Abstract
Background/Aims:
Minimal hepatic encephalopathy (MHE) is common in patients with extrahepatic portal vein obstruction (EHPVO). There is no study on the treatment of MHE using lactulose in patients with EHPVO.
Patients and Methods:
Consecutive EHPVO patients were assessed by psychometric (number connection test (NCT-A and B), digit symbol test (DST), serial dot test (SDT), line tracing test (LTT)), and critical flicker frequency (CFF) at inclusion. Patients diagnosed as MHE were treated with lactulose and psychometric tests, CFF, and were reassessed after 3 months.
Results:
Of the 70 patients screened, the prevalence of abnormal psychometric test was as follows: NCT-A (41%), NCT-B (53%), DST (38%), SDT (40%), and LTT (44%). Thirty patients (43%) had two or more than two abnormal (>2 SD) psychometry tests. Lactulose improved MHE in 16/30 (53%) of patients after 3 months of treatment. Arterial ammonia decreased after lactulose treatment compared to baseline (83.7±19.1 vs. 65.1±19.3 μmol/l, P=0.001). A total of 9 (75%) of 12 patients with large spontaneous shunt and 7 (39%) of 18 patients without spontaneous shunt improved with lactulose (P=0.07). CFF in patients with MHE (n=30) was significantly lower than those without MHE (n=40) (38.1±2.4 vs. 41.5±3.1 Hz, P=0.01). CFF was less than 38 Hz in 21 (70%) of 30 patients before treatment and in 10 (33%) patients after lactulose therapy in MHE patients. All patients could tolerate lactulose without any significant side effects. Four patients (13%) developed transient diarrhea in whom dose needed reduction, 3 (10%) did not like its taste but have continued, and 2 (6%) developed abdominal bloating sensation.
Conclusions:
Lactulose is effective in the treatment of MHE in patients with EHPVO.
Keywords: Extrahepatic portal vein obstruction, lactulose, MHE
Extrahepatic portal vein obstruction (EHPVO) is a vascular disorder of the liver and is defined by obstruction of the extrahepatic portal vein with or without involvement of the intrahepatic portal veins or splenic or superior mesenteric veins.[1] EHPVO is a common cause of portal hypertension in the developing countries (up to 30% of all variceal bleeders) and is second to cirrhosis in the West (up to 5–10%).[2–4] Minimal hepatic encephalopathy (MHE) is characterized by subtle deficits and psychomotor abnormalities that can only be elicited by specialized psychometric tests.[5–7] MHE remains an important entity for clinicians to recognize because of its negative impact on a patient's health-related quality of life and association with driving impairment and vehicle accidents.[8,9] MHE has also been associated with an increased rate in the development of overt hepatic encephalopathy and increased mortality in patients with cirrhosis.[5–7] Hence the need of early identification and treatment of MHE exists. We and others have shown that MHE occurs in EHPVO patients who have either spontaneous or surgically created portosystemic shunt.[10–13] We have also shown that majority (2/3) of EHPVO patients with MHE continued to have MHE, and new-onset MHE developed in 5% over 1 year. Patients with EHPVO and MHE did not progress to overt encephalopathy over a follow-up of 1 year.[11] In a study by Goel et al.[12] 31 patients with EHPVO were studied and found MHE in 45%. On 1H-magnetic resonance (MR) spectroscopy and brain water content on diffusion tensor imaging and the magnetization transfer ratio (MTR) these patients had interstitial cerebral edema.
Lactulose and lactitol have been shown to be effective in MHE. It improved psychomotor tests and lowered ammonia levels as well as quality of life in patients with cirrhosis.[8,14–16] However there is no study on lactulose in EHPVO patients with MHE.
