Abstract
Cognitive dysfunction is one of the most typical characteristics in various neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease (advanced stage). Although several mechanisms like neuronal apoptosis and inflammatory responses have been recognized to be involved in the pathogenesis of cognitive dysfunction in these diseases, recent studies on neurodegeneration and cognitive dysfunction have demonstrated a significant impact of receptor modulation on cognitive changes. The pathological alterations in various receptors appear to contribute to cognitive impairment and/or deterioration with correlation to diversified mechanisms. This article recapitulates the present understandings and concepts underlying the modulation of different receptors in human beings and various experimental models of Alzheimer’s disease and Parkinson’s disease as well as a conceptual update on the underlying mechanisms. Specific roles of serotonin, adrenaline, acetylcholine, dopamine receptors, and N-methyl-D-aspartate receptors in Alzheimer’s disease and Parkinson’s disease will be interactively discussed. Complex mechanisms involved in their signaling pathways in the cognitive dysfunction associated with the neurodegenerative diseases will also be addressed. Substantial evidence has suggested that those receptors are crucial neuroregulators contributing to cognitive pathology and complicated correlations exist between those receptors and the expression of cognitive capacities. The pathological alterations in the receptors would, therefore, contribute to cognitive impairments and/or deterioration in Alzheimer’s disease and Parkinson’s disease. Future research may shed light on new clues for the treatment of cognitive dysfunction in neurodegenerative diseases by targeting specific alterations in these receptors and their signal transduction pathways in the frontal-striatal, fronto–striato–thalamic, and mesolimbic circuitries.
Keywords: Neurodegeneration, Alzheimer’s disease, Parkinson disease, receptors, cognitive dysfunction
1. Introduction
Neurodegenerative disorders are one of the most frequent causes of death and disability worldwide and have a significant clinical and socio-economic impact. Two of the most common types of neurodegenerative diseases are Alzheimer’s disease (AD) and Parkinson’s disease (PD). It has been well documented that in addition to motor disorders, cognitive dysfunction such as dementia, characterizes the clinical presentation common to these two diseases. Although different mechanisms such as neuronal apoptosis and inflammatory responses (Pascual, 2011) are involved in the pathogenesis of cognitive dysfunction in neurodegeneration, there is increasing evidence that shows that alterations in various receptors may account for the progression of cognitive decline. The activation of G protein-coupled receptors (GPCRs) modulates the intracellular signal transduction pathways (e.g., inhibiting the adenylyl cyclase/protein kinase A (PKA) signaling cascade or stimulating the phospholipase C (PLC)/protein kinase C (PKC) signaling cascade (Polter, 2010)), thus producing the secondary messenger, cAMP, and subsequently regulating calcium ion flux, membrane excitability, and cAMP-responsive transcription factors such as the cAMP response element binding protein (CREB) (Nichols and Nichols, 2008). The 5-HT6 receptor modulation results in the release of different neurotransmitters, such as glutamate and acetylcholine, which facilitates learning and memory processes (Schechter et al., 2008; West et al., 2009). The modification of these receptors may be associated with amyloid plaque formation and tau neurotoxicity and may mediate the subsequent oxidative stress related to neuronal toxicity, which results in cognitive impairment (Xiong et al., 2004). This review highlights the current findings of the effects of receptor alterations on cognitive dysfunction in neurodegenerative diseases such as AD and PD and provides a conceptual update on the multiple underlying mechanisms of neurodegenerative pathology.
2. Alterations in neurotransmitter receptors in Alzheimer’s disease
2.1. Serotonin receptors and Alzheimer’s disease
There are at least 16 different types of serotonin receptors, which can be broadly divided into seven sub-families, 5-HT1 to 5-HT7, based on their primary physiological mechanisms (Hoyer, 1997). Inoue et al. (1985) presented a positron emission tomographic atlas of the serotonin receptor distribution in the normal and abnormal human brain in 1985 (Inoue et al., 1985). Most of these receptors belong to the GPCR family of receptors, with the exception of the 5-HT3 receptor (a ligand-gated ion channel). Their activations stimulate intracellular responses through distinct signal transduction pathways, such as the inhibition of the adenylyl cyclase/protein kinase A (PKA) signaling cascade or the stimulation of the phospholipase C (PLC)/protein kinase C (PKC) signaling cascade, which regulates extracellular signal-regulated mitogen-activated protein kinase and proliferation of related pathways (Li, 2010; Polter, 2010), which can subsequently influence cognitive impairment in neurodegenerative diseases. Although various characteristics and the potential psychopharmacological significance of 5-HT receptor subtypes have been identified, a major aim of this present review is to summarize the current knowledge of the potential associations between 5-HT receptors and cognitive deficits in neurological disorders.
Several lines of evidence from animal and clinical studies have indicated the role of 5-HT and its receptors in different aspects of cognitive dysfunction, such as cognitive deficits, learning, and memory decline (Meneses and Hong, 1999; Sumiyoshi, 2007). In one study, Yasuno et al. demonstrated a negative correlation between verbal memory and the binding potential of 5-HT1A receptors in the hippocampus, where a dose-dependent decline in explicit verbal memory in healthy volunteers upon the administration of tandospirone, a 5-HT1A partial agonist, was observed (Yasuno et al., 2003). It was shown that increased 5-HT1A receptor density correlated with the cognitive impairment observed in AD and provided the basis for the use of 5-HT1A antagonists in AD treatment (Lai et al., 2002). Similarly, 5-HT1B/1D was also found to be associated with cognitive dysfunction in AD. A recent study confirmed that 5-HT1B/1D receptor density was significantly reduced in the frontal and temporal cortex of AD patients with impaired Mini-Mental State Examination (MMSE) scores (Garcia-Alloza et al., 2004).
Lai et al. (2003b) reported that MMSE scores were more closely related to neuronal degeneration when compared to the alterations in 5-HT1A receptors as demonstrated by the similar pattern of 5-HT receptor binding affinity and density in both the AD and control group. The dementia severity was measured by patient MMSE scores, which corresponded to the neuronal degeneration (Lai et al., 2003a). In contrast, Truchot et al. found a significant decrease in 5-HT1A receptor density in the hippocampus, inferior temporal gyrus, and inferior occipital gyrus of AD patients using positron emission tomography (PET) (Truchot et al., 2008). These diverging results can, however, be explained by the stark differences in the investigational methodologies adopted and the tested groups used in each study.
Similar to the 5-HT1A receptor, the 5-HT2 receptor is also widely distributed in the brain and is closely related to cognitive dysfunction. Using [18F]setoperone PET, Blin and colleagues reported a significant reduction in the 5-HT2 receptor binding in the cerebral cortex of AD patients compared to healthy controls (Blin et al., 1993). Similar findings were reported by Lai and colleagues who found that the 5-HT2A receptor density in frontal and temporal cortical neurons was reduced in severely demented AD patients compared to controls, This finding also suggested that the amount of neocortical 5-HT2A receptor loss could predict the rate of cognitive decline in AD (Lai et al., 2005). Using MRI and [18F]altanserin PET imaging, Hasselbalch and colleagues investigated sixteen patients with mild cognitive impairment (MCI) and observed widespread reductions in 5-HT2A receptor binding in the neocortical areas of amnesic MCI patients when compared to control subjects (Hasselbalch et al., 2008). Hasselbalch, Lai, and Blin’s studies indicated an important association between 5-HT2 receptors and the cognitive decline in AD (Blin et al., 1993; Lai et al., 2005; Hasselbalch et al., 2008). However, unlike the 5-HT2 and 5-HT6 receptors, the 5-HT4 receptors are not significantly involved in the regulation of cognition. Using [3H]GR113808, no significant changes in 5-HT4 receptor binding were observed in the postmortem frontal and temporal cortex of AD patients compared with those of the control subjects (Lai et al., 2003b).
The serotonin 5-HT6 receptor is another interesting receptor subtype due to its unique distribution and multiple modulating mechanisms in the CNS. In the central nervous system (CNS), this receptor is mainly distributed in the striatal, hippocampal, and cortical areas of the brain (Ruat et al., 1993). Increasing evidence shows that the blockade of the 5-HT6 receptor leads to an improvement in learning and memory impairments (Mitchell and Neumaier, 2005; Perez-García and Meneses, 2005). Da Silva Costa et al. found that the 5-HT6 receptor antagonist, SB-271046, significantly improves spatial recognition memory and reverses age-related deficits in the spatial recognition memory of aged mice (Da Silva Costa et al., 2009). Marcos et al. indicated that acute and subchronic treatment with the selective 5-HT6 receptor antagonist, SB-271046, in unimpaired rats significantly improved memory retention and acquisition in rats, suggesting a potential role of 5-HT6 receptor antagonists in the treatment of cognitive dysfunction (Marcos et al., 2008). In addition, a similar conclusion was drawn from Rosse and Schaffhauser’s study (Rosse and Schaffhauser, 2010). Moreover, West et al. reported that the activation of the 5-HT6 receptor, by employing the selective 5-HT6 receptor agonist, WAY-181187, may modulate synaptic plasticity via attenuating long-term potentiation (LTP). In addition, this effect could be dose-dependently prevented by treatment with the selective 5-HT6 receptor antagonist, SB-399885 (West et al., 2009).
2.2. Serotonin receptor-mediated signaling mechanisms in Alzheimer’s disease
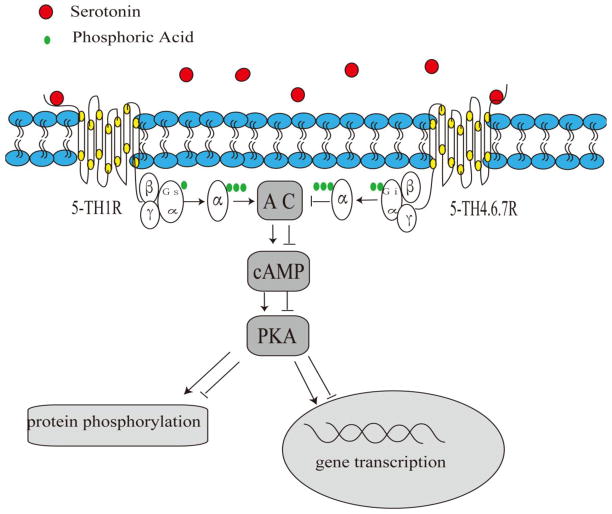
Several signaling pathways have been recognized for their involvement in the mechanisms that underlie 5-HT receptor-mediated improvement of cognitive deficits. The 5-HT6 receptor activates G(s) proteins, which stimulate adenylyl cyclase and, consequently, the production of the secondary messenger cAMP. cAMP activates protein kinase A (PKA), which phosphorylates downstream effectors and subsequently regulates calcium flux, membrane excitability, and cAMP-responsive transcription factors, such as CREB (Nichols and Nichols, 2008). Cumulative data support the expression of 5-HT6 receptors on GABAergic neurons (Upton et al., 2008). 5-HT6 receptors modulate a wide variety of neurotransmitters, such as glutamate and acetylcholine, and facilitate learning and memory processes (Hirst et al., 2006; Schechter et al., 2008; West et al., 2009). Moreover, the activation of microglia and astrocytes by Aβ promotes neuronal injury through the disruption of synaptic integrity and alterations in neurotransmitter and secondary messenger signaling (Hardy and Selkoe, 2002). A schematic overview of the involvement of 5-HT receptors in cognitive dysfunction in AD is shown in Fig. 1.
Fig 1. Potential serotonin receptor-mediated signaling in AD.
Stimulation of serotonin receptors potentially activates the secondary messenger, cAMP, and subsequently stimulates the PKA-mediated pathway in AD.
2.3. Adrenergic receptors and Alzheimer’s disease
Adrenergic receptors, a class of metabotropic GPCRs, are sensitized to catecholamines, specifically norepinephrine and epinephrine. There are two main groups of adrenergic receptors, α and β, with several subtypes, including α1, α2, β1, β2, and β3.
Several lines of evidence have demonstrated that adrenergic receptors are closely associated with cognitive declines in AD patients (Laureys et al., 2010). Two studies conducted by Shimohama et al. demonstrated that both the beta and alpha-adrenergic receptors were present in the human brain and significantly correlated with cognitive impairment in AD patients (Shimohama et al., 1986, 1987). A series of clinical studies by Kalaria et al. showed that beta 2 receptors were significantly increased in the cerebral microvessels, prefrontal cortex, and hippocampus in AD patients (Kalaria et al., 1989a; Kalaria et al., 1989b; Kalaria and Harik, 1989). One genetic study demonstrated that Gly16Arg and Gln27Glu, two polymorphisms of the β2-AR gene, interacted with the epsilon4 allele and markedly increased AD susceptibility and risk (Yu et al., 2008). Changes in the different subtypes of adrenergic receptors as well as different changes in adrenergic receptor subtypes displayed different behaviors, such as the presence or absence of aggression in AD patients. For instance, there was a higher density of beta1 and beta2 adrenergic receptors in the cerebellar cortex in aggressive AD patients, while no significant differences were observed in the adrenergic receptors of the frontal cortex or hypothalamus in these AD patients (Russo-Neustadt and Cotman, 1997). Another study by Kalaria and Andorn reported a lower density of α2 receptors in the prefrontal cortex of AD patients (Kalaria and Andorn, 1991), which was consistent with a study conducted by Meana and colleagues (Meana et al., 1992). Several lines of evidence demonstrated that the α2 adrenoceptors in the hippocampus and frontal cortex in AD were dramatically decreased (Pascual et al., 1992), and that the α 1D/2C-AR mRNA expression in the hippocampus were significantly reduced (Szot et al., 2006). This may be due to compensatory changes in the remaining noradrenergic neurons in the locus ceruleus and hippocampus of AD patients (Szot et al., 2006). However, some studies have reported conflicting results, demonstrating an upregulation of alpha1-adrenoceptors in AD patients with dementia and aggressive behavior (Sharp et al., 2007). Taken together, both animal and clinical studies have demonstrated a significant influence of adrenergic receptors in the cognitive impairment associated with AD. However, additional studies are needed to investigate the precise mechanisms involved.
