Rigoulet M, Penicaud L, Casteilla L. Inhibition of preadipocyte proliferation by mitochondrial reactive oxygen species. FEBS Lett. 2003;550(1-3):163–167. doi: 10.1016/s0014-5793(03)00862-7. [DOI] [PubMed] [Google Scholar]
- 45.Song JS, Cho HH, Lee BJ, Bae YC, Jung JS. Role of Thioredoxin 1 and Thioredoxin 2 on Proliferation of Human Adipose Tissue-Derived Mesenchymal Stem Cells. Stem cells Dev. 2011 doi: 10.1089/scd.2010.0364. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 48.Yamanaka S. Induction of pluripotent stem cells from mouse fibroblasts by four transcription factors. Cell prolif. 2008;41(Suppl 1):51–56. doi: 10.1111/j.1365-2184.2008.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JH, Lee MR, Kim JH, Jee MK, Kang SK. IFATS collection: Selenium induces improvement of stem cell behaviors in human adipose-tissue stromal cells via SAPK/JNK and stemness acting signals. Stem cells. 2008;26(10):2724–2734. doi: 10.1634/stemcells.2008-0184. [DOI] [PubMed] [Google Scholar]
- 50.Kim JH, Jee MK, Lee SY, Han TH, Kim BS, Kang KS, Kang SK. Regulation of adipose tissue stromal cells behaviors by endogenic Oct4 expression control. PloS One. 2009;4(9):e7166. doi: 10.1371/journal.pone.0007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tesio M, Golan K, Corso S, Giordano S, Schajnovitz A, Vagima Y, Shivtiel S, Kalinkovich A, Caione L, Gammaitoni L, Laurenti E, Buss EC, Shezen E, Itkin T, Kollet O, Petit I, Trumpp A, Christensen J, Aglietta M, Piacibello W, Lapidot T. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood. 2011;117(2):419–428. doi: 10.1182/blood-2009-06-230359. [DOI] [PubMed] [Google Scholar]
- 52.Carrière A, Carmona MC, Fernandez Y, Rigoulet M, Wenger RH, Pénicaud L, Casteilla L. Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: a mechanism for hypoxia-dependent effect. J Biol Chem. 2004;279(39):40462–40469. doi: 10.1074/jbc.M407258200. [DOI] [PubMed] [Google Scholar]
- 53.Mouche S, Mkaddem SB, Wang W, Katic M, Tseng YH, Carnesecchi S, Steger K, Foti M, Meier CA, Muzzin P, Kahn CR, Ogier-Denis E, Szanto I. Reduced expression of the NADPH oxidase NOX4 is a hallmark of adipocyte differentiation. Biochim Biophys Acta. 2007;1773(7):1015–1027. doi: 10.1016/j.bbamcr.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Efimenko A, Starostina E, Kalinina N, Stolzing A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med. 2011;9:10. doi: 10.1186/1479-5876-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cramer C, Freisinger E, Jones RK, Slakey DP, Dupin CL, Newsome ER, Alt EU, Izadpanah R. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev. 2010;19(12):1875–1884. doi: 10.1089/scd.2010.0009. [DOI] [PubMed] [Google Scholar]
- 56.Kim HK, Kim YJ, Kim JT, Kwon CH, Kim YK, Bae YC, Kim DH, Jung JS. Alterations in the proangiogenic functions of adipose tissue-derived stromal cells isolated from diabetic rats. Stem Cells Dev. 2008;17(4):669–680. doi: 10.1089/scd.2007.0141. [DOI] [PubMed] [Google Scholar]
- 57.Kim JH, Jung M, Kim HS, Kim YM, Choi EH. Adipose-derived stem cells as a new therapeutic modality for ageing skin. Exp Dermatol. 2011;20(5):383–387. doi: 10.1111/j.1600-0625.2010.01221.x. [DOI] [PubMed] [Google Scholar]
- 58.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150–61. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Suga H, Eto H, Aoi N, Kato H, Araki J, Doi K, Higashino T, Yoshimura K. Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg. 2010;126(6):1911–23. doi: 10.1097/PRS.0b013e3181f4468b. [DOI] [PubMed] [Google Scholar]
- 60.Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204(1):184–91. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- 61.George SK, Jiao Y, Bishop CE, Lu B. Mitochondrial peptidase IMMP2L mutation causes early onset of age-associated disorders and impairs adult stem cell self-renewal. Aging Cell. 2011;10(4):584–94. doi: 10.1111/j.1474-9726.2011.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanda Y, Hinata T, Kang SW, Watanabe Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 2011;89(7-8):250–8. doi: 10.1016/j.lfs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 63.Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109(5):656–63. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 64.Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20(2):205–16. doi: 10.3727/096368910X520065. [DOI] [PubMed] [Google Scholar]
- 65.Merceron C, Vinatier C, Portron S, Masson M, Amiaud J, Guigand L, Chérel Y, Weiss P, Guicheux J. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am J Physiol Cell Physiol. 2010;298(2):C355–64. doi: 10.1152/ajpcell.00398.2009. [DOI] [PubMed] [Google Scholar]
- 66.He J, Genetos DC, Yellowley CE, Leach JK. Oxygen tension differentially influences osteogenic differentiation of human adipose stem cells in 2D and 3D cultures. J Cell Biochem. 2010;110(1):87–96. doi: 10.1002/jcb.22514. [DOI] [PubMed] [Google Scholar]
- 67.Pilgaard L, Lund P, Duroux M, Lockstone H, Taylor J, Emmersen J, Fink T, Ragoussis J, Zachar V. Transcriptional signature of human adipose tissue-derived stem cells (hASCs) preconditioned for chondrogenesis in hypoxic conditions. Exp Cell Res. 2009;315(11):1937–52. doi: 10.1016/j.yexcr.2009.01.020. [DOI] [PubMed] [Google Scholar]