PATIENTS AND METHODS
Consecutive patients (from January 2009 to July 2010) with a diagnosis of EHPVO as per APASL guidelines were enrolled.[1] Patients were excluded if they had associated hepatitis B, C, history of alcohol intake or history of variceal bleed and lactulose intake in the previous 6 weeks, patients with jaundice and portal biliopathy, any shunt surgery in the past and history of beta-blockers intake, significant comorbid illness such as heart, respiratory, or renal failure and any neurologic diseases such as Alzheimer's disease, Parkinson's disease, and nonhepatic metabolic encephalopathies. Patients on psychoactive drugs, such as antidepressants or sedatives and those who restarted alcohol during follow-up were also excluded. The study was approved by ethics committee of the institute and informed written consent was taken from every patient before enrolling in the study.
Psychometric testing
All patients underwent a combination of psychometric tests including the number connection tests – A and B, digit symbol test, line tracing test, and serial dotting test. These tests were easy to administer and could be performed in 30-40 minutes. In the NCT - A, which measures cognitive motor abilities, patients connect numbers from 1 to 25 printed on paper as quickly as possible. In the NCT B, letters were also included and the patients connect alternating numbers and letters (1-A-2-B-3…L-13). In the digit symbol test (DST) subjects have to accurately and quickly transcribe symbols corresponding to numbers looking at a key in a timed manner over 90 seconds. The number of correctly transcribed symbols indicates performance, i.e., a low score means poor performance. In the serial dot test (SDT) subjects place a dot exactly in the center of 10 rows of large circles beginning each row on the left side and work to the right. In line tracing tests subjects need to draw a line between two lines on the paper and must stay between, neither touching nor drawing over the printed lines. The test score is the time required to complete the test, including the time needed to correct any errors. Tests were considered abnormal when the test score was more than mean ±2 S.D. from the age- and education-matched controls (n=150) which were taken from a healthy population.[17]
Measurement of critical flicker frequency threshold
CFF was done by a HEPAtonorm analyzer (Hepatonorm Analyzer; RandR Medi-Business Freiburg GmbH, Freiburg, Germany). It was measured in a quiet semidarkened room. Patients were first instructed and trained about the procedure. Flicker frequencies were measured eight times and the mean value was calculated. Measurement of the CFF thresholds was done by intrafoveal stimulation with a luminous diode. Decreasing the frequency of the light pulses from 60 Hz downward, the CFF threshold was determined as the frequency when the impression of fused light turned to a flickering one. The critical flicker frequency was considered abnormal when the value was <38 Hz.[18]
Assessment of minimal hepatic encephalopathy and hepatic encephalopathy
HE was defined according to West-Haven criteria. MHE was diagnosed if two or more psychometric tests were abnormal.[19]
Study design
All patients with MHE received 30-60 ml of lactulose in two or three divided doses so that the patient passed 2-3 semisoft stools per day along with their previous treatment and prophylaxis of variceal bleed if any with endoscopic variceal ligation. All subjects were followed up every month for treatment compliance and for development of any complications. Compliance with the therapy was assured primarily by ensuring increased stool frequency and a change to a softer consistency and by counting the number of bottles of lactulose consumed. Patients who did not turn up were contacted on telephone and instructed for regular check-up. All MHE patients were assessed by psychometry, CFF and arterial ammonia at inclusion into the study and then at month 3. The primary endpoint was reversal of MHE at 3 months of treatment.
Blood tests, imaging, and biochemical examinations
After overnight fasting, patient venous blood was taken and analyzed for routine liver function tests and hematologic parameters by conventional methods at baseline and then at 3 months. Repeat relevant investigations were done on the as- and when-required basis. All patients underwent Doppler ultrasound/MR and MR angiography for the diagnosis and evaluation of portal cavernoma, collaterals, and spontaneous shunts. Arterial ammonia was measured by the ammonia Test Kit II for the PocketChem BA device (Arkay, Inc., Kyoto, Japan) at baseline and at the end of follow-up. The blood sample was collected onto ice and tested immediately after collection. The continuous measurement range was 7-286 μmol/l; the normal blood ammonia level for healthy adults for this device was less than 54 μmol/l.