2.4. Acetylcholine receptors and Alzheimer’s disease
The acetylcholine receptor (AChR) is an integral membrane protein that is the target of the neurotransmitter, acetylcholine. According to their specific affinities and sensitivities to different molecules, acetylcholine receptors can be classified as either nicotinic receptors or muscarinic receptors. Nicotinic receptors are located in the skeletal neuromuscular junction and autonomic ganglia, whereas muscarinic receptors are expressed in the brain and parasympathetic effector organs (Kato and Agid, 1979; Paterson and Nordberg, 2000). Our previous study indicated that muscarinic receptors were widely distributed in cerebral areas, such as the piriform cortex, secondary somatosensory cortex, cingulate cortex, caudate putamen, and some limbic regions, including the nucleus accumbens, hippocampus, prefrontal cortex, and primary motor cortex, suggesting the involvement of muscarinic receptors in cognitive dysfunction (Wang et al., 2008).
Although the pathological hallmarks of AD are the formation of amyloid beta-peptide and neurofibrillary tangles (NFTs) (Miranda et al., 2000), there exists considerable evidence, which implicates defects in acetylcholine receptors in the pathological mechanisms of AD (Barrantes et al., 2010; Medeiros et al., 2011). Growing evidence shows the importance of muscarinic receptors in AD. Tsang et al. showed that M1 receptor densities did not change in AD patients, but M1/G-protein coupling in the frontal cortex was significantly decreased and correlated with the severity of cognitive decline (Tsang et al., 2006). Poulin et al. demonstrated that M3-muscarinic receptor knockout mice showed a deficit in conditioned fear learning and memory in a receptor phosphorylation/arrestin-dependent manner, suggesting a functional role of muscarinic receptors in AD (Poulin, 2010). Another study demonstrated that the M1-muscarinic receptor agonist, TBPB, exhibited beneficial effects on the processing of the amyloid precursor protein and decreased Aβ production in vitro, suggesting that selective activation of the M1 receptor may provide a novel approach for the treatment of symptoms associated with AD (Jones, 2008).
The nicotinic receptor is another acetylcholine receptor subtype and the two main nAChR subtypes expressed in the CNS are the α7 and α4β2 receptors (Wevers and Schroder, 1999). Perry et al. and Paterson and Nordberg described a substantial reduction in nicotinic receptor expression in AD (Perry et al., 1986; Paterson and Nordberg, 2000). One postmortem study demonstrated that the number of nicotinic receptors declined in all of the examined brain areas in AD patients (Rinne et al., 1991). Consistent with previous reports, recent studies found a decrease in nicotinic receptor expression in AD. A clinical study compared AD to mild cognitive impairment (MCI) and normal aging and found a substantial decrease in nicotinic receptor binding (Sabbagh et al., 2006). Sabri et al observed changes in α4 and β2 nicotinic receptor expression in patients with AD and MCI and found a profound reduction in α4β2 nicotinic receptors that were closely correlated with the severity of cognitive impairment (Sabri et al., 2008). In contrast, Mitsis et al. reported that nAChRs did not change during the prodromal and early stages but was reduced in the late stage of AD (Mitsis et al., 2009). Ellis et al. also examined the distribution of α4β2 nAChRs in 15 patients with early AD and did not observed any association between α4β2 nAChRs and cognitive decline in the early stage of AD (Ellis et al., 2008). Findings obtained from animal studies were also consistent with human results. One transgenic animal study confirmed that α7-nAChR knockout mice were significantly impaired in their odor span and showed impaired attention when compared to wild-type mice (Young et al., 2007). However, Dziewczapolski et al. reported that transgenic AD mice lacking the α7nAChR gene exhibited neuroprotective effects and improved learning and memory behavior compared with control groups. It was suggested that Aβ1–42 binds to α7 nAChR, which mediates Aβ-induced tau protein phosphorylation (Dziewczapolski et al., 2009). In an early AD mouse model, α7 nAChRs also provided neuroprotection through the maintenance of the septo-hippocampal cholinergic phenotype, the preservation of hippocampal integrity, and decreasing the Aβ accumulation and oligomerization (Hernandez et al., 2010). Counts et al. found that α7 nAChR expression increased in the nucleus basalis of neurons and this trend was inversely associated with the MMSE score, suggesting a compensatory response to the decline of cholinergic activity (Counts et al., 2007). More preclinical and clinical studies have demonstrated that α7 nAChR agonists and partial agonists led to an improvement in cognitive deficits (Faghih et al., 2007; Roncarati et al., 2009; Chen et al., 2010).
2.5. Acetylcholine receptor-mediated signaling pathways in Alzheimer’s disease
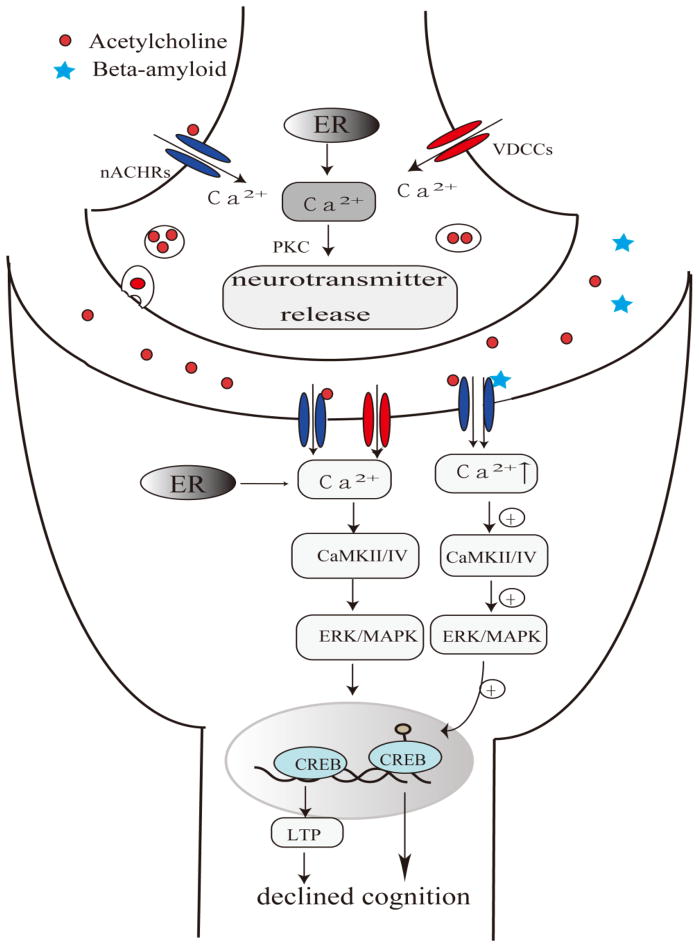
Recent studies have focused on studying the mechanisms through which acetycholine receptors are associated with the improvement in cognitive deficits in AD patients. nAChR-mediated calcium signaling have been suggested to play important roles in learning and memory in aging (Miwa et al., 2006; Gubbins et al., 2010). Miwa et al. found that lynx1 null mutant mice exhibited an enhanced performance in learning and memory via the hyperactivation of nAChRs (Miwa et al., 2006). The activation of nAChRs elevated cytoplasmic calcium levels and triggered a series of calcium-dependent intracellular processes, such as neurotransmitter release and gene expression, that mediate learning and memory processes (Turner, 2004). The activation of nAChRs mediates three types of cytoplasmic calcium signals: instantaneous, short-term (through cellular signaling cascades), and long-term effects (via gene expression) (Shen and Yakel, 2009). One study by Soliakov showed that presynaptic nAChR activation could modulate transmitter release such as striatal dopamine through protein kinase C (PKC) (Soliakov and Wonnacott, 2001). Long-term effects mainly rely on calcium influx increases to activate CaMKII/IV, ERK/MAPK and CREB, which would alter gene expression or induce long-term potentiation (LTP) (Chang and Berg, 2001; Hu et al., 2002), subsequently underlying the contribution of nAChRs to cognitive dysfunction (Ji et al., 2001; Bitner, 2007). The accumulation of Aβ and tau protein in the brain is a key feature in AD pathogenesis. Moreover, Aβ peptides negatively alter the cholinergic system at multiple sites, including ACh synthesis, ACh release, and AChRs (Auld et al., 2002). Aβ oligomers may modulate calcium homeostasis, LTP and synaptic plasticity by binding to nAChRs, and stimulating downstream signaling pathways (Lilja et al., 2011), which subsequently leads to declined cognition. A schematic overview of various mechanisms of nAChRs in cognitive dysfunction in AD is shown in Fig. 2.
Fig 2. Proposed acetylcholine receptor-mediated pathways involved in AD.
The activation of the acetylcholine receptor might elevate the cytoplasmic calcium inflow and trigger a series of calcium-dependent signaling processes such as CaMKII/IV and ERK/MAPK pathways.
2.6. Dopamine receptors and Alzheimer’s disease
Dopaminergic receptors are a class of metabotropic GPCRs and are involved in many neurological processes, such as motivation, cognition, and learning. There are two different classes of dopamine receptors, D1-like and D2-like, with five subtypes: D1, D2, D3, D4, and D5 (Contreras et al., 2002). Both D1 and D2 dopamine receptors are critical for learning and memory processes, which primarily function in the prefrontal cortex (Beaulieu and Gainetdinov, 2011). Recent studies have demonstrated significant roles of D1 and D2 receptors in modulating synaptic plasticity and mediating cognitive dysfunction via cAMP/PKA signaling cascades, DARPP-32, and CREB modulation (Chang and Berg, 2001; Hu et al., 2002). In addition, increasing evidence has shown a close dopaminergic-cholinergic and dopaminergic-glutamatergic interaction, which may play an important role in anxiety, and learning and memory (El-Ghundi et al., 2007).
Although abundant studies have investigated the correlation between dopamine receptors and PD, few studies have investigated the association of dopamine receptors and AD. Using PET, Pizzolato et al. showed that D2 receptor binding was significantly reduced in AD patients without overt extra-pyramidal symptoms (Pizzolato et al., 1996). However, Kemppainen et al. presented conflicting evidence that D1, but not D2, receptor binding in either the putamen and caudate nucleus in AD was decreased in AD patients (Kemppainen et al., 2000) and that no significant association was observed between the binding density of dopamine D1/D2 receptors in the putamen and caudate nucleus and cognitive deficits (Kemppainen et al., 2000). However, Kemppainen and colleagues found that the availability of the D2 receptor was reduced in the hippocampus and that the alteration of D2 receptor binding potentials in the right hippocampus was significantly and positively associated with verbal memory performance (Kemppainen et al., 2003). In addition, Tanaka et al. found that the reduction of striatal D2 receptor density was associated with severe behavioral abnormalities in AD (Tanaka et al., 2003). Taken together, it seems the reduction of dopamine receptors was positively correlated with severity of cognitive dysfunction in AD patients.
2.7. N-methyl-D-aspartate receptors and Alzheimer’s disease
N-methyl-D-aspartate (NMDA) receptors, one of the families of ionotropic glutamate receptors, are widely studied and abundant in the cerebral cortex, hippocampus, nucleus accumbens and striatum (Yu et al., 1997; Janssen et al., 2005; Nilsson et al., 2007). Changes in NMDA receptor populations in the brain are closely associated with many important brain functions, including neuronal apoptosis (Yu et al., 1999), attention and movement (Bi and Sze, 2002) as well as anxiety and depression (Johnson and Shekhar, 2006; Wang et al., 2009). Recent studies have demonstrated that NMDA receptors in different brain regions, such as the amygdala and hippocampus mediate anxiety and fear-related activity (Blundell and Adamec, 2007; Harré et al., 2008), respectively.
