Abstract
Background/Aim:
Gastro duodenal ulcer is a common disorder of the gastrointestinal tract. Several Indian medicinal plants have been traditionally and extensively used to prevent different diseases. In the present research studies, Bael fruit (Aegle marmelos (AM), family: Rutaceae) which are also called as Bilva in ancient Sanskrit was used as a herbal drug and its antioxidative role in aspirin- induced gastroduodenal ulceration in albino rat was evaluated using essential biochemical parameters.
Patients and Methods:
Mucosal thickness (MT), ulcer index (UI), different biochemical parameters, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), catalase (CAT), superoxide dismutase (SOD), reduced glutathione (GSH), and lipid peroxidation (LPO) were measured in all the groups, to study the possible involvement of antioxidants with gastroduodenal protection.
Results:
A significant decrease in MT, SOD and CAT activities and GSH level and a significant increase in UI, AST, ALT, and ALP activities and LPO level were observed in aspirin treated stomach and duodenum of albino rats.
Conclusions:
Pretreatment with AM fruit pulp extract for 14 consecutive days showed the reverse effects of aspirin suggesting gastro-duodenal protective and anti- ulcerogenic properties of AM through its antioxidant mechanism.
Keywords: Aegle marmelos, albino rats, aspirin, bael fruits, gastro duodenal ulcer, herbal drugs
Gastroduodenal ulcer is a common disorder of the gastrointestinal tract.[1] Gastric ulcer develops when there is imbalance between the defensive and aggressive factors on the mucosa resulting from either potentiation of aggressive factors and/or lowering of mucosal protection.[2,3] It is now accepted that gastric ulcer is mainly caused by oxidative stress.[1,2,4,5]
Aegle marmelos (AM), commonly known as Bael, is a spiny tree belonging to the family Rutaceae. It is an indigenous tree found in India, Myanmar, Pakistan, and Bangladesh. The leaves, roots, bark, seeds, and fruits are edible and have medicinal value. Eugenol (C10H12O2), present in AM leaf extract, has potent antioxidant property[6–8] and inhibits lipid peroxidation.[8,9] AM leaf extract was found to be a potential antioxidant drug, which reduces the blood sugar level in alloxan induced diabetic rats.[10] Several vitamins, such as Vitamin A, Vitamin C, and Vitamin E, have been isolated from AM.[11] These vitamins have been reported to have a role in protection against gastric mucosal damage. Stress, non steroidal anti-inflammatory drugs (NSAIDs) and Helicobacter pylori are the most common causes of ulceration.[1] Aspirin a well known NSAID,[12,13] which induces erosions and ulcers in gastroduodenal tract through different processes, such as inhibition of cyclo–oxygenase - mediated prostaglandin synthesis, generation of reactive oxygen species (ROS) and induction of apoptosis. Inflammatory injury is associated in part with increased generation of ROS.
Repeated use of this drug may cause gastro–intestinal bleeding and large doses can provoke a host of reactions, including ulcer, vomiting, diarrhea, vertigo, and hallucinations. Chronic NSAID users have a 42% - 44% risk of developing a symptomatic ulcer, gastrointestinal bleeding, or even perforation.[14] To treat a gastric ulcer the doctor may prescribe drugs that will lower the rate of stomach acid secretion or protect tissues that line the stomach. The most effective suppressors of gastric acid secretion are the proton pump inhibitors such as omeprazole, lansoprazole and pantoprazole.
The present study was under taken to evaluate the antioxidative role of AM in aspirin– induced gastroduodenal ulceration in experimental rat models.
PATIENTS AND METHODS
Collection of plant materials
The fruits of AM were purchased from the local market and the identity of the plant was authenticated by the Botanical Survey of India, Howrah, and kept in the Department of Physiology, Katwa College, University of Burdwan.
Preparation of aqueous extract from the pulp of AM
The pulp of AM fruit was used throughout the experimental study. The fruits of AM were cut into pieces, sun-dried and ground with the help of an electrical grinder to get a free-flowing powder. This powder was subjected to extraction with water (1:3) at room temperature for 48 h. The extract obtained was filtered through Whatman filter paper and vacuum dried at 40°C – 50°C to get a dry powder, which was dissolved in double-distilled water for final use.[15]
Chemicals
Aspirin and omeprazole were obtained as gift samples from Krishnath College (University of Kalyani), Berhampore, Murshidabad, West Bengal, India.