Statistical analysis and data management
Data processing was performed by using the software packages SPSS. Data were expressed as mean±SD. For a comparison of categorical variables, the chi-square and Fisher's exact tests were used, and for continuous variables, a Mann-Whitney test for unpaired data and a Wilcoxon rank sum test for paired data were used as appropriate. The probability level of P<0.05 was set for statistical significance.
RESULTS
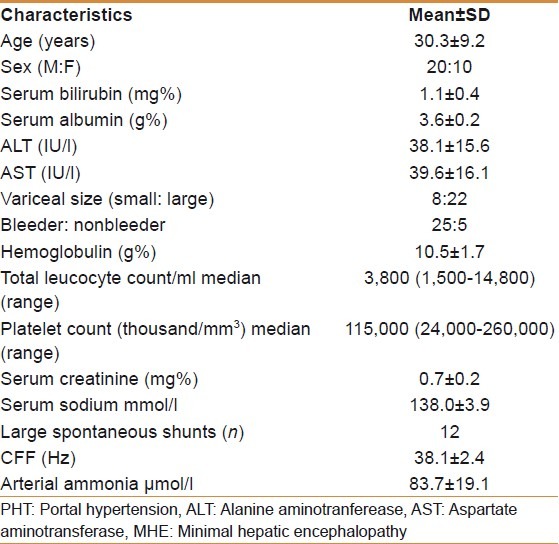
Of 90 patients screened, 70 patients met the inclusion criteria. Twenty patients were excluded as chronic hepatitis B, n=7, chronic hepatitis C, n=2, recent bleeder, n=8, and not willing for participation, n=3. Hence 70 patients underwent psychometry tests. Baseline characteristics of these patients are shown in Table 1. Of these 70 patients 30 (43%) patients had MHE and these patients were treated with lactulose and repeat psychometry done at 3 months. Baseline characteristics of patients with MHE are shown in Table 2.
Table 1.
Baseline characteristics of EHPVO patients screened for minimal hepatic encephalopathy (n=70)
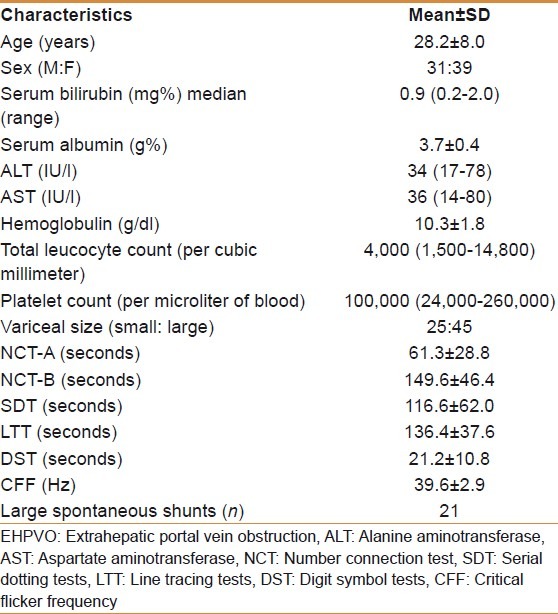
Table 2.
Baseline characteristics of patients with MHE (n=30)
Result of psychometric tests
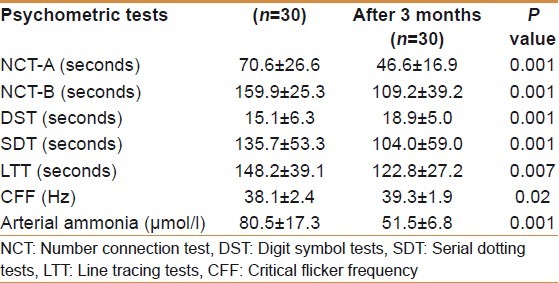
The normative of psychometric tests was derived from age and the education-matched group comprised 125 men and 25 women. The normative for NCT-A was 31±10 seconds, for NCT-B 56.0±16 seconds, for SDT 55.0±10 seconds, for DST 37±9 seconds, and for LTT 84±16.0 seconds. The prevalence of the abnormal psychometric test in 70 patients screened were NCT-A 41%, NCT-B 53%, DST 38%, SDT 40%, and LTT 44%. Thirty patients (43%) had two or more than two abnormal psychometry tests [Table 3]. Lactulose improved MHE in 16/30 (53%) of patients after 3 months of treatment [Table 4]. Twenty-one (30%) of 70 patients had large spontaneous shunts; of these 21 patients, 12 (57%) had MHE.