Glutamatergic modulation plays an important role in the pathogenesis of neuronal death in neurodegenerative diseases such as AD (Proctor et al., 2011). In addition to the roles of glutamate transmissions in neurodegenerative diseases, the mediation of NMDA receptor play key roles in the development of AD (Del Bel and Slater, 1991; Proctor et al., 2011). Because of the importance of NMDA receptors in the mechanisms of AD, many studies focus on the relationship between NMDA receptors and AD. Mishizen-Eberz et al reported that markedly reduced NMDA receptor binding levels were observed in the hippocampus and striatum of aged mice and AD patients (Mishizen-Eberz et al., 2004) in association with cognitive decline and anxiety. In a clinical study, Tsang et al demonstrated that the NMDA receptor NR2A subunit was significantly reduced in the orbitofrontal gyrus of high-anxiety Alzheimer’s patients in comparison to low anxiety patients, indicating that changes in the expression of NMDA receptors in the brain potentially regulate anxiety-like activity (Tsang et al., 2008). One autoradiographic study conducted by Ulas and colleagues revealed that NMDA receptor binding sites decreased in the CA1 region in AD patients (Ulas et al., 1992). In severe AD cases, the intensity of NMDA NR1 expression within the CA fields was greater than that in the controls and mild AD groups, while no difference was observed between the controls and mild/modest AD patients (Ikonomovic et al., 1999). Bi and Sze showed that NR2A and NR2B mRNA levels decreased in the entorhinal cortex and hippocampus of AD patients but that NR1 mRNA expression levels did not change when compared to control subjects (Bi and Sze, 2002). Similarly, in another AD postmortem study, Panegyres et al. observe no significant differences of NR1 receptor expression in brain areas such as the frontal lobe, superior temporal gyrus, and CA1 regions when compared with controls. However, he did find a slight reduction of NR1 receptors in the hippocampus and an increase in the frontal and superficial temporal gyrus (Panegyres et al., 2002). Gong et al. also found that NR1 expression levels were significantly reduced in 12 patients with AD (Gong et al., 2009). Another study demonstrated a reduction of NMDA NR2 receptors in the postmortem brain tissue of AD patients (Hynd et al., 2004). In addition to the hippocampus, NMDA receptor changes have also been found in other brain regions. Fang et al. found increases in NMDA receptor-positive cells in the deep cortical layers of the frontal cortex of AD patients using immunohistochemical methods (Fang et al., 2005). However, Fang’s study demonstrated that co-localization of the NMDA receptor with caspase-3 could contribute to the neuronal apoptosis and dementia of AD (Fang et al., 2005).
The effects of NMDA receptors on cognitive dysfunction have been well documented and various mechanisms are involved in NMDA receptor-mediated modulation in AD pathogenesis. Sun et al. showed that a reduction in the levels of NMDA NR1 and NR2B proteins in the hippocampi of a chronic cerebral hypoperfusion rat model, suggesting a close correlation between NMDA receptors and cognitive deficits (Sun et al., 2010a). Our previous study showed a significant positive correlation between hyperlocomotive as well as anxiolytic-like activities and the upregulation of NMDA receptors in different brain regions, which suggests the regulation of psycho-neurodegenerative processes by NMDA receptors (Wang et al., 2009). Snyder and colleagues confirmed enhanced endocytosis of NMDA receptors by Aβ in AD (Snyder et al., 2005). In addition to changes in the NMDA receptors, the genetic variation encoding the receptors could also affect cognitive dysfunction in AD. Data from a case-control study conducted by Liu et al. confirms that the exonic polymorphisms of NR3A, but not NR3B, are an important risk factor for AD pathogenesis among the Taiwanese population (Liu et al., 2009). One stereotaxic and genome-wide study confirmed that one SNP, rs10845840, which encodes the NMDA receptor NR2B subunit, is associated with temporal lobe volume and has proven to be a more informative phenotype than hippocampal volumes in AD (Stein et al., 2010). Taken together, genetic polymorphisms encoding NMDA receptors are associated with AD development.
2.8. N-methyl-D-aspartate receptor-mediated mechanisms in Alzheimer’s disease
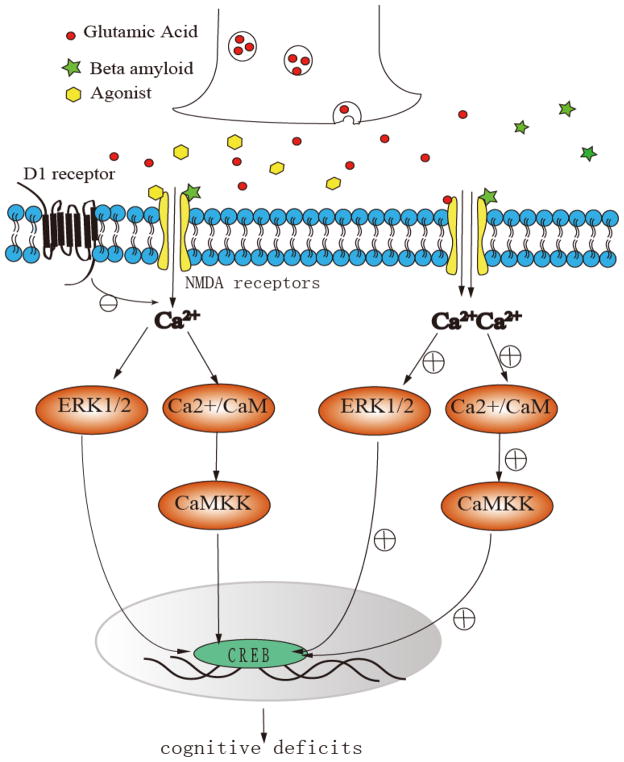
AD is characterized by cortical atrophy, the profound loss of neurons and synapses, apoptosis, reactive gliosis, intraneuronal neurofibrillary tangles, and extracellular senile amyloid plaques (Xiong et al., 2004; Gulyás et al., 2011). Several studies have elucidated that amyloid plaques lead to neuronal loss in AD and is mediated by NMDA receptors. In AD development, tau and amyloid plaques overactivate NMDA receptors and thus can cause CA2+ overflow into neurons and the activation key enzymes (Paoletti and Neyton, 2007; Fortin et al., 2010; Guetg et al., 2010). Xiong et al. found that the NMDA receptor antagonist can block inward currents in Xenopus oocytes that expressed NR1A/NR2B. The APP-stimulated monocyte-derived macrophages have been suggested to be responsible for the NR1A/NR2B activation due to the direct activation of NMDA receptor subtypes, which is relevant to the pathogenesis of AD, by APPs/Aβ-stimulated monocyte-derived macrophages conditioned media (Xiong et al., 2004). In addition to amyloid plaques, tau protein can also cause NMDA receptor activation and, consequently, NMDA receptor-mediated neurotoxicity in AD. Amadoro showed that Tau (tau 441) toxicity involves extrasynaptic NMDA NR2B receptors and ERK1/2 activation in both primary cultured cortical neurons and cerebellar granule cells (Amadoro et al., 2006). A schematic overview of the various mechanisms of NMDA receptors in cognitive dysfunction of AD is shown in Fig. 4.
Fig 4. Proposed NMDA receptor-mediated mechanisms in cognitive dysfunction in AD.
In AD development, tau and amyloid plaques overactivate NMDA receptors and thus can cause Ca2+ overflow into dopaminergic neurons and activate several key enzymes, such as the calmodulin-dependent protein kinase kinase (CaMKK).
It has been well documented that there is a close interaction between brain glutamatergic NMDA receptors and the monoamine dopaminergic system (de Bartolomeis, 2005). Dopaminergic disturbances in the brain can lead to glutamatergic NMDA receptor changes (Hallett, 2006) and vice versa (Hallett and Standaert, 2004). NMDA receptors are mediated dopamine-glutamatergic interactions by direct coupling between both NR1 and NR2A subunits with the C terminus of dopamine D1 receptor, and dopamine depletion can change the level of NMDA receptor expression (Hallett and Standaert, 2004). Oxidative stress is another significant pathology in AD. Aβ oligomers induce neuronal oxidative stress through an NMDA receptor-dependent mechanism, which can be blocked by memantine, an NMDA receptor antagonist (De Felice et al., 2007). Decker demonstrated that NMDA receptors can mediate synaptic deterioration by the reduction of NMDA receptor expression through RNA interference, which blocks neuronal oxidative stress induced by Aβ oligomers (Decker et al., 2010). The ubiquitination of the tyrosine phosphatase 61 (STEP 61) was found to be involved in NMDA receptor endocytosis in AD (Kurup et al., 2010). However, further studies are needed to elucidate the mechanisms through which NMDA receptors influence cognitive dysfunction in AD. A hypothetical schematic depicting the association of NMDA receptors and AD is shown in Fig. 4
3. Alterations in neurotransmitter receptors in Parkinson’s disease
3.1. Adrenergic receptors and Parkinson’s disease
In the 1980s, Cash et al. established a link between different types of adrenergic receptors and PD. He found an increase in the adrenergicα1 and β1 receptor density in demented PD patients and a decrease in α2 adrenergic receptors (Cash et al., 1984). Studies of the adrenergic receptor binding pattern showed a decrease in the binding sites of α1 adrenergic receptors in the cerebral microvessels of the prefrontal cortex and putamen regions of PD patients (Cash et al., 1985). In the following year, Cash et al. found that adrenergic α1 receptors increased in the synaptosomal fraction, while adrenergic β receptors increased in the synaptosomal and microsomal fractions in PD patients (Cash et al., 1986). Visanji et al. found that the α1-adrenoceptor antagonist prazosin significantly attenuated L-DOPA-induced hyperactivity in MPTP-lesioned macaques in a dose-dependent manner, suggesting that α1-adrenoceptors are involved in the pathological response to dopaminergic neurodegeneration (Visanji et al., 2009). Furthermore, Belujon et al. showed that the activation of α1 and α2 adrenergic receptors in the subthalamic nucleus (STN) in 6-hydroxydopamine (6-OHDA)-lesioned rats markedly decreased locomotor activity by controlling STN firing, thereby indicating the role of α1 and α2 adrenergic receptors in the regulation of STN neurons (Belujon et al., 2007). Many studies have explored the relationship between adrenergic receptors and PD, but few studies have been successful in revealing the mechanism of cognitive impairment in PD patients. Therefore, future studies are highly desired to gain further understanding of these correlations and mechanisms.
3.2. Acetylcholine receptors and Parkinson’s disease
Although many studies have investigated the relationship between AD and AChRs, only a limited number of studies have reported a similar association between PD and AchRs. Ward et al indicated that nicotinic acetycholine receptors were abundant in the nigrostriatal area and may be of relevance to occurrence of PD (Ward et al., 2008). Meyer et al. observed that the density of α4β2-nAChRs significantly decreased in patients with PD and correlated with the severity of cognition decline (Meyer et al., 2009). Oishi et al also found that the density of nAChRs showed a significant decrease in the brainstem and frontal cortex of PD patients without dementia compared with the healthy individuals using (123)I-5IA SPECT (Oishi et al., 2007). A postmortem study on PD patients conducted by Rinne and colleagues indicated that nicotinic receptor expression declined in all areas of the brain and had a negative correlation with the degree of dementia in PD (Rinne et al., 1991).
3.3. Dopamine receptors and Parkinson’s disease
In addition to motor dysfunction, PD patients also manifest non-motor symptoms such as sleep disorders, constipation, and dementia. Constituting the major part of non-motor dysfunction, cognitive deficits in advanced PD patients have recently drawn wider attention (Nieoullon, 2002). It has been reported that as a motor disorder, PD patients normally display cognitive deficits not in the early stages, but in the more advanced stages of PD (Péron et al., 2009). More studies have focused on the relationship between dopamine receptors and dementia in PD and several lines of evidence have shown the association between the disturbance of dopaminergic receptors in brain regions and cognitive deficits in elderly people, PD, and AD (Fetsko et al., 2005; Reeves et al., 2005; Reeves et al., 2009; Reeves et al., 2010; Rieckmann et al., 2011). In addition to severe nigrostriatal denervation, PD patients also show temporal processing deficits and prefrontal cortical dysfunction, which are closely correlated with cognitive impairments (Harrington et al., 1998; Nagano-Saito et al., 2004).
Animal studies have shown that dopamine D1/D2 receptor expression were significantly decreased in the frontal cortex in Sprague–Dawley rats with a unilateral lesion in the medial forebrain bundle by 6-hydroxydopamine (Wang et al., 2005a); while stimulation of the dopamine D1/D2 receptor was also associated with cAMP alterations (Wang et al., 2005b). Namba’s group found a dynamic change of D2 receptors in medial forebrain bundle (MFB)-lesioned PD rats. Moreover, at early stages of PD (4 weeks after lesion), striatal D2 receptors were upregulated, while the expression of striatal D2 receptors reduced at more advanced stages of PD (6 months after lesion). However, in the unilaterally striatal-lesioned rats, the D2 receptors were consistently downregulated (Sun et al., 2010b; Sun et al., 2011). In striatal-lesioned PD rat models, Fang et al. found that the PD rats demonstrated declined learning and memory at early stages (3–4 weeks after lesion) of PD (Fang et al., 2006). The combined findings obtained by Fang and Sun (Fang et al., 2006; Sun et al., 2010b; Sun et al., 2011) strongly suggested that the impaired learning and memory in the striatal PD rats were closely associated with the down-regulation of dopamine D2 receptors, most likely mediated by unknown mechanisms involving important changes in dopaminergic receptors.
