Abstract
A carbon nanofiber (CNF) electrode array was integrated with the Wireless Instantaneous Neurotransmitter Sensor System (WINCS) for detection of dopamine using fast scan cyclic voltammetry (FSCV). Dopamine detection performance by CNF arrays was comparable to that of traditional carbon fiber microelectrodes (CFMs), demonstrating that CNF arrays can be utilized as an alternative carbon electrodes for neurochemical monitoring.
1. Introduction
Over several decades, electrochemical detection methodologies have been applied to the central nervous system (CNS), increasing both our understanding of various nervous system disorders (e.g. neurodegenerative diseases such as Parkinson’s disease) as well as possible treatment options.1, 2 Parkinson’s disease and many other debilitating neuropathologies3 are successfully treated by deep brain stimulation (DBS). Despite well-established clinical efficacy, the mechanism of DBS remains poorly understood. Several hypotheses have been proposed, including the popular hypothesis that DBS produces evoked release of neurochemicals.4, 5 The detection and monitoring of these released chemicals has the potential to improve treatment and patient outcome. The Wireless Instantaneous Neurotransmitter Concentration Sensor System (WINCS) was developed to measure the DBS-stimulated release of neurochemicals.6 Carbon Fiber Microelectrodes, (CFMs) in conjunction with WINCS, have proven successful in monitoring neurochemicals in vitro and in vivo,7–9 and have led to better understanding of the clinical efficiency of DBS.6, 10 While the small diameter of the CFM permits high spatial resolution at the electrode, the CFM is limited to recording only one localized region of the brain at a time. A sensor array, such as a carbon nanofiber (CNF) nanoelectrode array, can improve therapies like DBS by providing high spatial resolution of neurochemical release in a larger tissue area, while permitting more flexibility in the spatial design of neurochemical recording electrodes.
Carbon nanotubes (CNTs) and CNFs have shown promise as neurochemical recording electrodes due to their high sensitivity, rapid electron transfer kinetics, wide potential window, biocompatibility and manufacturing versatility.11 CNTs are made up of concentric graphitic sheets rolled into a tube while CNFs consist of closed graphitic shells along the tube axis that prevent transport of electrolytes through the CNF core.11 CNT-modified macro and mico electrodes have been used to detect dopamine with good stability and excellent reversibility in vitro12–14 and in vivo,14 but do not allow for simple multiplexing due to the nonspecific solution electrode coating processes that are commonly employed. Susbstrate-grown materials have simple multiplexing ability due to integration with standard silicon microprocessing.18 Substrate-grown CNTs have been used to detect dopamine, but are difficult to insulate and isolate from one another due to poor vertical alignment during growth.15 CNFs are grown vertically aligned on metal-coated substrates, and are easily isolated from one another and the underlying metal using silicon dioxide or parylene C, which improves the signal-to-noise ratio and decreases capacitance.16–18 Electrically isolated vertically aligned CNFs have been used to detect DNA,11, 19, 20 and their rapid electron transport has been studied using cyclic voltammetry, AC voltammetry, differential pulse voltammetry and electrochemical impedance spectroscopy.11, 16, 17
Fast scan cylic voltammetry (FSCV) is a particularly powerful technique used to monitor very rapid events such as neurochemical release.21, 22 Due to the small electrode size, both micro and nano electrodes exhibit a small double-layer capacitance which aids the rapid change in electrode potentials and eliminates distortions in the applied voltage waveform that are observed with larger electrodes.23, 24 WINCS,6 which was developed at Mayo Clinic, has been used in conjunction with a CFM to detect dopamine, glutamate, adenosine and serotonin in vitro and in vivo.10, 25–28 However, a CFM electrode allows for detection in one location only. A sensor containing an array of microelectrodes will allow for higher spatial resolution of neurochemical release. In this study, we demonstrate the use of WINCS with a patterned 3 × 3 array containing vertically aligned CNFs to detect dopamine. The successful integration of these two technologies opens the door for carbon nanofiber-based electrode arrays of novel design to be used in studies of CNS electrical and chemical activity.