Animals used and maintenance
Thirty-six male Holtzman strain adult albino rats of age approximately 120 days and weighing 250-300 g were used in the following studies. The animals were individually housed and maintained under standard laboratory conditions with natural dark and light cycle (approximately 12- h light/12-hour dark cycle) and room temperature (27±1°C) and constant humidity (60%) in accordance with the -“Institutional Ethical Committee” - rules and regulations. Food and water were freely available except during testing. Drinking water was supplied ad libitum.
Animals were randomly divided equally into 6 groups of 6 each, as follows: control group, aspirin- treated experimental group, AM-treated control group, AM-pretreated aspirin treated experimental group, omeprazole-treated control group, and omeprazole-pretreated aspirin treated experimental group. The control rats were given distilled water orally with approximately the same volume of AM extract. Groups - III and IV rats were treated with aqueous pulp extract of AM fruit at a dose of 250 mg/kg body weight[15] through orogastric cannula once daily for 14 consecutive days at a particular time (10:30-11:30 hours) every day. In the 5th and 6th groups, i.e., omeprazole treated control group and omeprazole pretreated aspirin treated experimental group, omeprazole (250 mg/kg body weight) was administered through orogastric cannula once daily for 14 consecutive days at a particular time (10:30-11:30 hours) every day. On the 14th day, after feeding the extract, food was withdrawn from rats of Groups - III, IV, V, and VI but had free access to water. On the 15th day, aspirin (German - Remedies Ltd, Mumbai, Maharashtra, India) was dissolved in distilled water and given at a dose of 500 mg/kg body weight orally and waited for 4 h.[12] After 4 h the experiment was terminated and rats were sacrificed by an overdose of thiopentone sodium (NEON, Laboratories Ltd, Mumbai, Maharashtra, India). Body weights of the rats were recorded everyday and maintained in the laboratory throughout the experimental period.[16]
Biochemical estimations
Collection of serum
The blood was collected by heart puncture and serum was separated by centrifugation (3000 rpm at 4°C for 10 min). The stomach and duodenum tissues were immediately removed.
Estimation of ALT, AST, and ALP levels
AST and ALT activities were measured according to the method of Kind and King,[17] alkaline phosphatase (ALP) activity was measured according to the procedure of Reitman and Frankel.[18]
Measurement of superoxide dismutase
Superoxide dismutase (SOD) was estimated by the method of Mishra and Fridovich and Roy et al.,[19,20] Tissue samples were homogenised with 5 mL of ice-cold 0.1 M phosphate buffer (pH-7.4). The homogenates were then centrifuged at 3000 rpm for 10 min. Then, 0.1 mL of sample was mixed with 0.8 ml of Trehalose- 6, 6 dibehenate (TDB). Reaction was started by the addition of 4 μL of nicotinamide adenine dinucleotide phosphate (NADPH). Then, 25 μL of ethylenediaminetetraacetic acid manganese chloride (EDTA-MnCl2) mixture was added to it. Thereafter, spectrophotometric readings were recorded at 340 nm. After recording of the spectrophotometric readings, 0.1 ml of mercaptoethanol was added to this mixture and again spectrophotometric readings were recorded at 340 nm.
Measurement of lipid peroxidation
Lipid peroxidation (LPO) was measured according to the method of Roy et al. and Hazra et al.,[20,21] Tissue samples were homogenized with 5 mL of ice-cold 0.1 M phosphate buffer (pH-7.4). The homogenates were then centrifuged at 3000 rpm for 10 min. Then, 0.5 mL of sample was mixed with 1 ml of TDB and then the mixture was incubated at 37°C for 1 hour. To this, 0.5 mL of trichloroacetic acid (TCA) was added, vortexed and the absorbance was read at 350 nm. After recording of the spectrophotometric reading, 1 mL sample was mixed with 500 ml mercaptoethanol and again the absorbance was read at 350 nm.