Clinical findings demonstrated similar findings with the results obtained from animal studies. Clinically, PET/SPECT data has been used to investigate the alterations in dopaminergic receptors in PD patients. At early stages of PD, the dopamine D2 receptor binding as measured by PET/SPET was elevated in some brain regions, while the progression of PD with dementia occurrence (over years rather than months) has been found to correlate with lower dopamine D2 receptor binding in these patients, strongly implying the association of dopaminergic receptors and cognitive impairment (Antonini et al., 1995; Antonini et al., 1997b; Antonini et al., 1997a; Piggott et al., 1999). With disease progression, dopamine receptor expression declines at a relatively faster annual rate in the brain compared to the rate in healthy individuals. Kaasinen et al. demonstrated that dopamine D2 receptors profoundly decreased in the dorsolateral prefrontal cortex, temporal cortex, and medial thalami with the progression of PD (corresponding to an annual decline of 6–11%) (Kaasinen et al., 2003). In advanced, but not early, stages of PD, dopamine D2 and D3 receptor bindings were significantly decreased in the dorsolateral prefrontal cortex, anterior cingulate cortex, and medial thalamus compared with healthy controls (Kaasinen et al., 2000). In the hypothalamus, the D2 receptor binding potentials were also significantly reduced without a correlation with age and levodopa equivalent dose (Politis et al., 2008). Mizukawa et al. reported a significant reduction in the density of dopamine receptors in both the caudate and putamen of advanced PD patients (Mizukawa et al., 1993); while Boileau et al. found decreased dopamine D3 receptor binding in the ventral striatum and globus pallidus in drug-naive PD patients (Boileau et al., 2009).
However, conflicting results have also been reported in several studies. One study by Verstappen at al. indicated that D2 receptor expression increased in the corpora striata during the first year of the disease (Verstappen et al., 2007). Another study by Piggott and colleagues showed that PD patients with dementia exhibited an increase in D2 receptor density in the ventro-intermedius in correlation with cognitive decline as measured by MMSE scores (Piggott et al., 2007). Similar results have been reported by Knudsen et al., demonstrating that the ratio of caudate dopamine transporters (DAT) and D2 receptor binding was significantly higher in patients with PD when compared with healthy subjects (Knudsen et al., 2004). The reasons for these contrasting results are still unclear, but may result from the different methodologies used in measuring dopamine receptor levels and the variability exhibited by the selected PD subjects; further investigation is required.
In addition to the studies that demonstrate alterations in dopaminergic receptors in PD patients, there is increasing evidence that indicates a close relationship between the alteration in dopamine receptors and cognition in PD. Due to the activation of dopamine receptors in PD, dopamine receptor agonists were usually used to ameliorate the cognitive dysfunction observed in PD. Nigrostriatal 6-hydroxydopamine-lesioned PD rats develop attentional deficits, and this decline in cognition was significantly recovered after subchronic treatment with the dopamine D2/3 receptor agonist, piribedil (Turle-Lorenzo et al., 2006). Consistent with Turle-Lorenzo’s study, Costa et al. treated advanced PD patients with the dopamine receptor agonists pergolide and pramipexole and found a significant improvement in the visual–spatial, visual-object, and verbal working memory tasks in PD patients; this suggests that the stimulation of dopaminergic receptors may ameliorate the cognitive impairment demonstrated in PD patients, potentially by modulating activity in frontal–striatal circuits (Costa et al., 2009). In contrast, Mehta et al. showed that the dopamine D2 receptor antagonist sulpiride could reduce spatial recognition, spatial working memory, planning, and attention set-shifting in young healthy male volunteers and in patients with PD, which strongly implies that the impairments observed in PD depend, in part, on dopamine D2 receptors, most likely involved in the frontostriatal pathway (Mehta et al., 1999).
In contrast with to D2 receptors, D1 receptors present different characteristics in PD. One study by Sawaguchi showed that the local activation of the dopamine D1 receptors in the frontal cortical areas of monkeys played a facilitating role in working memory-guided directional movements (Sawaguchi, 2000). In non-demented PD patients, Cropley et al. conducted a PET study to examine the association between cognitive disturbances and D1 receptor density in fifteen non-demented patients with PD. They showed that the D1 receptor density in the frontal cortical regions did not change compared with that of healthy subjects (Cropley et al., 2008). Mattila et al. observed that the decrease in the density of D1 dopamine receptors in the caudate nucleus and putamen of PD patients was associated with the cognitive decline as measured by Reisberg’s global deterioration scale (Mattila et al., 2001). To investigate the correlation of working memory decline with dopamine D1 receptor density in PD patients, Costa et al. treated PD patients with the D1 receptor agonist, pergolide, and found a clear improvement in working memory function, implying the importance of D1 receptor modulation in frontal-striatal circuits (Costa et al., 2009). In a randomized prospective multi-center study, Rektorova et al. indicated that dopamine D1 and D2 receptor agonists displayed positive effects on cognitive dysfunction in advanced PD patients (Rektorová et al., 2005; Rektorová, 2010).
3.4. Dopamine receptor-mediated signaling pathways in cognitive dysfunction in Parkinson’s disease
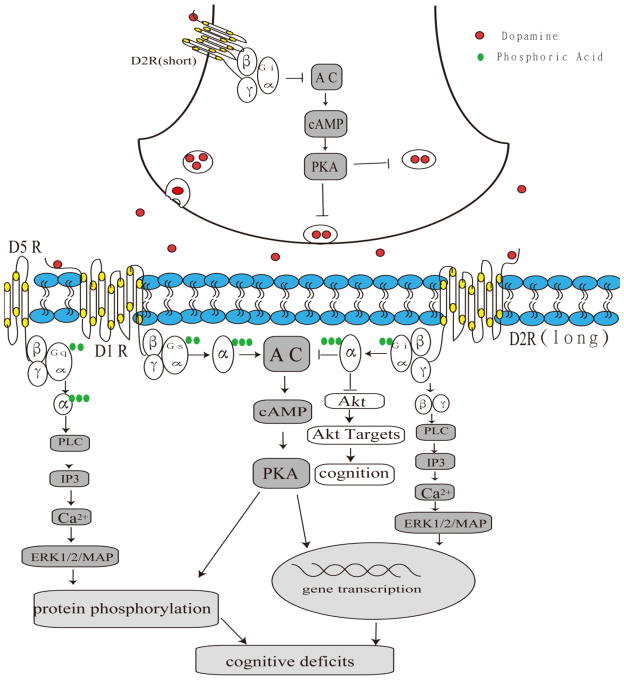
It has become increasingly apparent that dopamine receptor-mediated signaling cascades are altered as a consequence of the dopaminergic denervation that occurs in PD and that the functional reactions of dopamine receptors may result from a variety of alterations in the signaling mechanisms (Hurley and Jenner, 2006). This view is supported by a number of recent studies. The simultaneous activation of the dopamine D2 receptor suppresses transmembrane Ca2+ currents, activated phospholipase C (PLC) and the calcium-dependent phosphatase calcineurin (Hernandez-Lopez et al., 2000). The functions of the dopamine receptors were associated with the regulation of the cyclic adenosine monophosphate (cAMP)–protein kinase A (PKA)–dopamine and cAMP-regulated phosphoprotein through GPCR-mediated signaling pathways. After the denervation of nigrostriatal dopaminergic neurons, extracellular-signal-regulated kinase1/2 (ERK1/2)/MAP kinase was activated in response to D1 dopamine receptor agonists, either alone or in combination with the stimulation of corticostriatal afferents (Gerfen et al., 2002). The inhibition of MAP kinase was responsible for the phosphorylation of ERK1/2/MAP kinase and the blockage of the dopamine D1 receptor mediated the activation of ERK1/2/MAP kinase in the dopamine-depleted striatum (Gerfen et al., 2002). Several studies have linked ERK with the acute and chronic responses to dopaminergic drugs (Valjent et al., 2005; Beaulieu et al., 2006; Valjent et al., 2006; Beaulieu et al., 2007a). The activation of PI3-kinase and AKT can mediate D2 receptor neuroprotection against oxidative stress in PC12 cells (Nair and Sealfon, 2003). Moreover, D2 receptor activation of ERK can cause DNA synthesis and mitogenesis in different cell types (Ghahremani et al., 2000). Furthermore, the activation of MAP kinases is associated not only with cell survival and synaptic plasticity in post-mitotic neurons but also with acute behavioral responses to dopamine receptor stimulation (Impey et al., 1999; Otani et al., 1999). The inhibition of AKT1 and the potentiation of the behavioral effects of amphetamine in AKT1-KO mice by the stimulation of dopamine D2-like receptors further supports the involvement of AKT in dopamine-related behavioral responses (Beaulieu et al., 2007b). The stimulation of D2 receptors induced a partial inhibition of protein tyrosine phosphatase (PTP) activity and mitogen-activated protein kinase phosphatase (MKP) pathway in a 6-hydroxydopamine-lesioned PD rat model (Zhen et al., 2002). The D4MN9D cells that stably expressed D4 dopamine receptors were treated by the D4 dopamine receptor agonist PD168077, which induced a time- and dose-dependent activation of AKT and ERK (Zhen et al., 2001). The study demonstrated that D4 receptor-stimulated cell proliferation is mediated by the Src/SHC/Ras/ERK pathway (Zhen et al., 2001). A schematic overview of several mechanisms of dopamine receptors in cognitive dysfunction is shown in Fig. 3.
Fig 3. Proposed dopamine receptor-mediated signaling pathways in cognitive dysfunction in PD.
The simultaneous activation of dopamine receptors suppresses transmembrane Ca2+ currents and activates phospholipase C (PLC) and the calcium-dependent phosphatase calcineurin. ERK1/2/MAP, AKT and PKA signal pathways would be activated after the denervation of nigrostriatal dopaminergic neurons following dopamine receptor modulation.
3.5. NMDA receptors and Parkinson’s disease
Unlike the association between NMDA receptors and AD, there are few studies that establish a correlation between the cognitive dysfunction found in PD and NMDA receptors. Our recent work indicates that in PD rats, 6-hydroxydopamine induced anxiety and the downregulation of NMDA receptors in the hippocampus, CA1, amygdala, and caudate putamen, as observed in 6-hydroxydopamine-lesioned rats, whereas simvastatin significantly ameliorated the anxiety-like activity and restored the expression of NMDA receptors in several examined brain regions (Yan et al., 2011). A significant positive correlation was identified between the anxiolytic-like activity and the restoration of NMDA receptor expression in the hippocampus, amygdala and CA1 following simvastatin administration, which accompanied NMDA-mediated anti-inflammatory responses (Yan et al., 2011). Several lines of evidence have investigated the changes of NMDA receptors in experimental animal models of PD and human postmortem tissue from PD patients. However, the alterations of NMDA receptors in PD in both experimental models and human tissue showed contradictory results. Using autoradiographic methods, Wullner et al. found that NMDA binding sites were increased in the lesioned striatum of the 6-OHDA-lesioned rat (Wullner et al., 1994). Consistent with this finding, Hallett and Standaert reported a bilateral decrease in NMDA binding, which was more pronounced on the lesioned side, after an interval of 6 months (Hallett and Standaert, 2004). However, data from postmortem studies in humans present contrasting results. Meoni et al. reported that glutamate binding to the NMDA receptor was significantly reduced in the striatum of the post-mortem PD brain, while in the prefrontal cortex of the PD group, a decreased expression of NMDA NR1 mRNA was observed, with a maximal decrease observed in cortical layer IV (Meoni et al., 1999). In animal experiments, Dunah et al. employed co-immunoprecipitation methods and demonstrated the abundance of NR1 and a decrease in NR2B subunits in lesioned striatal synaptosomal membranes obtained from a 6-OHDA-lesioned rat model. Moreover, the NR2A expression remained unchanged and a chronic treatment with L-dopa restored the abundance of the NMDA receptors in the striatal membranes (Dunah et al., 2000). However, the reasons for these conflicting observations still remain unclear. Further studies using different methodologies are needed to explore the changes of NMDA receptors and the relationship between NMDA receptors and cognitive dysfunction in PD.; while NMDA receptors would be a potential and important target in the treatment of PD (Gubellini et al., 2004).
4. Conclusion
The role of receptor alterations in the central nervous system has become increasingly recognized as a key element in the pathological progression of neurodegenerative diseases such as AD and PD. Whether this receptor modulation is destructive or beneficial depends on the severity and stage of the disease. Indeed, cognitive dysfunction found in AD and PD are determined by very complicated mechanisms, which are reflected not only in the disturbances of various receptors, but in the chemical neurotransmitters as well, such as glutamate, serotonin, and dopamine transmissions (Danbolt, 2001; Pralong et al., 2002; Piccini et al., 2003; Cheesman et al., 2005; Sawamoto et al., 2008; Huot et al., 2011). Nonetheless, the precise relationship between receptors and neurotransmission necessitates additional clarification. Future studies are needed to explore the interactions and alterations in different receptors and the various chemical neurotransmitters in different neural circuits such as the frontal-striatal, fronto–striato–thalamic and mesolimbic circuitries. Moreover, additional studies are required to understand how the associations between receptors and neurotransmitters mediate cognitive impairment in AD and PD. In the future, new approaches such as dynamic brain functional imaging will shed light on the roles of neurotransmission and receptors in regulating cognitive dysfunction in AD and PD. Additional studies focused on the optimal timing of processing is worthwhile to explore in light of recent research regarding timing mechanisms of cognitive pathology in neural circuitries. Future perspectives should also address the optimal timing of different receptors that mediate interventions as well as elucidate how these receptors function to modulate cognitive dysfunction in AD and PD. Novel research strategies should consider these complicated relationships and potentially integrate these elements into drug treatments to allow better management of cognitive pathology in AD and PD.