2. Experimental
Electrode fabrication
Sensor devices of patterned CNF nanoelectrode arrays were prepared on a 4-inch silicon wafer. Each device consists of 3 × 3 electrode pads, 200 µm square, that contain CNFs spaced at 1-µm intervals. Detailed fabrication protocols have been described previously.18 Vertically aligned CNFs were grown from 100-nm diameter, electron-beam defined nickel catalyst spots in a DC-biased plasma-enhanced chemical vapor deposition (PECVD) reactor (BlackMagic Nanoinstruments, Cambridge UK) using ethylene feedstock (125 sccm) and ammonia etchant (444 sccm) at 700 °C, 4.7 Torr and 180 W. CNF length typically ranged from 2.5 to 3.5 µm. The CNFs were encapsulated with dual-RF PECVD-deposited silicon dioxide using O2 (6000 sccm) and tetraethylorthosilicate (2–3 mL/min) at 400° C, 3 Torr and 1000 W. The oxide was polished using a 0.5-µm alumina slurry to expose the tips of the CNFs. Finally, the silicon dioxide covering the contact pads was removed using a dilute 7:1 water:hydrofluoric acid solution (Sigma Aldrich, Saint Louis, MO).
The CFMs were assembled by aspirating a single flame-etched polyacrylonitrile-based carbon fiber (T300, Amoco Inc., Greenville, SC; 5-µm diameter) into a borosilicate glass capillary which was then pulled to a finely tapered tip by a laser pipette puller (P-2000, Sutter Instruments, CA, USA). The exposed carbon fiber was trimmed with a scalpel to a length of ~50 µm, under a microscope. The Ag/AgCl reference electrodes were prepared by chlorination in bleach a 31-AWG Teflon-insulated Ag wire (World Precision Instruments, Sarasota, FL, USA).
Microscopy characterization of electrodes
A Hitachi S-4800 field emission scanning electron microscope (SEM) (Hitachi, Pleasanton, CA), operated with 10-kV accelerating voltage and 10-µA beam current, was utilized to visualize the CNF array and CFM. CFM length and diameter and CNF diameters were measured from SEM images using ImageJ software (NIH, version 1.36b), via the freehand selection tool. A Nanoscope atomic force microscope (AFM) (Digital Instruments, Santa Barbara, CA) was used to visualize the protruding CNFs. Images were acquired using a multiwalled carbon nanotube AFM probe (CCHAR, Carbon Design Innovations, Burlingame, CA) in tapping mode by dampening the amplitude 30%. AFM height and amplitude images were collected at a 0.8-Hz scan rate and 512 × 512 pixels/frame. CNF height was measured using Gwyddion (SourceForge.net) software, using the freehand profile extraction function.
WINCS
WINCS integrates FSCV and digital telemetry to display electrochemical measurements on a personal computer in nearly real time. WINCS hardware incorporates front-end analog circuitry for FSCV, a Bluetooth transceiver and a microcontroller on a small (3.3 cm by 6.5 cm) multilayer printed circuit board (PCB), shown in Fig. 1. The PCB and a rechargeable lithium-ion battery are sealed in a polycarbonate case that can be sterilized by the Sterrad® gas plasma process. A digital-to-analog converter in the microcontroller impresses the FSCV waveform upon the electrochemical sensor, while a transimpedance amplifier converts the resulting current to a signal that is digitized by the microcontroller at up to 100,000 samples/s. The data stream is transmitted to the base station via a Bluetooth® radio link. Custom software, “WincsWare,” controls the operational parameters for FSCV, records the transmitted data stream, and displays the data in various formats, including a background-subtracted, three-dimensional color plot of sequential FSCV scans. Data are displayed nearly in real time. WincsWare can replay stored data files, which may also be ported to applications such as MATLAB®.