Measurement of catalase
Catalase (CAT) activity was estimated by the method of Roy et al. and Cohen et al., and[20,22] Tissue samples were homogenized with 5 mL of ice-cold 0.1 M phosphate buffer (pH-7.4). The homogenates were then centrifuged at 3000 rpm for 10 mins. The precipitate was then stirred with the addition of 15 mL of ice-cold 0.1 M phosphate buffer and allowed to stand in cold condition with occasional shaking. The shaking procedure was repeated three times. 1 milliliter of the sample was added to 9 mL of H2O2. The rate of decomposition of H2O2 was measured spectrophotometrically from the changes in absorbance at 350 nm. The activity of CAT was expressed as % inhibition unit.
Measurement of glutathione
Reduced glutathione (GSH) was measured according to the method of Ellman.[23] Equal quantity of homogenate was mixed with 10% TCA and centrifuged to separate the proteins. To 0.01 mL of this supernatant, 2 mL of phosphate buffer (pH- 8.4), 0.5 ml of 5,- 5- dithiobis- (2-nitrobenzoic acid) and 0.4 mL of double- distilled water were added. The mixture was vortexed and the absorbance was read at 412 nm within 15 min. The concentration of GSH was expressed as μg/g of tissue.
Statistical analysis
The data were expressed as mean±SEM and were analysed statistically using one–way analysis of variance, followed by multiple comparison t-test, which was used for statistical evaluation of the data. In addition to this, two–tailed Student's t-test was performed to determine the level of significance between the means. Difference below the probability level 0.05 was considered statistically significant.
RESULTS
After completion of the experiment, the AST, ALT, ALP, SOD and CAT activities, GSH and LPO levels were estimated and both ulcer index (UI) and mucosal thickness (MT) were estimated.
The control group, AM- treated control group and omeprazole- treated control group showed the lowest (zero) UI. There was a sharp decline (P<0.001) in mean ulcer index both in the AM- pretreated aspirin- treated experimental group and omeprazole- pretreated aspirin– treated experimental group as compared to the aspirin-treated experimental group. The MT was significantly (P<0.001) increased in AM- treated control group compared to the control group. AM significantly (P<0.001) increased MT in AM- pretreated aspirin- treated experimental group compared to the aspirin- treated experimental group. There was a sharp rise (P<0.001) in MT in the omeprazole- treated control group compared to the control group. The MT was significantly (P<0.001) increased in omeprazole– treated control group in comparison to AM- treated control group. The MT was significantly (P<0.001) increased in omeprazole- pretreated aspirin- treated experimental group when compared with aspirin- treated experimental group. The results are shown in Table 1.
Table 1.
Effect of Aegle marmelos extract on ulcer index and mucosal thickness of stomach and duodenum tissues
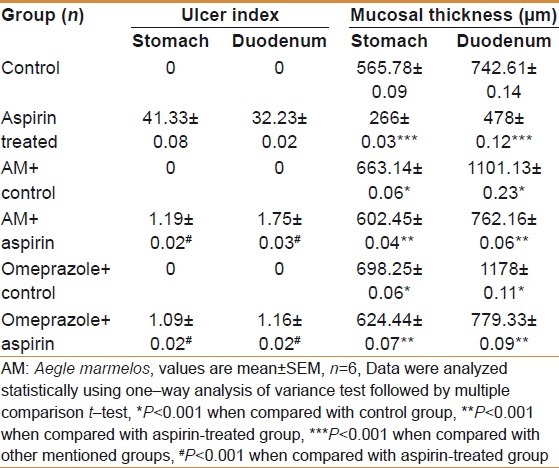
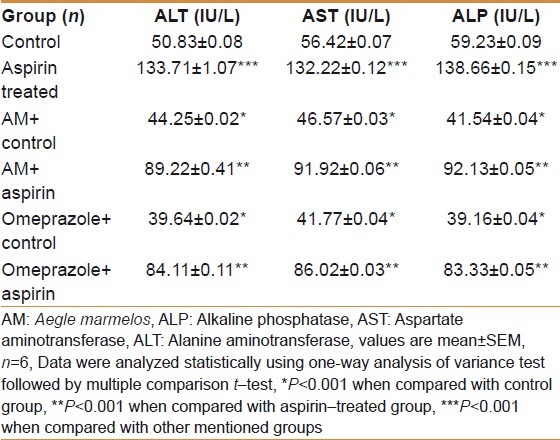
There was a sharp rise (P<0.001) in AST, ALT and ALP activities in the aspirin- treated experimental group as compared to the control group. The AST, ALT and ALP activities were significantly (P<0.001) decreased in AM- treated control group compared to the control group. AM significantly (P<0.001) decreased AST, ALT and ALP activities in AM- pretreated aspirin– treated experimental group compared with the aspirin- treated experimental group. There was a sharp decline (P<0.001) in AST, ALT and ALP activities in the omeprazole- treated control group compared to the control group. The AST, ALT and ALP activities were significantly (P<0.001) decreased in omeprazole- treated control group in comparison to AM- treated control group. The AST, ALT and ALP activities were significantly (P<0.001) decreased in omeprazole pretreated—aspirin- treated experimental group when compared to aspirin- treated experimental group. The results are shown in Table 2.