Table 3.
Prevalence of psychometry tests in patients with EHPVO (n=70)
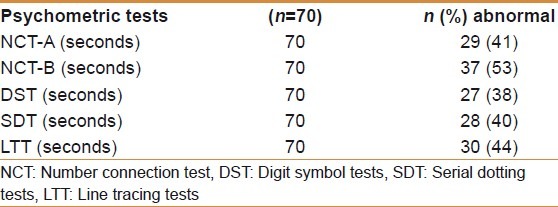
Table 4.
Psychometry tests, CFF and arterial ammonia before and after lactulose treatment
Critical flicker frequency
All patients could do CFF with little training. CFF in patients with MHE (n=30) was significantly lower than in those without MHE (n=40) (38.1±2.4 vs. 41.5±3.1 Hz, P=0.01). CFF was less than 38 Hz in 21 (70%) of 30 patients before treatment and in 10 (33%) patients after lactulose therapy in MHE patients. After 3 months of treatment CFF increased significantly from 38.1±2.4 Hz to 39.3±1.9Hz, P=0.01. Patients who did not recover (n=14) after lactulose therapy continued to have low CFF compared to patients who recovered (38.3±1.6 vs. 40.2±1.9 Hz, P=0.007).
Arterial ammonia
Arterial ammonia decreased after lactulose treatment compared to baseline (83.7±19.1 vs. 65.1±19.3 μmol/l, P=0.001). Patients in whom MHE improved (n=16) after lactulose had significant reduction in arterial ammonia (84.5±21.5 vs. 51.5±6.8 μmol/l, P=0.001) than those who did not improve (n=14) (82.7±16.7 vs. 80.5±17.3 μmol/l, P=0.46).
Patients with large spontaneous shunt (n=12) had significantly higher arterial ammonia compared to patients with no spontaneous shunts (95.5±18.7 vs. 75.8±15.2 μmol/l, P=0.001). Of 12 patients with large shunt 9 (75%) responded to lactulose while 7(39%) patients responded among those with no spontaneous shunt (P=0.07).
Compliance and side effects
All the patients were followed up for 3 months; hence the follow-up was 100%. All patients could tolerate lactulose without any significant side effects. Four patients (13%) developed transient diarrhea in whom dose needed reduction, 3 (10%) did not like its taste but have continued, and 2 (6%) developed abdominal bloating sensation.
DISCUSSION
This study shows that incidence of MHE in patients with EHPVO was 43% and lactulose improved MHE in 53% of these patients.
The prevalence of MHE in our outpatient EHPVO population was 43%. Similar percentages were found in our previous study[10] and in studies by other investigators.[12,13] The prevalence of MHE has been reported to vary between 30% and 84% in patients with liver cirrhosis.[5–8,20] Lactulose improved MHE (40-70%) in various studies in patients with cirrhosis depending upon the criteria used to assess MHE.[8,14–16] In this study we found lactulose improved MHE in 53% of patients which was equal to its efficacy in cirrhotic patients. We have not taken any control limb for this study as we had shown earlier in a cohort of 32 EHPVO patients (age, 23.2+/-10.8 years; M/F 22:10) who were followed up for 1 year in whom 12 patients who had MHE at baseline, 9 (75%) patients continued to have MHE, and in 3 (25%) patients it disappeared. One (5%) of the remaining 20 patients developed MHE during the follow-up.[11] Hence lactulose significantly improved MHE in patients with EHPVO. No patient developed overt encephalopathy in the 3-month follow-up. We have shown earlier that patients with EHPVO with large spontaneous shunts had higher venous ammonia level than those who did not have spontaneous shunts[10] and in this study we found the same. In this study we found patients with MHE and with spontaneous shunts had better response (75% vs. 39%) to lactulose than MHE patients without any spontaneous shunts though it did not achieve statistical significance (P=0.07). We also found that patients who responded to lactulose (84.5±21.5 vs. 51.5±6.8 μmol/l, P=0.001) had significant fall in arterial ammonia compared to patients who did not respond (82.7±16.7 vs. 80.5±17.3 μmol/l, P=0.46).