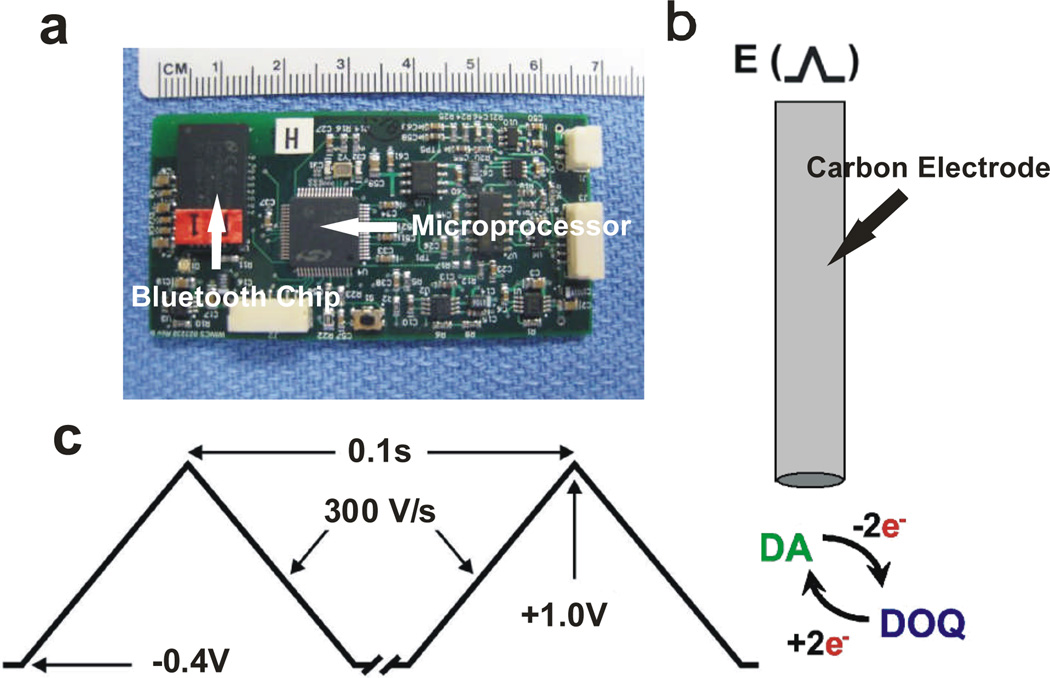
Fig. 1.
WINCS device for dopamine detection. (a) Printed circuit board indicating the FSCV microcontroller and the Bluetooth module for wireless communication with a data-acquisition computer. (b) Schematic of carbon electrode and dopamine redox reaction. (c) Applied voltage waveform for dopamine detection.
The typical FSCV waveform for dopamine detection is a pyramidal excursion (“scan”) from a baseline potential of −0.4 V to a peak of +1.0 V and back to baseline, at a “scan rate” of 300 V/s (Fig. 1c). The duration of each scan is thus approximately 9.3 ms. Voltage scans are typically repeated 10 times per second.
Dopamine detection
All electrochemical measurements were made with WINCS. A custom-designed flow cell and a FIAlab 2500 injection system (FIAlab Instruments, Seattle, WA) were utilized to introduce electroactive molecules to the electrode. Dopamine (Sigma Aldrich, Saint Louis, MO), in concentrations ranging from 500 nM to 2.5 µM, was prepared in 150-mM sodium chloride (Sigma Aldrich, Saint Louis, MO) and 12-mM Tris (Sigma Aldrich, Saint Louis, MO); the flow cell was flushed with this sodium chloride and Tris buffer for at least 30 seconds prior to neurotransmitter injection. The flow was operated at a rate of 2 mL/min for all experiments, which were performed using FSCV. The applied waveform for dopamine (Fig. 1c) was performed in a two-electrode configuration against an Ag/AgCl reference.