Table 2.
Effect of AM pulp extract on AST, ALT and ALP activity
There was a sharp decline (P<0.001) in SOD activity both in stomach and duodenum tissues in the aspirin– treated experimental group as compared to the control group. The SOD activity was significantly (P<0.001) increased in AM- treated control group compared with the control group, both in stomach and duodenum tissues. AM significantly (P<0.001) increased SOD activity in AM- pretreated aspirin- treated experimental group in comparison to aspirin- treated experimental group, both in stomach and duodenum tissues. There was a sharp increase (P<0.001) in SOD activity both in stomach and duodenum tissues in the omeprazole- treated control group compared to the control group. The SOD activity was significantly (P<0.001) increased in omeprazole- treated control group in comparison to AM- treated control group both in stomach and duodenum tissues. The SOD activity was significantly (P<0.001) increased in omeprazole- pretreated aspirin- treated experimental group compared with the aspirin- treated experimental group both in stomach and duodenum tissues. The results are shown in Tables 3 and 4.
Table 3.
Effect of AM pulp extract on antioxidant enzymatic changes of stomach tissue
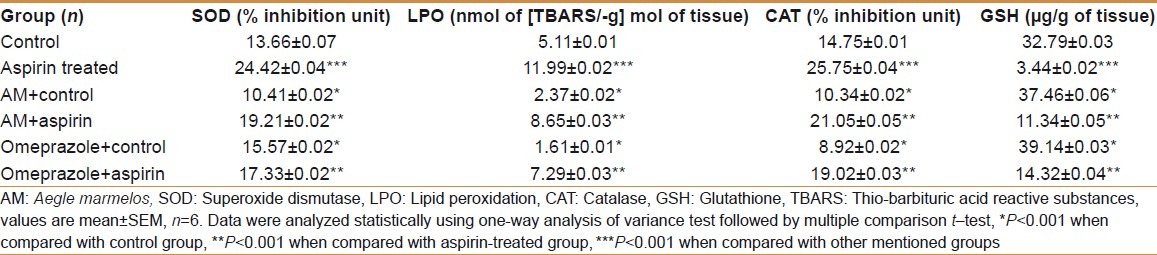
Table 4.