Highlights.
Cognitive dysfunction is a characteristic of neurodegenerative diseases, especially AD and PD.
Pathophysiological alterations in monoamine, acetylcholine and glutamate receptors contribute to cognitive impairment and/or deterioration.
Abnormalities of serotonin-, adrenaline-, dopamine-, acetylcholine- and NMDA- signaling pathways differentially contribute to the pathogenesis of such cognitive dysfunction.
Future research may shed light on new solutions of cognitive dysfunction in neurodegenerative diseases by targeting specific alterations in these receptors and their signal transduction in the frontal-striatal, fronto–striato–thalamic, and mesolimbic circuitries.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (09ykpy19 and 10ykzd08), 985 Project (2012–2013), Program for New Century Excellent Talents in University (NCET2010, P.R. China), National Natural Science Foundation of Guangdong China (S2011010004711), and National Natural Science Foundation of China (81071031) to Qing Wang. Ying Xia is supported by NIH (R01 HD-034852, R01 AT-004422).
Abbreviations
- AD
Alzheimer Disease
- PD
Parkinson’s disease
- NMDA receptors
N-methyl-D-aspartate receptors
- GPCRs
G protein-coupled receptors
- PKA
protein kinase A
- PLC
phospholipase C
- cAMP
Cyclic Advenosine Monophosphato
- CREB
cAMP response element binding protein
- 5-HT
5-hydroxytryptamine
- PKC
protein kinase C
- MMSE
mini-mental state examination
- PET
positron emission tomography
- SPECT
Single-Photon Emission Computed Tomography
- CNS
central nervous system
- MCI
mild cognitive impairment
- CNS
central nerve system
- LTP
long-term potentiation
- GABA
gamma-aminobutyric acid
- AchR
acetylcholine receptor
- NFTs
neurofibrillary tangles
- nAChR
nicotinic acetylcholine receptor
- WT
wild-type
- CaMKII/IV
calmodulin-dependent protein kinases II/IV
- ERK
external signal-regulated kinase
- MAPK
mitogen-activated protein kinases
- DARPP
dopamine and adenosine 3′5′-monophosphate-regulated phospho-protein
- DAT
Dopamine transporters
- APP
Amyloid precursor protein
- MPTP
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- STN
subthalamic nucleus
- 6-OHDA
6-hydroxydopamine
- MFB
medial forebrain bundle
- DAT
dopamine transporters
- PI3-kinase
1-Phosphatidylinositol 3-Kinase
- DNA
Deoxyribonucleic acids
- CA1
hippocampus 1
Footnotes
Potential Conflicts of Interest: The authors have declared that no competing interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci U S A. 2006;103:2892–2897. doi: 10.1073/pnas.0511065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Schwarz J, Oertel WH, Pogarell O, Leenders KL. Long-term changes of striatal dopamine D2 receptors in patients with Parkinson’s disease: a study with positron emission tomography and [11C]raclopride. Mov Disord. 1997a;12:33–38. doi: 10.1002/mds.870120107. [DOI] [PubMed] [Google Scholar]
- Antonini A, Vontobel P, Psylla M, Günther I, Maguire PR, Missimer J, Leenders KL. Complementary positron emission tomographic studies of the striatal dopaminergic system in Parkinson’s disease. Arch Neurol. 1995;52(12):1183–1190. doi: 10.1001/archneur.1995.00540360061017. [DOI] [PubMed] [Google Scholar]
- Antonini A, Leenders KL, Vontobel P, Maguire RP, Missimer J, Psylla M, Gunther I. Complementary PET studies of striatal neuronal function in the differential diagnosis between multiple system atrophy and Parkinson’s disease. Brain. 1997b;120 ( Pt 12):2187–2195. doi: 10.1093/brain/120.12.2187. [DOI] [PubMed] [Google Scholar]
- Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer’s disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol. 2002;68:209–245. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- Barrantes FJ, Borroni V, Vallés S. Neuronal nicotinic acetylcholine receptor-cholesterol crosstalk in Alzheimer’s disease. FEBS Lett. 2010;584(9):1856–1863. doi: 10.1016/j.febslet.2009.11.036. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007a;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice. J Biol Chem. 2006;281:32072–32080. doi: 10.1074/jbc.M606062200. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007b;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Bezard E, Taupignon A, Bioulac B, Benazzouz A. Noradrenergic modulation of subthalamic nucleus activity: behavioral and electrophysiological evidence in intact and 6-hydroxydopamine-lesioned rats. J Neurosci. 2007;27(36):9595–9606. doi: 10.1523/JNEUROSCI.2583-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H, Sze CI. N-methyl-D-aspartate receptor subunit NR2A and NR2B messenger RNA levels are altered in the hippocampus and entorhinal cortex in Alzheimer’s disease. J Neurol Sci. 2002;200(1–2):11–18. doi: 10.1016/s0022-510x(02)00087-4. [DOI] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Buccafusco J, et al. Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. J Neurosci. 2007;27(39):10578–10587. doi: 10.1523/JNEUROSCI.2444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin J, Baron JC, Dubois B, Crouzel C, Fiorelli M, Attar-Levy D, Pillon B, Fournier D, Vidailhet M, Agid Y. Loss of brain 5-HT2 receptors in Alzheimer’s disease. In vivo assessment with positron emission tomography and [18F]setoperone. Brain. 1993;116 ( Pt 3):497–510. doi: 10.1093/brain/116.3.497. [DOI] [PubMed] [Google Scholar]
- Blundell J, Adamec R. The NMDA receptor antagonist CPP blocks the effects of predator stress on pCREB in brain regions involved in fearful and anxious behavior. Brain Res. 2007;1136(1):59–76. doi: 10.1016/j.brainres.2006.09.078. [DOI] [PubMed] [Google Scholar]
- Boileau I, Guttman M, Rusjan P, Adams JR, Houle S, Tong J, Hornykiewicz O, Furukawa Y, Wilson AA, Kapur S, Kish SJ. Decreased binding of the D3 dopamine receptor-preferring ligand [11C]-(+)-PHNO in drug-naive Parkinson’s disease. Brain. 2009;132:1366–1375. doi: 10.1093/brain/awn337. [DOI] [PubMed] [Google Scholar]
- Cash R, Ruberg M, Raisman R, Agid Y. Adrenergic receptors in Parkinson’s disease. Brain Res. 1984;322:269–275. doi: 10.1016/0006-8993(84)90117-3. [DOI] [PubMed] [Google Scholar]
- Cash R, Lasbennes F, Sercombe R, Seylaz J, Agid Y. Adrenergic receptors on cerebral microvessels in control and parkinsonian subjects. Life Sci. 1985;37:531–536. doi: 10.1016/0024-3205(85)90465-5. [DOI] [PubMed] [Google Scholar]
- Cash R, Raisman R, Lanfumey L, Ploska A, Agid Y. Cellular localization of adrenergic receptors in rat and human brain. Brain Res. 1986;370:127–135. doi: 10.1016/0006-8993(86)91112-1. [DOI] [PubMed] [Google Scholar]
- Chang KT, Berg DK. Voltage-gated channels block nicotinic regulation of CREB phosphorylation and gene expression in neurons. Neuron. 2001;32:855–865. doi: 10.1016/s0896-6273(01)00516-5. [DOI] [PubMed] [Google Scholar]
- Cheesman AL, Barker RA, Lewis SJ, Robbins TW, Owen AM, Brooks DJ. Lateralisation of striatal function: evidence from 18F-dopa PET in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;76:1204–1210. doi: 10.1136/jnnp.2004.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang H, Zhang Z, Li Z, He D, Sokabe M. DMXB (GTS-21) ameliorates the cognitive deficits in beta amyloid(25–35(−) ) injected mice through preventing the dysfunction of alpha7 nicotinic receptor. J Neurosci Res. 2010;88:1784–1794. doi: 10.1002/jnr.22345. [DOI] [PubMed] [Google Scholar]
- Contreras F, Fouillioux C, Bolivar A, Simonovis N, Hernandez-Hernandez R, Armas-Hernandez MJ, Velasco M. Dopamine, hypertension and obesity. J Hum Hypertens. 2002;16(Suppl 1):S13–17. doi: 10.1038/sj.jhh.1001334. [DOI] [PubMed] [Google Scholar]
- Costa A, Peppe A, Dell’Agnello G, Caltagirone C, Carlesimo GA. Dopamine and cognitive functioning in de novo subjects with Parkinson’s disease: effects of pramipexole and pergolide on working memory. Neuropsychologia. 2009;47(5):1374–1381. doi: 10.1016/j.neuropsychologia.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Counts SE, He B, Che S, Ikonomovic MD, DeKosky ST, Ginsberg SD, Mufson EJ. Alpha7 nicotinic receptor up-regulation in cholinergic basal forebrain neurons in Alzheimer disease. Arch Neurol. 2007;64:1771–1776. doi: 10.1001/archneur.64.12.1771. [DOI] [PubMed] [Google Scholar]
- Cropley VL, Fujita M, Bara-Jimenez W, Brown AK, Zhang XY, Sangare J, Herscovitch P, Pike VW, Hallett M, Nathan PJ, Innis RB. Pre- and post-synaptic dopamine imaging and its relation with frontostriatal cognitive function in Parkinson disease: PET studies with [11C]NNC 112 and [18F]FDOPA. Psychiatry Res. 2008;163:171–182. doi: 10.1016/j.pscychresns.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Da Silva Costa V, Duchatelle P, Boulouard M, Dauphin F. Selective 5-HT6 receptor blockade improves spatial recognition memory and reverses age-related deficits in spatial recognition memory in the mouse. Neuropsychopharmacology. 2009;34(2):488–500. doi: 10.1038/npp.2008.94. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- de Bartolomeis A, Fiore G, Iasevoli F. Dopamine-glutamate interaction and antipsychotics mechanism of action: implication for new pharmacological strategies in psychosis. Curr Pharm Des. 2005;11(27):3561–3594. doi: 10.2174/138161205774414538. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Decker H, Jurgensen S, Adrover MF, Brito-Moreira J, Bomfim TR, Klein WL, Epstein AL, De Felice FG, Jerusalinsky D, Ferreira ST. N-methyl-D-aspartate receptors are required for synaptic targeting of Alzheimer’s toxic amyloid-beta peptide oligomers. J Neurochem. 2010;115:1520–1529. doi: 10.1111/j.1471-4159.2010.07058.x. [DOI] [PubMed] [Google Scholar]
- Del Bel EA, Slater P. Binding to the glycine site of the NMDA receptor complex in brains of patients with Alzheimer’s disease. Neurosci Lett. 1991;131:75–78. doi: 10.1016/0304-3940(91)90340-y. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Wang Y, Yasuda RP, Kameyama K, Huganir RL, Wolfe BB, Standaert DG. Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl-D-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson’s disease. Mol Pharmacol. 2000;57:342–352. [PubMed] [Google Scholar]
- Dziewczapolski G, Glogowski CM, Masliah E, Heinemann SF. Deletion of the alpha 7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2009;29:8805–8815. doi: 10.1523/JNEUROSCI.6159-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghundi M, O’Dowd BF, George SR. Insights into the role of dopamine receptor systems in learning and memory. Rev Neurosci. 2007;18:37–66. doi: 10.1515/revneuro.2007.18.1.37. [DOI] [PubMed] [Google Scholar]
- Ellis JR, Villemagne VL, Nathan PJ, Mulligan RS, Gong SJ, Chan JG, Sachinidis J, O’Keefe GJ, Pathmaraj K, Wesnes KA, Savage G, Rowe CC. Relationship between nicotinic receptors and cognitive function in early Alzheimer’s disease: a 2-[18F]fluoro-A-85380 PET study. Neurobiol Learn Mem. 2008;90:404–412. doi: 10.1016/j.nlm.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Faghih R, Gfesser GA, Gopalakrishnan M. Advances in the discovery of novel positive allosteric modulators of the alpha7 nicotinic acetylcholine receptor. Recent Pat CNS Drug Discov. 2007;2:99–106. doi: 10.2174/157488907780832751. [DOI] [PubMed] [Google Scholar]
- Fang M, Li J, Tiu SC, Zhang L, Wang M, Yew DT. N-methyl-D-aspartate receptor and apoptosis in Alzheimer’s disease and multiinfarct dementia. J Neurosci Res. 2005;81:269–274. doi: 10.1002/jnr.20558. [DOI] [PubMed] [Google Scholar]