3. Results and Discussion
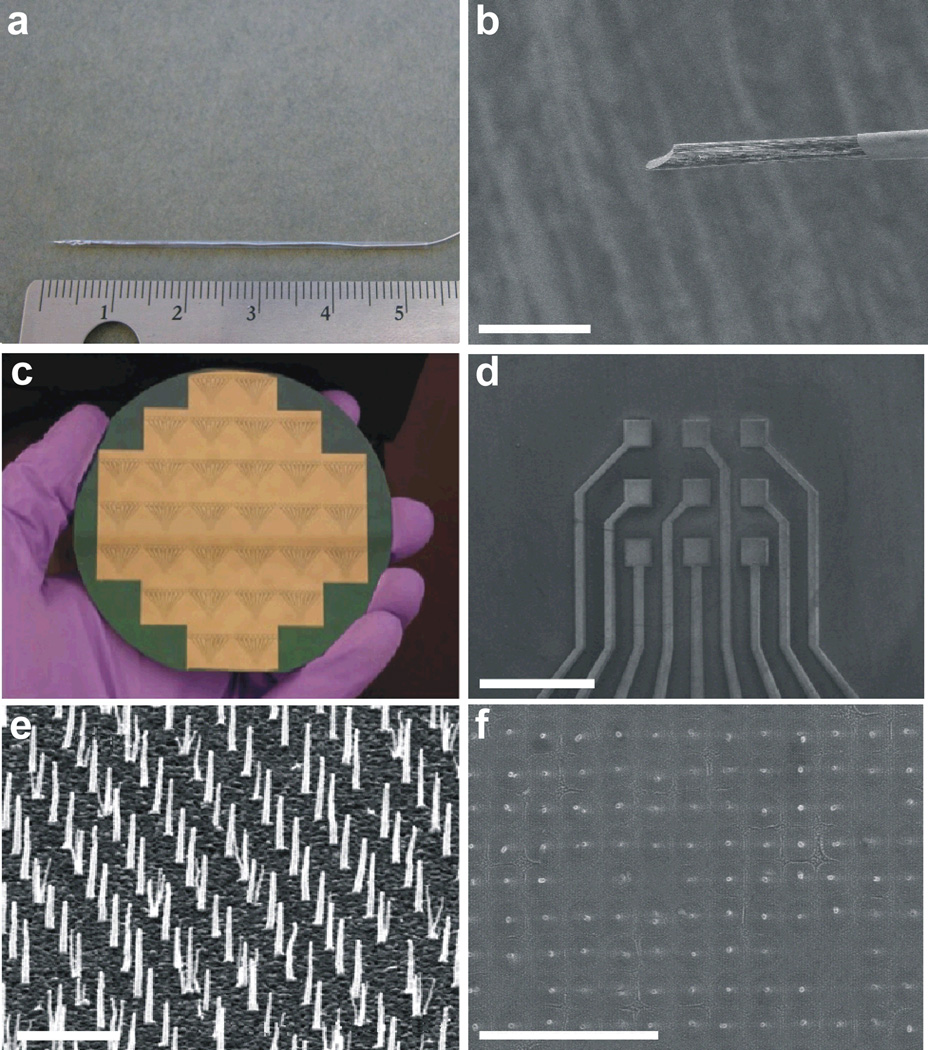
The tested CFM and CNF electrode configurations are shown in Fig. 2. CFM fabrication is a serial process in which an individual carbon fiber is insulated in a borosilicate capillary (Figs. 2a and 2b). The exposed tip of the carbon fiber acts as an individual microelectrode. CNF sensor devices are fabricated in lots of 30 devices per 4-inch silicon wafer, using standard silicon processing technology (Fig. 2c). These devices are insulated with silicon dioxide, with only the tips of the CNFs exposed for sensing, as shown in Fig. 2f. Unlike the single electrode of the CFM, the CNF device contains 9 discrete sensing pads, allowing for simultaneous neurochemical detection in 9 locations (Fig. 2d), thereby providing high spatial resolution over a larger area of interrogation.
Fig. 2.