Effect of AM pulp extract on antioxidant enzymatic changes of duodenum tissue
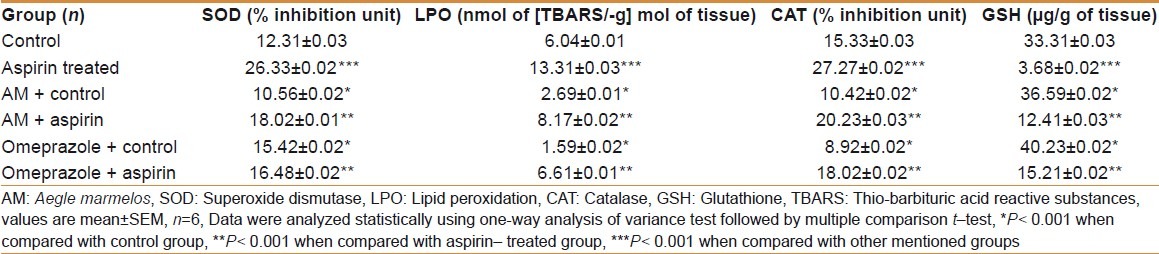
There was a sharp rise (P<0.001) in LPO level both in stomach and duodenum tissues in the aspirin- treated experimental group as compared with the control group. The LPO level was significantly (P<0.001) decreased in AM- treated control group compared with the control group, both in stomach and duodenum tissues. AM significantly (P<0.001) decreased LPO level in AM- pretreated aspirin- treated experimental group in comparison to aspirin- treated experimental group, both in stomach and duodenum tissues. There was a sharp decline (P<0.001) in LPO level both in stomach and duodenum tissues in the omeprazole- treated control group when compared with the control group. The LPO level was significantly (P<0.001) decreased in omeprazole- treated control group compared to the AM- treated control group, both in stomach and duodenum tissues. The LPO level was significantly (P<0.001) decreased in omeprazole- pretreated aspirin- treated experimental group when compared to the aspirin- treated experimental group, both in stomach and duodenum tissues. The results are shown in Tables 3 and 4.
There was a sharp decline (P<0.001) in CAT activity both in stomach and duodenum tissues in the aspirin- treated experimental group as compared with the control group. The CAT activity was significantly (P<0.001) increased in AM- treated control group when compared with the control group, both in stomach and duodenum tissues. AM significantly (P<0.001) increased CAT activity in AM- pretreated aspirin- treated experimental group in comparison to aspirin- treated experimental group, both in stomach and duodenum tissues. There was a sharp increase (P<0.001) in CAT activity, both in stomach and duodenum tissues in the omeprazole- treated control group compared with the control group. The CAT activity was significantly (P<0.001) increased in omeprazole- treated control group in comparison to AM- treated control group, both in stomach and duodenum tissues. The CAT activity was significantly (P<0.001) increased in omeprazole- pretreated aspirin- treated experimental group when compared with the aspirin- treated experimental group, both in stomach and duodenum tissues. The results are shown in Tables 3 and 4.
There was a sharp decline (P<0.001) in GSH level both in stomach and duodenum tissues in the aspirin- treated experimental group as compared with the control group. The GSH level was significantly (P<0.001) increased in AM- treated control group compared with the control group, both in stomach and duodenum tissues. AM significantly (P<0.001) increased GSH level in AM- pretreated aspirin- treated experimental group in comparison with aspirin- treated experimental group, both in stomach and duodenum tissues. There was a sharp increase (P<0.001) in GSH level, both in stomach and duodenum tissues in the omeprazole– treated control group compared with the control group. The GSH level was significantly (P<0.001) increased in omeprazole- treated control group when compared with the AM- treated control group, both in stomach and duodenum tissues. The GSH level was significantly (P<0.001) increased in omeprazole- pretreated aspirin- treated experimental group compared with aspirin- treated experimental group both in stomach and duodenum tissues. The results are shown in Tables 3 and 4.