- Fang X, Sugiyama K, Akamine S, Namba H. The stepping test and its learning process in different degrees of unilateral striatal lesions by 6-hydroxydopamine in rats. Neurosci Res. 2006;55(4):403–409. doi: 10.1016/j.neures.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Fetsko LA, Xu R, Wang Y. Effects of age and dopamine D2L receptor-deficiency on motor and learning functions. Neurobiol Aging. 2005;26(4):521–530. doi: 10.1016/j.neurobiolaging.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Davare MA, Srivastava T, Brady JD, Nygaard S, Derkach VA, Soderling TR. Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J Neurosci. 2010;30(35):11565–11575. doi: 10.1523/JNEUROSCI.1746-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alloza M, Hirst WD, Chen CP, Lasheras B, Francis PT, Ramirez MJ. Differential involvement of 5-HT(1B/1D) and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer’s disease. Neuropsychopharmacology. 2004;29:410–416. doi: 10.1038/sj.npp.1300330. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci. 2002;22(12):5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani MH, Forget C, Albert PR. Distinct roles for Galpha(i)2 and Gbetagamma in signaling to DNA synthesis and Galpha(i)3 in cellular transformation by dopamine D2S receptor activation in BALB/c 3T3 cells. Mol Cell Biol. 2000;20:1497–1506. doi: 10.1128/mcb.20.5.1497-1506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Lippa CF, Zhu J, Lin Q, Rosso AL. Disruption of glutamate receptors at Shank-postsynaptic platform in Alzheimer’s disease. Brain Res. 2009;1292:191–198. doi: 10.1016/j.brainres.2009.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbins EJ, Gopalakrishnan M, Li J. Alpha7 nAChR-mediated activation of MAP kinase pathways in PC12 cells. Brain Res. 2010;1328:1–11. doi: 10.1016/j.brainres.2010.02.083. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Pisani A, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptors and striatal synaptic plasticity: implications for neurological diseases. Prog Neurobiol. 2004;74(5):271–300. doi: 10.1016/j.pneurobio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Guetg N, Abdel Aziz S, Holbro N, Turecek R, Rose T, Seddik R, Gassmann M, Moes S, Jenoe P, Oertner TG, Casanova E, Bettler B. NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1. Proc Natl Acad Sci. 2010;107(31):13924–13929. doi: 10.1073/pnas.1000909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás B, Pavlova E, Kása P, Gulya K, Bakota L. Activated MAO-B in the brain of Alzheimer patients, demonstrated by [11C]-L-deprenyl using whole hemisphere autoradiography. Neurochem Int. 2011;58(1):60–68. doi: 10.1016/j.neuint.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Standaert DG. Rationale for and use of NMDA receptor antagonists in Parkinson’s disease. Pharmacol Ther. 2004;102:155–174. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW. Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. J Neurosci. 2006;26(17):4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harré EM, Galic MA, Mouihate A, Noorbakhsh F, Pittman QJ. Neonatal inflammation produces selective behavioural deficits and alters N-methyl-D-aspartate receptor subunit mRNA in the adult rat brain. Eur J Neurosci. 2008;27(3):644–653. doi: 10.1111/j.1460-9568.2008.06031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY, Hermanowicz N. Temporal processing in the basal ganglia. Neuropsychology. 1998;12(1):3–12. doi: 10.1037//0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- Hasselbalch SG, Madsen K, Svarer C, Pinborg LH, Holm S, Paulson OB, Waldemar G, Knudsen GM. Reduced 5-HT2A receptor binding in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1830–1838. doi: 10.1016/j.neurobiolaging.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Kayed R, Zheng H, Sweatt JD, Dineley KT. Loss of alpha7 nicotinic receptors enhances beta-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2010;30:2442–2453. doi: 10.1523/JNEUROSCI.5038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst WD, Stean TO, Rogers DC, Sunter D, Pugh P, Moss SF, Bromidge SM, Riley G, Smith DR, Bartlett S, Heidbreder CA, Atkins AR, Lacroix LP, Dawson LA, Foley AG, Regan CM, Upton N. SB-399885 is a potent, selective 5-HT6 receptor antagonist with cognitive enhancing properties in aged rat water maze and novel object recognition models. Eur J Pharmacol. 2006;553:109–119. doi: 10.1016/j.ejphar.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Martin G. 5-HT receptor classification and nomenclature: towards a harmonization with the human genome. Neuropharmacology. 1997;36(4–5):419–428. doi: 10.1016/s0028-3908(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Hu M, Liu QS, Chang KT, Berg DK. Nicotinic regulation of CREB activation in hippocampal neurons by glutamatergic and nonglutamatergic pathways. Mol Cell Neurosci. 2002;21:616–625. doi: 10.1006/mcne.2002.1202. [DOI] [PubMed] [Google Scholar]
- Huot P, Fox SH, Brotchie JM. The serotonergic system in Parkinson’s disease. Prog Neurobiol. 2011;95(2):163–212. doi: 10.1016/j.pneurobio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Hurley MJ, Jenner P. What has been learnt from study of dopamine receptors in Parkinson’s disease? Pharmacol Ther. 2006;111:715–728. doi: 10.1016/j.pharmthera.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR. Differential expression of N-methyl-D-aspartate receptor NR2 isoforms in Alzheimer’s disease. J Neurochem. 2004;90:913–919. doi: 10.1111/j.1471-4159.2004.02548.x. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Mizukami K, Warde D, Sheffield R, Hamilton R, Wenthold RJ, Armstrong DM. Distribution of glutamate receptor subunit NMDAR1 in the hippocampus of normal elderly and patients with Alzheimer’s disease. Exp Neurol. 1999;160:194–204. doi: 10.1006/exnr.1999.7196. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Storm DR. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999;23:11–14. doi: 10.1016/s0896-6273(00)80747-3. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Wagner HN, Jr, Wong DF, Links JM, Frost JJ, Dannals RF, Rosenbaum AE, Takeda K, Di Chiro G, Kuhar MJ. Atlas of dopamine receptor images (PET) of the human brain. J Comput Assist Tomogr. 1985;9:129–140. doi: 10.1097/00004728-198501000-00024. [DOI] [PubMed] [Google Scholar]
- Janssen WG, Vissavajjhala P, Andrews G, Moran T, Hof PR, Morrison JH. Cellular and synaptic distribution of NR2A and NR2B in macaque monkey and rat hippocampus as visualized with subunit-specific monoclonal antibodies. Exp Neurol. 2005;191(Suppl 1):S28–44. doi: 10.1016/j.expneurol.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Shekhar A. Panic-prone state induced in rats with GABA dysfunction in the dorsomedial hypothalamus is mediated by NMDA receptors. J Neurosci. 2006;26(26):7093–7104. doi: 10.1523/JNEUROSCI.0408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, et al. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci. 2008;28(41):10422–10433. doi: 10.1523/JNEUROSCI.1850-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Aalto S, KNA, Hietala J, Sonninen P, Rinne JO. Extrastriatal dopamine D(2) receptors in Parkinson’s disease: a longitudinal study. J Neural Transm. 2003;110:591–601. doi: 10.1007/s00702-003-0816-x. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nagren K, Hietala J, Oikonen V, Vilkman H, Farde L, Halldin C, Rinne JO. Extrastriatal dopamine D2 and D3 receptors in early and advanced Parkinson’s disease. Neurology. 2000;54:1482–1487. doi: 10.1212/wnl.54.7.1482. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Harik SI. Increased alpha 2- and beta 2-adrenergic receptors in cerebral microvessels in Alzheimer disease. Neurosci Lett. 1989;106:233–238. doi: 10.1016/0304-3940(89)90231-0. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Andorn AC. Adrenergic receptors in aging and Alzheimer’s disease: decreased alpha 2-receptors demonstrated by [3H]p-aminoclonidine binding in prefrontal cortex. Neurobiol Aging. 1991;12:131–136. doi: 10.1016/0197-4580(91)90051-k. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Andorn AC, Harik SI. Alterations in adrenergic receptors of frontal cortex and cerebral microvessels in Alzheimer’s disease and aging. Prog Clin Biol Res. 1989a;317:367–374. [PubMed] [Google Scholar]
- Kalaria RN, Andorn AC, Tabaton M, Whitehouse PJ, Harik SI, Unnerstall JR. Adrenergic receptors in aging and Alzheimer’s disease: increased beta 2-receptors in prefrontal cortex and hippocampus. J Neurochem. 1989b;53:1772–1781. doi: 10.1111/j.1471-4159.1989.tb09242.x. [DOI] [PubMed] [Google Scholar]
- Kato G, Agid Y. Acetylcholine receptors (author’s transl) Nouv Presse Med. 1979;8:2407–2411. [PubMed] [Google Scholar]
- Kemppainen N, Ruottinen H, Nagren K, Rinne JO. PET shows that striatal dopamine D1 and D2 receptors are differentially affected in AD. Neurology. 2000;55:205–209. doi: 10.1212/wnl.55.2.205. [DOI] [PubMed] [Google Scholar]
- Kemppainen N, Laine M, Laakso MP, Kaasinen V, Nagren K, Vahlberg T, Kurki T, Rinne JO. Hippocampal dopamine D2 receptors correlate with memory functions in Alzheimer’s disease. Eur J Neurosci. 2003;18:149–154. doi: 10.1046/j.1460-9568.2003.02716.x. [DOI] [PubMed] [Google Scholar]
- Knudsen GM, Karlsborg M, Thomsen G, Krabbe K, Regeur L, Nygaard T, Videbaek C, Werdelin L. Imaging of dopamine transporters and D2 receptors in patients with Parkinson’s disease and multiple system atrophy. Eur J Nucl Med Mol Imaging. 2004;31:1631–1638. doi: 10.1007/s00259-004-1578-x. [DOI] [PubMed] [Google Scholar]
- Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, Nairn AC, Lombroso PJ. Abeta-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30:5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MK, Tsang SW, Francis PT, Esiri MM, Keene J, Hope T, Chen CP. Reduced serotonin 5-HT1A receptor binding in the temporal cortex correlates with aggressive behavior in Alzheimer disease. Brain Res. 2003a;974:82–87. doi: 10.1016/s0006-8993(03)02554-x. [DOI] [PubMed] [Google Scholar]
- Lai MK, Tsang SW, Francis PT, Keene J, Hope T, Esiri MM, Spence I, Chen CP. Postmortem serotoninergic correlates of cognitive decline in Alzheimer’s disease. Neuroreport. 2002;13:1175–1178. doi: 10.1097/00001756-200207020-00021. [DOI] [PubMed] [Google Scholar]
- Lai MK, Tsang SW, Francis PT, Esiri MM, Hope T, Lai OF, Spence I, Chen CP. [3H]GR113808 binding to serotonin 5-HT(4) receptors in the postmortem neocortex of Alzheimer disease: a clinicopathological study. J Neural Transm. 2003b;110:779–788. doi: 10.1007/s00702-003-0825-9. [DOI] [PubMed] [Google Scholar]
- Lai MK, Tsang SW, Alder JT, Keene J, Hope T, Esiri MM, Francis PT, Chen CP. Loss of serotonin 5-HT2A receptors in the postmortem temporal cortex correlates with rate of cognitive decline in Alzheimer’s disease. Psychopharmacology (Berl) 2005;179:673–677. doi: 10.1007/s00213-004-2077-2. [DOI] [PubMed] [Google Scholar]
- Laureys G, Clinckers R, Gerlo S, Spooren A, Wilczak N, Kooijman R, Smolders I, Michotte Y, De Keyser J. Astrocytic beta(2)-adrenergic receptors: from physiology to pathology. Prog Neurobiol. 2010;91(3):189–199. doi: 10.1016/j.pneurobio.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang XF, Deng C, Meyer B, Wu A, Yu Y, Ying W, Yang GY, Yenari MA, Wang Q. Alterations in 5-HT2A receptor binding in various brain regions among 6-hydroxydopamine-induced Parkinsonian rats. Synapse. 2010;64(3):224–230. doi: 10.1002/syn.20722. [DOI] [PubMed] [Google Scholar]
- Lilja AM, Porras O, Storelli E, Nordberg A, Marutle A. Functional interactions of fibrillar and oligomeric amyloid-beta with alpha7 nicotinic receptors in Alzheimer’s disease. J Alzheimers Dis. 2011;23:335–347. doi: 10.3233/JAD-2010-101242. [DOI] [PubMed] [Google Scholar]
- Liu HP, Lin WY, Liu SH, Wang WF, Tsai CH, Wu BT, Wang CK, Tsai FJ. Genetic variation in N-methyl-D-aspartate receptor subunit NR3A but not NR3B influences susceptibility to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2009;28:521–527. doi: 10.1159/000254757. [DOI] [PubMed] [Google Scholar]
- Marcos B, Chuang TT, Gil-Bea FJ, Ramirez MJ. Effects of 5-HT6 receptor antagonism and cholinesterase inhibition in models of cognitive impairment in the rat. Br J Pharmacol. 2008;155:434–440. doi: 10.1038/bjp.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PM, Roytta M, Lonnberg P, Marjamaki P, Helenius H, Rinne JO. Choline acetytransferase activity and striatal dopamine receptors in Parkinson’s disease in relation to cognitive impairment. Acta Neuropathol. 2001;102:160–166. doi: 10.1007/s004010100372. [DOI] [PubMed] [Google Scholar]