Images of CFM and CNF electrodes. Photographs of (a) CFM and and (c) CNF devices after fabrication. Scanning electron microscopy images of (b) CFM encased in a borosilicate capillary and (d) CNF-based 3 × 3 sensor pad array. High-resolution scanning electron microscopy images of (e) CNFs on a sensor pad prior to dielectric encapsulation and (f) CNFs on a sensor pad after dielectric encapsulation. Scale bars are 20 µm, 800 µm, 2 µm and 5 µm, respectively. An imposed tilt of 30° was utilized in (e).
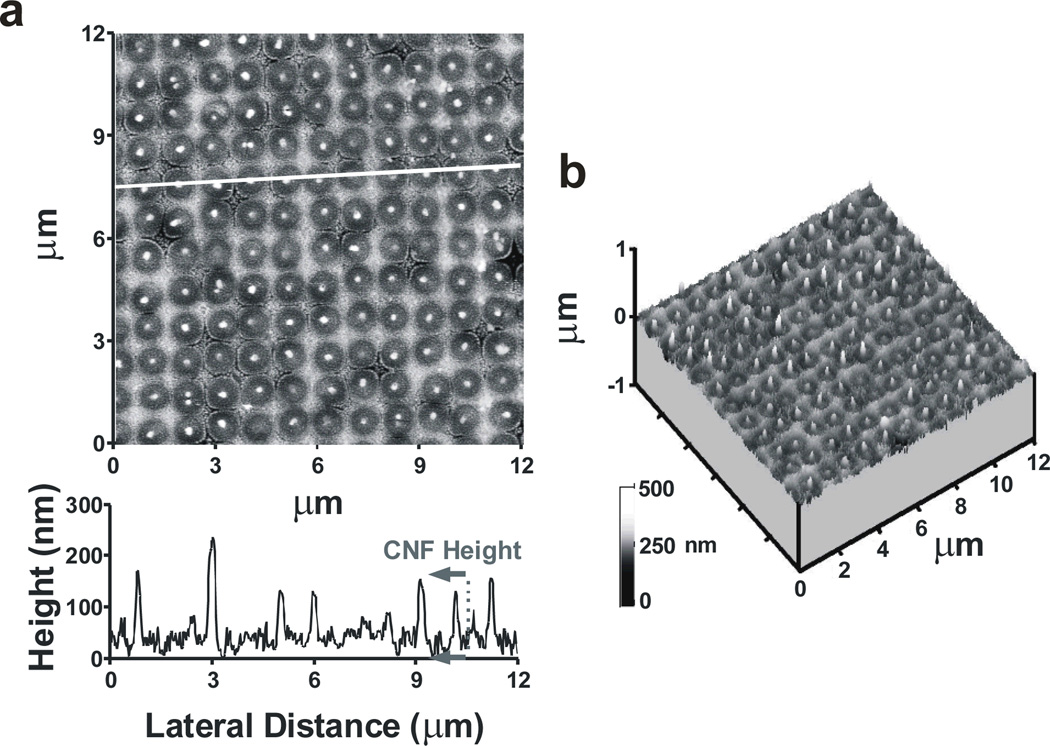
To choose CNF and CFM electrodes with similar surface areas, electrode dimensions are measured by SEM and AFM (Figs. 2 and 3). The SEM images of the CFM are used to estimate the total exposed surface area of the carbon fiber. Modeled as a cylinder intersected by an ellipse, the carbon fiber has an estimated surface area of 1300 µm2. SEM is used to determine the number of CNFs per sensing pad, estimated to be 32,560, based on a total of 27 images. CNF diameters are estimated to be 102 ± 12 nm, based on 14 line measurements across 3 SEM images. This dimension is consistent with the size of the nickel catalyst spots defined by electron beam lithography, as confirmed by AFM line scans. Because the CNF array contains high aspect-ratio protruding CNFs, a high aspect-ratio AFM imaging probe is required to minimize tip-induced broadening of the CNFs during AFM analysis. AFM is used to determine the height of CNFs protruding from the SiO2 matrix (Fig. 3). Height measurements are performed using 2-dimensional line scans from the height topograph (Fig. 3a). The CNF average height was determined to be 126 ± 43 nm, measured from 100 line scans. The fact that CNFs protrude from the SiO2 (Fig. 3) is presumably due to the high mechanical resilience of the CNF, and is consistent with previous results.11 The aggregate surface area of the CNFs on one sensing pad, where CNFs are modeled as cylinders, is determined to be 1583 µm2 for the electrode utilized in this study. Here, the electrochemical responses are scaled to electrode surface area for a clear comparison of CNF and CFM electrodes.