DISCUSSION
The present study evaluates the protective role of aqueous fruit extract of AM on aspirin-induced gastric and duodenal ulcer in albino rat model with the possible involvement of the antioxidants. AST, ALT, and ALP are the most sensitive tests employed in the diagnosis of ulcer disease. Analysis of the results showed that aspirin produced extensive increase in UI, decrease in MT, and decrease in AST, ALT, and ALP activities in rat stomach and duodenal tissues. The decrease of ALP activity seems to be a general property of all chemicals which are known to provoke severe ulcer. The activity of ALP may be relevant to duodenal ulcer pathogenesis or healing.[24]
The pathogenesis of ulcer disease is believed to reflect an imbalance between increased aggressive factors and decreased protective factors.[25] The decreased ALP activity imbalances are one of the first lines of defense protecting the gastro-duodenal mucosa and the mucus bicarbonate barrier overlying the epithelium.[26] The increase in UI, decrease in MT, and decrease in AST, ALT, and ALP activities observed in the present study after oral administration of aspirin treatment may be due to failure in gastro-protective and repair mechanisms leading to disrupted mucosal barrier.[27]
It is evident from the results of the present investigation that treatment with aqueous pulp extract of AM significantly decreased the AST, ALT, and ALP activities at a dose of 400 mg/kg body weight. These findings can be explained by alterations of the LPO level and antioxidant activities such as that of SOD, CAT and GSH, both in stomach and duodenum tissues. The ability of gastric mucosa to resist injury by ingested irritants (aspirin) is attributed to number of factors that have been collectively referred to as mucosal defense.[28] Some necrotizing agents, such as, aspirin, ethanol and strong alkalis induced gastric mucosal lesions by disrupting this defense mechanism.[29]
LPO can be used as an index for measuring the damage that occurs in membranes of tissue as a result of free radical generation.[30,31] In our present study, oral administration of aspirin significantly increased the LPO level. Significant elevation of LPO level observed in aspirin treated experimental-group is possibly due to the generation of free radicals via auto-oxidation or through metal ion or superoxide catalyzed oxidation process. In the present study, AM significantly decreased LPO level at a dose of 400 mg/kg body weight compared with other groups. The aqueous pulp extract of AM was found to have excellent scavenging effect on LPO, which was well comparable with the standard drug omeprazole. So, from the results obtained on LPO levels, it may be concluded that the protection by AM may be due to vitamin E, beta-carotene, flavonols, and flavonoids, which are present in AM pulp extract.
The defensive antioxidant enzyme next to SOD is CAT. CAT traps the harmful hydrogen peroxide and converts into water and oxygen. The activity of CAT was found to be decreased in aspirin treated rats. The inhibition of CAT activity during aspirin induced ulcer may be due to the increased generation of reactive free radicals, which can create an oxidative stress in the cells. The administration of herbal drug AM inversed the CAT activity in liver tissues and protected from the free radical induced oxidative stress.[32] This result supports that, the antioxidant properties of the herbal drug was excellent as compared with the standard drug omeprazole.
The destruction of superoxide radicals is catalyzed by SOD, which is an important defense system against oxidative damage. From our experimental results of the aforesaid antioxidant enzyme activities in stomach and duodenum tissues, it is clear that aspirin significantly decreased SOD, CAT, and GSH activities in aspirin- treated experimental group compared with control group, AM- treated control group, omeprazole- treated control group, omeprazole- pretreated aspirin- treated experimental group and AM- pretreated aspirin- treated experimental group. So, it may be concluded that the protection by AM may be due to vitamin E, beta-carotene, flavonols, and flavonoids, which are present in AM pulp extract.
In the present research work, we have observed the decreased level of glutathione in aspirin induced gastric and peptic ulcer rat model. Treatment with AM herbal drug had significantly improved the level of reduced glutathione both in stomach and duodenum tissues. Similar results were also obtained with the standard drug omeprazole. It prevents the hydroxyl radical generation by interacting with free radicals. The decreased level of GSH in aspirin- treated experimental group seen in our study indicates that there was an increased generation of free radicals and the GSH was depleted during the process of combating oxidative stress.[33,34] This has probably been possible either from the low level of reactive oxygen species (ROS) production or through a rapid dissolution of ROS that has further been strengthened by the elevated activities of important antioxidant defense enzymes CAT and SOD, studied in this experiment. AM has potential antioxidant action on cerebellar nodular lesion induced peptic ulcer rat model.[15] Stress, non steroidal anti- inflammatory drugs (NSAIDs), and Helicobacter pylori are the most common causes of ulceration.[1] Development of oxidative stress,[1,2,4,35] lowering gastroprotection, decrement of mucosal blood flow, delayed restitution, and regeneration, and so forth, play a vital role in the pathogenesis of ulcer.[2,3] The phenolic compounds, capable of scavenging peroxyl radicals, which are present in AM fruit extract are potent antioxidants[36] and have powerful antiulcer activities.[1] These compounds contain an OH group linked with the aromatic ring, and thus may possess potential antioxidant and antiulcer activities.
The salient findings of our present study suggest that phenolic compounds of the AM fruit extract provide a good source of antioxidants that could offer potential protective effects against aspirin- induced gastro–duodenal ulceration in albino rat model.