- Meana JJ, Barturen F, Garro MA, Garcia-Sevilla JA, Fontan A, Zarranz JJ. Decreased density of presynaptic alpha 2-adrenoceptors in postmortem brains of patients with Alzheimer’s disease. J Neurochem. 1992;58:1896–1904. doi: 10.1111/j.1471-4159.1992.tb10067.x. [DOI] [PubMed] [Google Scholar]
- Medeiros R, Kitazawa M, Caccamo A, Baglietto-Vargas D, Estrada-Hernandez T, Cribbs DH, Fisher A, Laferla FM. Loss of Muscarinic M(1) Receptor Exacerbates Alzheimer’s Disease-Like Pathology and Cognitive Decline. Am J Pathol. 2011 doi: 10.1016/j.ajpath.2011.04.041. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Sahakian BJ, McKenna PJ, Robbins TW. Systemic sulpiride in young adult volunteers simulates the profile of cognitive deficits in Parkinson’s disease. Psychopharmacology (Berl) 1999;146:162–174. doi: 10.1007/s002130051102. [DOI] [PubMed] [Google Scholar]
- Meneses A, Hong E. 5-HT1A receptors modulate the consolidation of learning in normal and cognitively impaired rats. Neurobiol Learn Mem. 1999;71:207–218. doi: 10.1006/nlme.1998.3866. [DOI] [PubMed] [Google Scholar]
- Meoni P, Bunnemann BH, Kingsbury AE, Trist DG, Bowery NG. NMDA NR1 subunit mRNA and glutamate NMDA-sensitive binding are differentially affected in the striatum and pre-frontal cortex of Parkinson’s disease patients. Neuropharmacology. 1999;38:625–633. doi: 10.1016/s0028-3908(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Meyer PM, Strecker K, Kendziorra K, Becker G, Hesse S, Woelpl D, Hensel A, Patt M, Sorger D, Wegner F, Lobsien D, Barthel H, Brust P, Gertz HJ, Sabri O, Schwarz J. Reduced alpha4beta2*-nicotinic acetylcholine receptor binding and its relationship to mild cognitive and depressive symptoms in Parkinson disease. Arch Gen Psychiatry. 2009;66:866–877. doi: 10.1001/archgenpsychiatry.2009.106. [DOI] [PubMed] [Google Scholar]
- Miranda S, Opazo C, Larrondo LF, Muñoz FJ, Ruiz F, Leighton F, Inestrosa NC. The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer’s disease. Prog Neurobiol. 2000;62(6):633–648. doi: 10.1016/s0301-0082(00)00015-0. [DOI] [PubMed] [Google Scholar]
- Mishizen-Eberz AJ, Rissman RA, Carter TL, Ikonomovic MD, Wolfe BB, Armstrong DM. Biochemical and molecular studies of NMDA receptor subunits NR1/2A/2B in hippocampal subregions throughout progression of Alzheimer’s disease pathology. Neurobiol Dis. 2004;15:80–92. doi: 10.1016/j.nbd.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Neumaier JF. 5-HT6 receptors: a novel target for cognitive enhancement. Pharmacol Ther. 2005;108:320–333. doi: 10.1016/j.pharmthera.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Mitsis EM, Reech KM, Bois F, Tamagnan GD, Macavoy MG, Seibyl JP, Staley JK, van Dyck CH. 123I-5-IA-85380 SPECT imaging of nicotinic receptors in Alzheimer disease and mild cognitive impairment. J Nucl Med. 2009;50:1455–1463. doi: 10.2967/jnumed.109.064030. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Stevens TR, King SL, Caldarone BJ, Ibanez-Tallon I, et al. The prototoxin lynx1 acts on nicotinic acetylcholine receptors to balance neuronal activity and survival in vivo. Neuron. 2006;51(5):587–600. doi: 10.1016/j.neuron.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Mizukawa K, McGeer EG, McGeer PL. Autoradiographic study on dopamine uptake sites and their correlation with dopamine levels and their striata from patients with Parkinson disease, Alzheimer disease, and neurologically normal controls. Mol Chem Neuropathol. 1993;18:133–144. doi: 10.1007/BF03160027. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Kato T, Arahata Y, Washimi Y, Nakamura A, Abe Y, Yamada T, Iwai K, Hatano K, Kawasumi Y, Kachi T, Dagher A, Ito K. Cognitive- and motor-related regions in Parkinson’s disease: FDOPA and FDG PET studies. Neuroimage. 2004;22(2):553–561. doi: 10.1016/j.neuroimage.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Nair VD, Sealfon SC. Agonist-specific transactivation of phosphoinositide 3-kinase signaling pathway mediated by the dopamine D2 receptor. J Biol Chem. 2003;278:47053–47061. doi: 10.1074/jbc.M303364200. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Nilsson A, Eriksson M, Muly EC, Akesson E, Samuelsson EB, Bogdanovic N, Benedikz E, Sundström E. Analysis of NR3A receptor subunits in human native NMDA receptors. Brain Res. 2007;1186:102–112. doi: 10.1016/j.brainres.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Oishi N, Hashikawa K, Yoshida H, Ishizu K, Ueda M, Kawashima H, Saji H, Fukuyama H. Quantification of nicotinic acetylcholine receptors in Parkinson’s disease with (123)I-5IA SPECT. J Neurol Sci. 2007;256:52–60. doi: 10.1016/j.jns.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Otani S, Auclair N, Desce JM, Roisin MP, Crepel F. Dopamine receptors and groups I and II mGluRs cooperate for long-term depression induction in rat prefrontal cortex through converging postsynaptic activation of MAP kinases. J Neurosci. 1999;19:9788–9802. doi: 10.1523/JNEUROSCI.19-22-09788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panegyres PK, Zafiris-Toufexis K, Kakulas BA. The mRNA of the NR1 subtype of glutamate receptor in Alzheimer’s disease. J Neural Transm. 2002;109:77–89. doi: 10.1007/s702-002-8238-9. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Pascual J, Grijalba B, Garcia-Sevilla JA, Zarranz JJ, Pazos A. Loss of high-affinity alpha 2-adrenoceptors in Alzheimer’s disease: an autoradiographic study in frontal cortex and hippocampus. Neurosci Lett. 1992;142:36–40. doi: 10.1016/0304-3940(92)90614-d. [DOI] [PubMed] [Google Scholar]
- Pascual M, Baliño P, Alfonso-Loeches S, Aragón CM, Guerri C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun. 2011;25(Suppl 1):S80–91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61(1):75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Perez-García G, Meneses A. Oral administration of the 5-HT6 receptor antagonists SB-357134 and SB-399885 improves memory formation in an autoshaping learning task. Pharmacol Biochem Behav. 2005;81(3):673–682. doi: 10.1016/j.pbb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Péron J, Vicente S, Leray E, Drapier S, Drapier D, Cohen R, Biseul I, Rouaud T, Le Jeune F, Sauleau P, Vérin M. Are dopaminergic pathways involved in theory of mind? A study in Parkinson’s disease. Neuropsychologia. 2009;47(2):406–414. doi: 10.1016/j.neuropsychologia.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH, Smith CJ, Purohit D, Bonham J, Dick DJ, Candy JM, Edwardson JA, Fairbairn A. Cholinergic receptors in cognitive disorders. Can J Neurol Sci. 1986;13:521–527. doi: 10.1017/s0317167100037240. [DOI] [PubMed] [Google Scholar]
- Piccini P, Pavese N, Brooks DJ. Endogenous dopamine release after pharmacological challenges in Parkinson’s disease. Ann Neurol. 2003;53(5):647–653. doi: 10.1002/ana.10526. [DOI] [PubMed] [Google Scholar]
- Piggott MA, Ballard CG, Dickinson HO, McKeith IG, Perry RH, Perry EK. Thalamic D2 receptors in dementia with Lewy bodies, Parkinson’s disease, and Parkinson’s disease dementia. Int J Neuropsychopharmacol. 2007;10:231–244. doi: 10.1017/S146114570600647X. [DOI] [PubMed] [Google Scholar]
- Piggott MA, Marshall EF, Thomas N, Lloyd S, Court JA, Jaros E, Burn D, Johnson M, Perry RH, McKeith IG, Ballard C, Perry EK. Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer’s and Parkinson’s diseases: rostrocaudal distribution. Brain. 1999;122 ( Pt 8):1449–1468. doi: 10.1093/brain/122.8.1449. [DOI] [PubMed] [Google Scholar]
- Pizzolato G, Chierichetti F, Fabbri M, Cagnin A, Dam M, Ferlin G, Battistin L. Reduced striatal dopamine receptors in Alzheimer’s disease: single photon emission tomography study with the D2 tracer [123I]-IBZM. Neurology. 1996;47:1065–1068. doi: 10.1212/wnl.47.4.1065. [DOI] [PubMed] [Google Scholar]
- Politis M, Piccini P, Pavese N, Koh SB, Brooks DJ. Evidence of dopamine dysfunction in the hypothalamus of patients with Parkinson’s disease: an in vivo 11C-raclopride PET study. Exp Neurol. 2008;214:112–116. doi: 10.1016/j.expneurol.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Polter AM, Li X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cell Signal. 2010;22(10):1406–1412. doi: 10.1016/j.cellsig.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin B, Butcher A, McWilliams P, Bourgognon JM, Pawlak R, et al. The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc Natl Acad Sci U S A. 2010;107(20):9440–9445. doi: 10.1073/pnas.0914801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong E, Magistretti P, Stoop R. Cellular perspectives on the glutamate-monoamine interactions in limbic lobe structures and their relevance for some psychiatric disorders. Prog Neurobiol. 2002;67(3):173–202. doi: 10.1016/s0301-0082(02)00017-5. [DOI] [PubMed] [Google Scholar]
- Proctor DT, Coulson EJ, Dodd PR. Post-synaptic scaffolding protein interactions with glutamate receptors in synaptic dysfunction and Alzheimer’s disease. Prog Neurobiol. 2011;93(4):509–521. doi: 10.1016/j.pneurobio.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Reeves S, Brown R, Howard R, Grasby P. Increased striatal dopamine (D2/D3) receptor availability and delusions in Alzheimer disease. Neurology. 2009;72:528–534. doi: 10.1212/01.wnl.0000341932.21961.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves S, Mehta M, Howard R, Grasby P, Brown R. The dopaminergic basis of cognitive and motor performance in Alzheimer’s disease. Neurobiol Dis. 2010;37:477–482. doi: 10.1016/j.nbd.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Reeves SJ, Grasby PM, Howard RJ, Bantick RA, Asselin MC, Mehta MA. A positron emission tomography (PET) investigation of the role of striatal dopamine (D2) receptor availability in spatial cognition. Neuroimage. 2005;28:216–226. doi: 10.1016/j.neuroimage.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Rektorová I. Effects of dopamine agonists on neuropsychiatric symptoms of Parkinson’s disease. Neurodegener Dis. 2010;7(1–3):206–209. doi: 10.1159/000295665. [DOI] [PubMed] [Google Scholar]
- Rektorová I, Rektor I, Bares M, Dostál V, Ehler E, Fanfrdlová Z, Fiedler J, Klajblová H, Kulist’ák P, Ressner P, Svátová J, Urbánek K, Velísková J. Cognitive performance in people with Parkinson’s disease and mild or moderate depression: effects of dopamine agonists in an add-on to L-dopa therapy. Eur J Neurol. 2005;12(1):9–15. doi: 10.1111/j.1468-1331.2004.00966.x. [DOI] [PubMed] [Google Scholar]
- Rieckmann A, Karlsson S, Karlsson P, Brehmer Y, Fischer H, Farde L, Nyberg L, Bäckman L. Dopamine D1 receptor associations within and between dopaminergic pathways in younger and elderly adults: links to cognitive performance. Cereb Cortex. 2011;21(9):2023–2032. doi: 10.1093/cercor/bhq266. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Myllykyla T, Lonnberg P, Marjamaki P. A postmortem study of brain nicotinic receptors in Parkinson’s and Alzheimer’s disease. Brain Res. 1991;547:167–170. doi: 10.1016/0006-8993(91)90588-m. [DOI] [PubMed] [Google Scholar]
- Roncarati R, Scali C, Comery TA, Grauer SM, Aschmi S, Bothmann H, Jow B, Kowal D, Gianfriddo M, Kelley C, Zanelli U, Ghiron C, Haydar S, Dunlop J, Terstappen GC. Procognitive and neuroprotective activity of a novel alpha7 nicotinic acetylcholine receptor agonist for treatment of neurodegenerative and cognitive disorders. J Pharmacol Exp Ther. 2009;329:459–468. doi: 10.1124/jpet.108.150094. [DOI] [PubMed] [Google Scholar]
- Rosse G, Schaffhauser H. 5-HT6 receptor antagonists as potential therapeutics for cognitive impairment. Curr Top Med Chem. 2010;10:207–221. doi: 10.2174/156802610790411036. [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R, Schwartz JC. A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun. 1993;193:268–276. doi: 10.1006/bbrc.1993.1619. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Cotman CW. Adrenergic receptors in Alzheimer’s disease brain: selective increases in the cerebella of aggressive patients. J Neurosci. 1997;17:5573–5580. doi: 10.1523/JNEUROSCI.17-14-05573.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh MN, Shah F, Reid RT, Sue L, Connor DJ, Peterson LK, Beach TG. Pathologic and nicotinic receptor binding differences between mild cognitive impairment, Alzheimer disease, and normal aging. Arch Neurol. 2006;63:1771–1776. doi: 10.1001/archneur.63.12.1771. [DOI] [PubMed] [Google Scholar]