Fig. 3.
AFM images of CNF array. (a) Height topograph of a CNF array and a corresponding line plot representing data under the white line. (b) 3-dimensional height image of a CNF array. The scans are 12 µm by 12 µm.
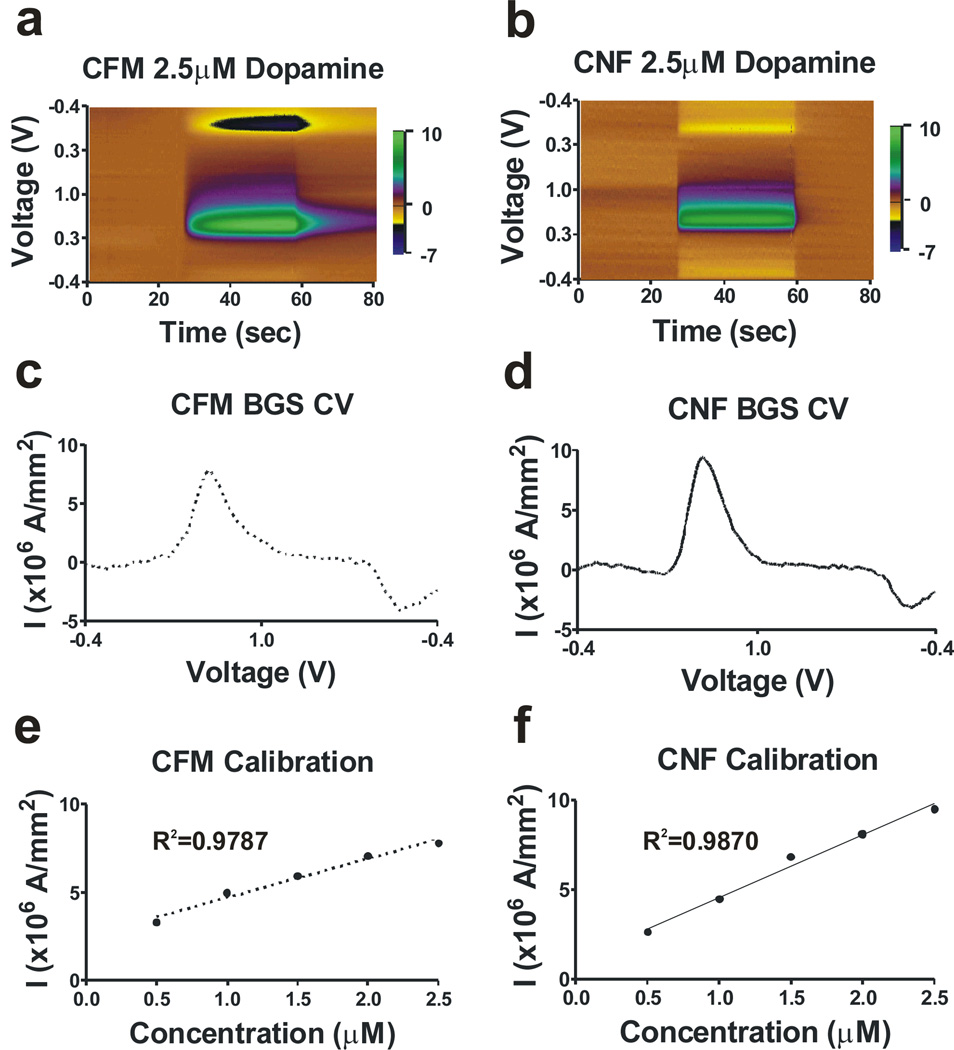
The CFM and CNF electrodes are used with WINCS to evaluate their response to variable dopamine concentrations, Fig. 4. Three-dimensional color plots (Figs. 4a–b) illustrate electrode response to injected dopamine in real time. For each electrode, a clear transition is observed upon dopamine injection, occurring at 25 seconds after the start of the data acquisition and ending at 60 seconds. Background subtraction is performed in each injection experiment by the WincsWare application to isolate the neurotransmitter redox currents from the much larger background currents associated with the charging and discharging of the electrode’s double layer of charge. Figs. 4c and 4d show the background-subtracted oxidation currents for a 2.5-µM dopamine injection, recorded using CFM and CNF electrodes, respectively. As shown in Figs. 4e–f, the dopamine oxidation currents of the CFM and CNF electrodes are comparable over a range of dopamine concentrations. CNF electrodes, which can be incorporated in electrode arrays of widely varying design, are thus shown to be a viable option for neurochemical monitoring by WINCS.
Fig. 4.
Dopamine detection by CFM (left side) or CNF (right side), using WINCS. (a–b) 3-dimensional color plots for a 2.5-µM dopamine injection. (c–d) Background-subtracted cyclic voltammograms for a 2.5-µM dopamine injection. (e–f) Measured current densities at varied dopamine concentrations.
Conclusions
The present study has demonstrated the integration of CNF electrode arrays with WINCS for neurochemical analysis. CNF electrode array performance was comparable to that of the CFM electrode in the detection of dopamine. The relative ease by which CNF arrays can be replicated in novel electrode designs is expected to facilitate the detection of neurochemical release over a large area of interrogation, yet with high spatial resolution.
Acknowledgements