CFF is a well-established neurophysiological technique with little bias for training effects, education, age, daytime, or interexaminate variability.[18,21–23] We also found that patients with MHE had significantly lower CFF than those who did not had MHE and CFF improved significantly after treatment with lactulose. Patients who did not have MHE after lactulose therapy continued to have lower CFF. So CFF alone could be used as an alternative for assessment of recovery of MHE as we had shown earlier.[22]
In our study compliance with lactulose was 100% and none discontinued either because of distaste, abdominal bloating or transient diarrhea though the dose needed manipulation. Prasad et al.[8] showed that lactulose improves health-related quality of life (HRQOL) in patients with MHE when treated with lactulose. We have not done HRQOF in EHPVO patients with MHE that would substantiate our belief that treating MHE in EHPVO helped them to live a better life. Other limitation of this study was the absence of the control arm to compare with the theraputic arm to further support the use of lactulose in the treatment of MHE in patients with EHPVO. To conclude, MHE is common in patients with EHPVO and lactulose is effective in the treatment of MHE in these patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sarin SK, Sollano JD, Chawla YK, Amarapurkar D, Hamid S, Hashizume M, et al. Consensus on extra-hepatic portal vein obstruction. Liver Int. 2006;26:512–9. doi: 10.1111/j.1478-3231.2006.01269.x. [DOI] [PubMed] [Google Scholar]
- 2.Yachha SK, Khanduri A, Sharma BC, Kumar M. Gastrointestinal bleeding in children. J Gastroenterol Hepatol. 1996;11:903–7. [PubMed] [Google Scholar]
- 3.Sarin SK, Agarawal SR. Extrahepatic portal vein obstruction. Semin Liver Dis. 2002;22:43–58. doi: 10.1055/s-2002-23206. [DOI] [PubMed] [Google Scholar]
- 4.Webb LJ, Sherlock S. The etiology, presentation and natural history of extra-hepatic portal venous obstruction. Q J Med. 1979;192:627–39. [PubMed] [Google Scholar]
- 5.Romero-Gomez M, Boza F, Garcia-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol. 2001;96:2718–23. doi: 10.1111/j.1572-0241.2001.04130.x. [DOI] [PubMed] [Google Scholar]
- 6.Das A, Dhiman RK, Saraswat VA, Verma M, Naik SR. Prevalence and natural history of subclinical hepatic encephalopathy in cirrhosis. J Gastroenterol Hepatol. 2001;16:531–5. doi: 10.1046/j.1440-1746.2001.02487.x. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann IJ, Groeneweg M, Quero JC, Beijeman SJ, de Man RA, Hop WC, et al. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol. 2000;95:2029–34. doi: 10.1111/j.1572-0241.2000.02265.x. [DOI] [PubMed] [Google Scholar]
- 8.Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45:549–59. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj JS, Hafeezullah M, Hoffmann RG, Saeian K. Minimal hepatic encephalopathy: A vehicle for accidents and traffic violations. Am J Gastroenterol. 2007;102:1903–9. doi: 10.1111/j.1572-0241.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- 10.Sharma P, Sharma BC, Puri V, Sarin SK. Minimal hepatic encephalopathy in patients with extrahepatic portal vein obstruction. Am J Gastroenterol. 2008;103:1406–12. doi: 10.1111/j.1572-0241.2008.01830.x. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Sharma BC, Puri V, Sarin SK. Natural history of minimal hepatic encephalopathy in patients with extrahepatic portal vein obstruction. Am J Gastroenterol. 2009;104:885–90. doi: 10.1038/ajg.2009.84. [DOI] [PubMed] [Google Scholar]