- Sabri O, Kendziorra K, Wolf H, Gertz HJ, Brust P. Acetylcholine receptors in dementia and mild cognitive impairment. Eur J Nucl Med Mol Imaging. 2008;35(Suppl 1):S30–45. doi: 10.1007/s00259-007-0701-1. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T. The role of D1-dopamine receptors in working memory-guided movements mediated by frontal cortical areas. Parkinsonism Relat Disord. 2000;7(1):9–19. doi: 10.1016/s1353-8020(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain. 2008;131:1294–1302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- Schechter LE, Lin Q, Smith DL, Zhang G, Shan Q, Platt B, Brandt MR, Dawson LA, Cole D, Bernotas R, Robichaud A, Rosenzweig-Lipson S, Beyer CE. Neuropharmacological profile of novel and selective 5-HT6 receptor agonists: WAY-181187 and WAY-208466. Neuropsychopharmacology. 2008;33:1323–1335. doi: 10.1038/sj.npp.1301503. [DOI] [PubMed] [Google Scholar]
- Sharp SI, Ballard CG, Chen CP, Francis PT. Aggressive behavior and neuroleptic medication are associated with increased number of alpha1-adrenoceptors in patients with Alzheimer disease. Am J Geriatr Psychiatry. 2007;15:435–437. doi: 10.1097/01.JGP.0000237065.78966.1b. [DOI] [PubMed] [Google Scholar]
- Shen JX, Yakel JL. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin. 2009;30:673–680. doi: 10.1038/aps.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimohama S, Taniguchi T, Fujiwara M, Kameyama M. Biochemical characterization of alpha-adrenergic receptors in human brain and changes in Alzheimer-type dementia. J Neurochem. 1986;47:1295–1301. [PubMed] [Google Scholar]
- Shimohama S, Taniguchi T, Fujiwara M, Kameyama M. Changes in beta-adrenergic receptor subtypes in Alzheimer-type dementia. J Neurochem. 1987;48:1215–1221. doi: 10.1111/j.1471-4159.1987.tb05649.x. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Soliakov L, Wonnacott S. Involvement of protein kinase C in the presynaptic nicotinic modulation of [(3)H]-dopamine release from rat striatal synaptosomes. Br J Pharmacol. 2001;132:785–791. doi: 10.1038/sj.bjp.0703873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Hua X, Morra JH, Lee S, Hibar DP, Ho AJ, Leow AD, Toga AW, Sul JH, Kang HM, Eskin E, Saykin AJ, Shen L, Foroud T, Pankratz N, Huentelman MJ, Craig DW, Gerber JD, Allen AN, Corneveaux JJ, Stephan DA, Webster J, DeChairo BM, Potkin SG, Jack CR, Jr, Weiner MW, Thompson PM. Genome-wide analysis reveals novel genes influencing temporal lobe structure with relevance to neurodegeneration in Alzheimer’s disease. Neuroimage. 2010;51:542–554. doi: 10.1016/j.neuroimage.2010.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi T, Park S, Jayathilake K, Roy A, Ertugrul A, Meltzer HY. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2007;95(1–3):158–168. doi: 10.1016/j.schres.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Sun H, Zhang J, Zhang L, Liu H, Zhu H, Yang Y. Environmental enrichment influences BDNF and NR1 levels in the hippocampus and restores cognitive impairment in chronic cerebral hypoperfused rats. Curr Neurovasc Res. 2010a;7:268–280. doi: 10.2174/156720210793180819. [DOI] [PubMed] [Google Scholar]
- Sun W, Sugiyama K, Fang X, Yamaguchi H, Akamine S, Magata Y, Namba H. Different striatal D2-like receptor function in an early stage after unilateral striatal lesion and medial forebrain bundle lesion in rats. Brain Res. 2010b;1317:227–235. doi: 10.1016/j.brainres.2009.12.048. [DOI] [PubMed] [Google Scholar]
- Sun W, Sugiyama K, Asakawa T, Yamaguchi H, Akamine S, Ouchi Y, Magata Y, Namba H. Dynamic changes of striatal dopamine D2 receptor binding at later stages after unilateral lesions of the medial forebrain bundle in Parkinsonian rat models. Neurosci Lett. 2011;496(3):157–162. doi: 10.1016/j.neulet.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci. 2006;26:467–478. doi: 10.1523/JNEUROSCI.4265-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Meguro K, Yamaguchi S, Ishii H, Watanuki S, Funaki Y, Yamaguchi K, Yamadori A, Iwata R, Itoh M. Decreased striatal D2 receptor density associated with severe behavioral abnormality in Alzheimer’s disease. Ann Nucl Med. 2003;17:567–573. doi: 10.1007/BF03006670. [DOI] [PubMed] [Google Scholar]
- Truchot L, Costes N, Zimmer L, Laurent B, Le Bars D, Thomas-Anterion C, Mercier B, Hermier M, Vighetto A, Krolak-Salmon P. A distinct [18F]MPPF PET profile in amnestic mild cognitive impairment compared to mild Alzheimer’s disease. Neuroimage. 2008;40:1251–1256. doi: 10.1016/j.neuroimage.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Tsang SW, Vinters HV, Cummings JL, Wong PT, Chen CP, Lai MK. Alterations in NMDA receptor subunit densities and ligand binding to glycine recognition sites are associated with chronic anxiety in Alzheimer’s disease. Neurobiol Aging. 2008;29:1524–1532. doi: 10.1016/j.neurobiolaging.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang SW, Lai MK, Kirvell S, Francis PT, Esiri MM, Hope T, Chen CP, Wong PT. Impaired coupling of muscarinic M1 receptors to G-proteins in the neocortex is associated with severity of dementia in Alzheimer’s disease. Neurobiol Aging. 2006;27:1216–1223. doi: 10.1016/j.neurobiolaging.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Turle-Lorenzo N, Maurin B, Puma C, Chezaubernard C, Morain P, Baunez C, Nieoullon A, Amalric M. The dopamine agonist piribedil with L-DOPA improves attentional dysfunction: relevance for Parkinson’s disease. J Pharmacol Exp Ther. 2006;319(2):914–923. doi: 10.1124/jpet.106.109207. [DOI] [PubMed] [Google Scholar]
- Turner TJ. Nicotine enhancement of dopamine release by a calcium-dependent increase in the size of the readily releasable pool of synaptic vesicles. J Neurosci. 2004;24(50):11328–11336. doi: 10.1523/JNEUROSCI.1559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulas J, Brunner LC, Geddes JW, Choe W, Cotman CW. N-methyl-D-aspartate receptor complex in the hippocampus of elderly, normal individuals and those with Alzheimer’s disease. Neuroscience. 1992;49:45–61. doi: 10.1016/0306-4522(92)90075-d. [DOI] [PubMed] [Google Scholar]
- Upton N, Chuang TT, Hunter AJ, Virley DJ. 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer’s disease. Neurotherapeutics. 2008;5:458–469. doi: 10.1016/j.nurt.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstappen CC, Bloem BR, Haaxma CA, Oyen WJ, Horstink MW. Diagnostic value of asymmetric striatal D2 receptor upregulation in Parkinson’s disease: an [123I]IBZM and [123I]FP-CIT SPECT study. Eur J Nucl Med Mol Imaging. 2007;34:502–507. doi: 10.1007/s00259-006-0258-4. [DOI] [PubMed] [Google Scholar]
- Visanji NP, Fox SH, Johnston TH, Millan MJ, Brotchie JM. Alpha1-adrenoceptors mediate dihydroxyphenylalanine-induced activity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaques. J Pharmacol Exp Ther. 2009;328(1):276–283. doi: 10.1124/jpet.108.144097. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang PH, McLachlan C, Wong PT. Simvastatin reverses the downregulation of dopamine D1 and D2 receptor expression in the prefrontal cortex of 6-hydroxydopamine-induced Parkinsonian rats. Brain Res. 2005a;1045:229–233. doi: 10.1016/j.brainres.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ting WL, Yang H, Wong PT. High doses of simvastatin upregulate dopamine D1 and D2 receptor expression in the rat prefrontal cortex: possible involvement of endothelial nitric oxide synthase. Br J Pharmacol. 2005b;144(7):933–939. doi: 10.1038/sj.bjp.0706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zengin A, Ying W, Newell KA, Wang P, Yeo W, Wong PT, Yenari MA, Huang XF. Chronic treatment with simvastatin upregulates muscarinic M1/4 receptor binding in the rat brain. Neuroscience. 2008;154(3):1100–1106. doi: 10.1016/j.neuroscience.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zengin A, Deng C, Li Y, Newell KA, Yang GY, Lu Y, Wilder-Smith EP, Zhao H, Huang XF. High dose of simvastatin induces hyperlocomotive and anxiolytic-like activities: The association with the up-regulation of NMDA receptor binding in the rat brain. Exp Neurol. 2009;216(1):132–138. doi: 10.1016/j.expneurol.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Lallemand F, de Witte P, Dexter DT. Neurochemical pathways involved in the protective effects of nicotine and ethanol in preventing the development of Parkinson’s disease: potential targets for the development of new therapeutic agents. Prog Neurobiol. 2008;85(2):135–147. doi: 10.1016/j.pneurobio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- West PJ, Marcy VR, Marino MJ, Schaffhauser H. Activation of the 5-HT(6) receptor attenuates long-term potentiation and facilitates GABAergic neurotransmission in rat hippocampus. Neuroscience. 2009;164:692–701. doi: 10.1016/j.neuroscience.2009.07.061. [DOI] [PubMed] [Google Scholar]
- Wevers A, Schroder H. Nicotinic acetylcholine receptors in Alzheimer’s disease. J Alzheimers Dis. 1999;1:207–219. doi: 10.3233/jad-1999-14-503. [DOI] [PubMed] [Google Scholar]
- Wullner U, Testa CM, Catania MV, Young AB, Penney JB., Jr Glutamate receptors in striatum and substantia nigra: effects of medial forebrain bundle lesions. Brain Res. 1994;645:98–102. doi: 10.1016/0006-8993(94)91642-x. [DOI] [PubMed] [Google Scholar]
- Xiong H, McCabe L, Costello J, Anderson E, Weber G, Ikezu T. Activation of NR1a/NR2B receptors by soluble factors from APP-stimulated monocyte-derived macrophages: implications for the pathogenesis of Alzheimer’s disease. Neurobiol Aging. 2004;25:905–911. doi: 10.1016/j.neurobiolaging.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Yan J, Xu Y, Zhu C, Zhang L, Wu A, Yang Y, Xiong Z, Deng C, Huang XF, Yenari MA, Yang YG, Ying W, Wang Q. Simvastatin prevents dopaminergic neurodegeneration in experimental parkinsonian models: the association with anti-inflammatory responses. PLoS One. 2011;6(6):e20945. doi: 10.1371/journal.pone.0020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno F, Suhara T, Nakayama T, Ichimiya T, Okubo Y, Takano A, Ando T, Inoue M, Maeda J, Suzuki K. Inhibitory effect of hippocampal 5-HT1A receptors on human explicit memory. Am J Psychiatry. 2003;160:334–340. doi: 10.1176/appi.ajp.160.2.334. [DOI] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, Finlayson K, Sharkey J. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007;17:145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Yu JT, Tan L, Ou JR, Zhu JX, Liu K, Song JH, Sun YP. Polymorphisms at the beta2-adrenergic receptor gene influence Alzheimer’s disease susceptibility. Brain Res. 2008;1210:216–222. doi: 10.1016/j.brainres.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Yu SP, Sensi SL, Canzoniero LM, Buisson A, Choi DW. Membrane-delimited modulation of NMDA currents by metabotropic glutamate receptor subtypes 1/5 in cultured mouse cortical neurons. J Physiol. 1997;499 (Pt 3):721–732. doi: 10.1113/jphysiol.1997.sp021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SP, Yeh C, Strasser U, Tian M, Choi DW. NMDA receptor-mediated K+ efflux and neuronal apoptosis. Science. 1999;284(5412):336–339. doi: 10.1126/science.284.5412.336. [DOI] [PubMed] [Google Scholar]
- Zhen X, Zhang J, Johnson GP, Friedman E. D(4) dopamine receptor differentially regulates Akt/nuclear factor-kappa b and extracellular signal-regulated kinase pathways in D(4)MN9D cells. Mol Pharmacol. 2001;60(4):857–864. [PubMed] [Google Scholar]
- Zhen X, Torres C, Cai G, Friedman E. Inhibition of protein tyrosine/mitogen-activated protein kinase phosphatase activity is associated with D2 dopamine receptor supersensitivity in a rat model of Parkinson’s disease. Mol Pharmacol. 2002;62:1356–1363. doi: 10.1124/mol.62.6.1356. [DOI] [PubMed] [Google Scholar]