The authors thank Ramsey Stevens of Carbon Design Innovations, Inc. for use of a CCB AFM probe. This work was supported by NIH (K08 NS 52232 award to KHL), the Mayo Foundation (2008–2011 Research Early Career Development Award for Clinician Scientists award to KHL) and NASA Ames Research Center.
References
- 1.Perry M, Li Q, Kennedy RT. Anal. Chim. Acta. 2009;653:1. doi: 10.1016/j.aca.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson GS, Johnson MA. Chem. Rev. 2008;108:2462. doi: 10.1021/cr068082i. [DOI] [PubMed] [Google Scholar]
- 3.Lee KH, Blaha CD, Garris PA, Mohseni P, Horne AE, Bennet KE, Agnesi F, Bledsoe JM, Lester DB, Kimble C, Min HK, Kim YB, Cho ZH. Neuromod. 2009;12:85. doi: 10.1111/j.1525-1403.2009.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KH, Blaha CD, Harris BT, Cooper S, Hitti FL, Leiter JC, Roberts DW, Kim U. Eur. J. Neurosci. 2006;23:1005. doi: 10.1111/j.1460-9568.2006.04638.x. [DOI] [PubMed] [Google Scholar]
- 5.Shon YM, Lee KH, Goerss SJ, Kim IY, Kimble C, Van Gompel JJ, Bennet K, Blaha CD, Chang SY. Neurosci. Lett. 2010;475:136. doi: 10.1016/j.neulet.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bledsoe JM, Kimble CJ, Covey DP, Blaha CD, Agnesi F, Mohseni P, Whitlock S, Johnson DM, Horne A, Bennet KE, Lee KH, Garris PA. J. Neurosurg. 2009;111:712. doi: 10.3171/2009.3.JNS081348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oneill RD. Analyst. 1994;119:767. [Google Scholar]
- 8.Robinson DL, Hermans A, Seipel AT, Wightman RM. Chem. Rev. 2008;108:2554. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kita JM, Wightman RM. Curr. Opin. Chem. Bio. 2008;12:491. doi: 10.1016/j.cbpa.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tawfik VL, Chang SY, Hitti FL, Roberts DW, Leiter JC, Jovanovic S, Lee KH. Neurosurg. 2010;67:367. doi: 10.1227/01.NEU.0000371988.73620.4C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Koehne JE, Cassell AM, Chen H, Ng HT, Ye Q, Fan W, Han J, Meyyappan M. Electroanal. 2005;17:15. [Google Scholar]
- 12.Valentini F, Amine A, Orlanducci S, Terranova ML, Palleschi G. Anal. Chem. 2003;75:5413. doi: 10.1021/ac0300237. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZH, Liu J, Liang QL, Wang YM, Luo G. Analyst. 2002;127:653. doi: 10.1039/b201060g. [DOI] [PubMed] [Google Scholar]