- 12.Goel A, Yadav S, Saraswat V, Srivastava A, Thomas MA, Pandey CM, et al. Cerebral oedema in minimal hepatic encephalopathy due to extrahepatic portal venous obstruction. Liver Int. 2010;30:1143–51. doi: 10.1111/j.1478-3231.2010.02289.x. [DOI] [PubMed] [Google Scholar]
- 13.Mínguez B, García-Pagán JC, Bosch J, Turnes J, Alonso J, Rovira A, et al. Noncirrhotic portal vein thrombosis exhibits neuropsychological and MR changes consistent with minimal hepatic encephalopathy. Hepatology. 2006;43:707–14. doi: 10.1002/hep.21126. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe A, Sakai T, Sato S, Imai F, Ohto M, Arakawa Y, et al. Clinical efficacy of lactulose in cirrhotic patients with and without subclinical hepatic encephalopathy. Hepatology. 1997;26:1410–4. doi: 10.1053/jhep.1997.v26.pm0009397979. [DOI] [PubMed] [Google Scholar]
- 15.Dhiman RK, Sawhney MS, Chawla YK, Das G, Ram S, Dilawari JB. Efficacy of lactulose in cirrhotic patients with subclinical hepatic encephalopathy. Dig Dis Sci. 2000;45:1549–52. doi: 10.1023/a:1005556826152. [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, Sharma BC, Puri V, Sarin SK. An open-label randomized controlled trial of Lactulose and probiotics in the treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2008;20:506–11. doi: 10.1097/MEG.0b013e3282f3e6f5. [DOI] [PubMed] [Google Scholar]
- 17.Sharma P, Singh S, Sharma BC, Kumar M, Garg H, Kumar A, et al. Propofol sedation during endoscopy in patients with cirrhosis and utility of psychometric and critical flicker frequency in assessment of recovery from sedation. Endoscopy. 2011;43:400–5. doi: 10.1055/s-0030-1256182. [DOI] [PubMed] [Google Scholar]
- 18.Romer Gómez M, Córdoba J, Jover R, del Olmo JA, Ramírez M, Rey R, et al. Value of the critical flicker frequency in patients with minimal hepatic encephalopathy. Hepatology. 2007;45:879–85. doi: 10.1002/hep.21586. [DOI] [PubMed] [Google Scholar]
- 19.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy–definition, nomenclature, diagnosis, and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–21. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 20.Amodio P, Del Piccolo F, Marchetti P, Angeli P, Iemmolo R, Caregaro L, et al. Clinical features and survival of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests. Hepatology. 1999;29:1662–7. doi: 10.1002/hep.510290619. [DOI] [PubMed] [Google Scholar]
- 21.Sharma P, Sharma BC, Puri V, Sarin SK. Critical flicker frequency: Diagnostic tool for minimal hepatic encephalopathy. J Hepatol. 2007;47:67–73. doi: 10.1016/j.jhep.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Sharma P, Sharma BC, Sarin SK. Critical flicker frequency for diagnosis and assessment of recovery from minimal hepatic encephalopathy in patients with cirrhosis. Hepatobiliary Pancreat Dis Int. 2010;9:27–32. [PubMed] [Google Scholar]
- 23.Kircheis G, Wettstein M, Timmermann L, Schnitzler A, Häussinger D. Critical flicker frequency for quantification of low–grade hepatic encephalopathy. Hepatology. 2002;35:357–66. doi: 10.1053/jhep.2002.30957. [DOI] [PubMed] [Google Scholar]