ACKNOWLEDGMENTS
We are highly indebted to B.Sc final year students, Krishnath College, University of Kalyani, and B.Sc final year students, Katwa College, University of Burdwan, for their valuable cooperation. The authors have no conflicts of interest and there is no funding agency to support this study and its publication.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bandyopadhyay U, Biswas K, Chatterjee R, Bandyopadhyay D, Chattopadhyay I, Ganguly CK, et al. Gastroprotective effect of Neem (Azadirachta indica) bark extract: Possible involvement of H (+) - K (+) ATPase inhibition and scavenging of hydroxyl radical. Life Sci. 2002;71:2845–65. doi: 10.1016/s0024-3205(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 2.Biswas K, Bandyopadhyay U, Chattopadhyay I, Varadaraj A, Ali E, Banerjee RK. A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J Biol Chem. 2003;278:10993–1001. doi: 10.1074/jbc.M210328200. [DOI] [PubMed] [Google Scholar]
- 3.Wallace JL, Granger DN. The cellular and molecular basis of gastric mucosal defense. Faseb J. 1996;10:731–40. doi: 10.1096/fasebj.10.7.8635690. [DOI] [PubMed] [Google Scholar]
- 4.Chattopadhyay I, Bandyopadhyay U, Biswas K, Maity P, Banerjee RK. Indomethacin inactivates gastric peroxidase to induce reactive oxygen mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic Biol Med. 2006;40:1397–408. doi: 10.1016/j.freeradbiomed.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Maity P, Biswas K, Roy S, Banerjee RK, Bandyopadhyay U. Smoking and the pathogenesis of gastroduodenal ulcer—recent mechanistic update. Mol Cell Biochem. 2003;253:329–38. doi: 10.1023/a:1026040723669. [DOI] [PubMed] [Google Scholar]
- 6.Jagetia GC, Venkatesh P, Baliga MS. Evaluation of the radioprotective effect of Aegle marmelos (L.) Correa in cultured human peripheral blood lymphocytes exposed to different doses of gamma-radiation: A micronucleus study. Mutagenesis. 2003;18:387–93. doi: 10.1093/mutage/geg011. [DOI] [PubMed] [Google Scholar]
- 7.Vidhya N, Devaraj SN. Antioxidant effect of eugenol in rat intestine. Indian J Exp Biol. 1999;37:1192–5. [PubMed] [Google Scholar]
- 8.Ogata M, Hoshi M, Urano S, Endo T. Antioxidant activity of Eugenol and related monomeric and dimeric compounds. Chem Pharm Bull (Tokyo) 2000;48:1467–9. doi: 10.1248/cpb.48.1467. [DOI] [PubMed] [Google Scholar]
- 9.Nagashima K. Inhibitory effect of Eugenol on Cu2+- catalyzed lipid peroxidation in human erythrocyte membranes. Int J Biochem. 1989;21:745–9. doi: 10.1016/0020-711x(89)90205-x. [DOI] [PubMed] [Google Scholar]
- 10.Sabu MC, Kuttan R. Antidiabetic activity of Aegle marmelos - and - its relationship with its antioxidant properties. Indian J Physiol Pharmacol. 2004;48:81–8. [PubMed] [Google Scholar]
- 11.Ladaniya MS. Citrus fruit biology technology and - evaluation. Waltham, Massachusetts: Academic Press; 2008. pp. 1–558. [Google Scholar]
- 12.Cho C H, Ogle C W. Cholinergic- mediated gastric mast cell degradation with subsequent histamine H1 and H2 receptor activation in stress ulceration in rats. Eur J Pharmacol. 1979;55:23–33. doi: 10.1016/0014-2999(79)90144-4. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar S, Buha D. Effect of ripe fruit pulp extract of Cucurbita pepo Linn. in aspirin induced gastric and duodenal ulcer in rats. Indian J Exp Biol. 2008;46:639–45. [PubMed] [Google Scholar]
- 14.La Corte R, Caselli M, Castellino G, Bajocchi G, Trotta F. Prophylaxis and treatment of NSAID induced gastroduodenal disorders. Drug Saf. 1999;20:527–43. doi: 10.2165/00002018-199920060-00006. [DOI] [PubMed] [Google Scholar]
- 15.Singh P, Guha D. Effect of Aegle marmelos in Cerebellar- nodular-lesion induced experimental peptic ulcer: Role of mucosal aggressive and defensive factors – Biochemical Study. Biogenic Amines. 2008;22:1–84. [Google Scholar]