- 14.Swamy BEK, Venton BJ. Analyst. 2007;132:876. doi: 10.1039/b705552h. [DOI] [PubMed] [Google Scholar]
- 15.Poh WC, Loh KP, De Zhang W, Triparthy S. Langmuir. 2004;20:5484. doi: 10.1021/la0490947. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui S, Arumugam PU, Chen H, Li J, Meyyappan M. ACS Nano. 2010;4:955. doi: 10.1021/nn901583u. [DOI] [PubMed] [Google Scholar]
- 17.Arumugam PU, Yu E, Riviere R, Meyyappan M. Chem. Phys. Lett. 2010;499:241. [Google Scholar]
- 18.Arumugam PU, Chen H, Siddiqui S, Weinrich JAP, Jejelowo A, Li J, Meyyappan M. Biosens. Bioelect. 2009;24:2818. doi: 10.1016/j.bios.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Koehne J, Chen H, Li J, Cassell AM, Ye Q, Ng HT, Han J, Meyyappan M. Nanotech. 2003;14:1239. doi: 10.1088/0957-4484/14/12/001. [DOI] [PubMed] [Google Scholar]
- 20.Cassell AM, Li J, Nguyen-Vu TB, Koehne JE, Chen H, Andrews R, Meyyappan M. J. Nanosci. Nanotechn. 2009;9:5038. doi: 10.1166/jnn.2009.gr06. [DOI] [PubMed] [Google Scholar]
- 21.Ewing AG, Bigelow JC, Wightman RM. Science. 1983;221:169. doi: 10.1126/science.6857277. [DOI] [PubMed] [Google Scholar]
- 22.Stamford JA, Kruk ZL, Millar J, Wightman RM. Neurosci. Lett. 1984;51:133. doi: 10.1016/0304-3940(84)90274-x. [DOI] [PubMed] [Google Scholar]
- 23.Howell JO, Wightman RM. Anal. Chem. 1984;56:524. [Google Scholar]
- 24.Wipf DO, Kristensen EW, Deakin MR, Wightman RM. Anal. Chem. 1988;60:306. [Google Scholar]
- 25.Agnesi F, Tye SJ, Bledsoe JM, Griessenauer CJ, Kimble CJ, Sieck GC, Bennet KE, Garris PA, Blaha CD, Lee KH. J. Neurosurg. 2009;111:701. doi: 10.3171/2009.3.JNS0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agnesi F, Blaha CD, Lin J, Lee KH. J. Neur. Eng. 2010;7 doi: 10.1088/1741-2560/7/2/026009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griessenauer CJ, Chang SY, Tye SJ, Kimble CJ, Bennet KE, Garris PA, Lee KH. J. Neurosurg. 2010;113:656. doi: 10.3171/2010.3.JNS091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shon YM, Chang SY, Tye SJ, Kimble CJ, Bennet KE, Blaha CD, Lee KH. J. Neurosurg. 2010;112:539. doi: 10.3171/2009.7.JNS09787. [DOI] [PMC free article] [PubMed] [Google Scholar]