- 16.Roy C, Ghosh TK, Guha D. The antioxidative role of Benincasa hispida on colchicine induced experimental rat model of Alzheimer's disease. Biogenic Amines. 2007;21:44–57. [Google Scholar]
- 17.Kind PR, King EJ. In: Method of practical clinical biochemistry. Invarley H, Gowenlock AH, editors. London: Bell Meditors; 1980. p. 899. [Google Scholar]
- 18.Reitman S, Frankel S. A Colourimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 19.Mishra HP, Fridovich I. The generation of radical during superoxide auto oxidation of hemoglobin. J Biol Chem. 1972;34:30–7. [Google Scholar]
- 20.Roy C, Ghosh TK, Guha D. Dose dependent activity of Benincasa hispida on colchicine induced experimental rat model of Alzheimer's disease. Int J Pharmacol. 2008;4:237–44. [Google Scholar]
- 21.Hazra R, Ray K, Guha D. Inhibitory role of Acorus calamus in ferric chloride induced epileptogenesis in rat. Hum Exp Toxicol. 2007;26:947–53. doi: 10.1177/0960327107087791. [DOI] [PubMed] [Google Scholar]
- 22.Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970;34:30–8. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 23.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 24.Japundzic I, Levi E, Rakic-Stojiljkovic LS. Effect of duodenal ulcerogens mepirizole and propionitrile on small intestinal and liver alkaline phosphatase activity in rats. Digestion. 1990;47:61–70. doi: 10.1159/000200478. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar N, Purkayastha S, Sarkar B, Guha D. Modulation of gastric mast cell population: Role of vestibule cerebellar lesion. Ind J Exp Biol. 2006;44:627–34. [PubMed] [Google Scholar]
- 26.Flemstrom G, Kivilaakso E. Demonstration of a pH gradient at the luminal surface of rat duodenum in vivo and its dependence on mucosal alkaline secretion. Gastroenterology. 1983;84:787–94. [PubMed] [Google Scholar]
- 27.Dhikav V, Singh S, Pande S, Chawla A, Singh AK. Non-steroidal drug-induced gastrointestinal toxicity: Mechanisms and management. J Indian Acad Clin Med. 2003;4:315–22. [Google Scholar]
- 28.Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15:691–703. doi: 10.1053/bega.2001.0229. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita M, Noto T, Tamaki H. Effect of a combination of ecabet sodium and cimetidine on experimentally induced gastric lesions and gastric mucosal resistance to ulcerogenic agents in rats. Biol Pharm Bull. 1995;18:223–6. doi: 10.1248/bpb.18.223. [DOI] [PubMed] [Google Scholar]
- 30.Dianzani MU. Lipid peroxidation in ethanol poisoning: A critical reconsideration. Alcohol. 1985;20:161–73. [PubMed] [Google Scholar]
- 31.Husain K, Somani SM. Interaction of exercise and ethanol on hepatic and plasma antioxidant system in rat. Pathophysiology. 1997;4:69–74. doi: 10.1002/(sici)1099-1263(199705)17:3<189::aid-jat431>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Rajashree CR, Rajmohan T, Augusti KT. Antiperoxidative effect of garlic in alcohol fed rats. Ind J Exp Biol. 1998;36:60–4. [PubMed] [Google Scholar]
- 33.Reiter RJ. Melatonin: Lowering the High Price of Free Radicals. News Physiol Sci. 2000;15:246–50. doi: 10.1152/physiologyonline.2000.15.5.246. [DOI] [PubMed] [Google Scholar]
- 34.Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–11. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 35.Szabo I, Tarnawski AS. Apoptosis in the gastric mucosa: Molecular mechanisms, basic and clinical implications. J Physiol Pharmacol. 2000;51:3–15. [PubMed] [Google Scholar]
- 36.Karakaya S. Bioavailability of phenolic compounds. Crit Rev Food Sci Nutr. 2004;44:453–64. doi: 10.1080/10408690490886683. [DOI] [PubMed] [Google Scholar]


