Abstract
Background/Aim:
To compare the quality of life (QOL) in patients undergoing transhiatal esophagectomy (THE) with or without chemotherapy, who were admitted to the Post Graduate Institute of Medical Education and Research, Chandigarh and enrolled in the study, from July 2004 to October 2005.
Patients and Methods:
Thirty patients of esophageal carcinoma by purposive sampling were randomized into two groups i.e., patients undergoing THE after chemotherapy and patients undergoing THE without chemotherapy. Two QOL questionnaires, one generic i.e., EORTC-QLQ C-30 (European Organization for Research and Treatment of Cancer) and other esophageal cancer-specific i.e., EORTC OES-18 were utilized to assess the QOL.
Result:
Physical functional scales were better in patients, who received neoadjuvant chemotherapy. The role and social aspects of functional scales deteriorated after completion of treatment in both groups. This was primarily due to the effect of surgery. However, they were better from an emotional and cognitive point of value after surgery and radiotherapy. Fourteen out of 30 patients experienced vomiting and diarrhea due to radiotherapy.
Conclusion:
THE in esophageal carcinoma improves global health scales and majority of symptom scales in all patients. QOL improvement in general was better in patients who were administered neoadjuvant chemotherapy along with surgery.
Keywords: Esophagectomy, functional scale, global health scale, neoadjuvant, quality of life, transhiatal
Carcinoma esophagus is one of the most aggressive malignancies of the gastrointestinal tract. Long-term survival is mostly stage dependent. The overall 5-year survival in esophageal carcinoma ranges from 15 to 22%.[1] Despite recent advances in early diagnosis, a number of patients are in advanced stage at the time of diagnosis. Patients are often elderly and have significant comorbid diseases.[2] In the past two decades there is growing interest in broadening the evaluation criteria employed in cancer clinical trials beyond the traditional parameters i.e., tumor response, 30-day mortality, morbidity, disease free and overall survival. Owing to dismal prognosis of esophageal carcinoma, one of the main objectives of surgery and adjuvant treatment is to maintain sufficient quality of life (QOL) during the limited survival time. The assessment of QOL in esophageal cancer is important because it provides detailed information about patient's perception of their health. Such QOL data can be used to describe relative effectiveness of treatment, enhance patient decision making and may even predict the survival.[3] Although there is no strict definition of elements that contribute to health-related QOL, it is generally accepted that they include physical, social and psychological aspects.[4] Few studies have examined these wider issues in patients of esophageal cancer and they have presumed that the most common presenting symptom, dysphagia, has an overwhelming influence on the patient's QOL.[5] Despite the disease being so common, no study has been reported from India that examines the physical, emotional, cognitive and social dimensions in patients with esophageal carcinoma undergoing esophagectomy. The objective of this study is to assess the QOL following transhiatal esophagectomy (THE) in patients with esophageal carcinoma.
PATIENTS AND METHODS
Thirty patients with esophageal carcinoma admitted to the department of general surgery at the Post Graduate Institute of Medical Education and Research, Chandigarh, were enrolled in the study, from July 2004 to October 2005. Approval to conduct the study was obtained from the ethics committee. Patients older than 18 years of age and with American Society of Anesthesiologists class I or II were included. Exclusion criteria were preoperative radiation therapy, tracheoesophageal fistula and patients unfit for surgery. Eligible patients with histologically confirmed carcinoma of middle or distal third esophagus were randomly allocated to one of the two groups - patients undergoing THE after chemotherapy and patients undergoing THE without chemotherapy with the help of numbered sealed envelope method. Randomization was done using computer-generated table of random numbers. Patients in chemotherapy arm received 2 cycles of Cisplatin and 5-Flurouracil based neoadjuvant chemotherapy. Neoadjuvant chemotherapy was followed by THE after 4 weeks. All patients received postoperative radiotherapy. QOL questionnaires were filled prior to neo adjuvant chemotherapy, before surgery and 16 weeks after completion of radiotherapy. Two QOL questionnaires, one generic i.e., EORTC-QLQ C-30 (European Organization for Research and Treatment of Cancer) and other esophageal cancer specific i.e., EORTC OES-18 were utilized to assess the QOL. Comparison of each item of all scales was performed by nonparametric test (Wilcoxan sign rank test). Statistical analysis was performed using SPSS 11.0. A significance level of P<0.05 was used throughout.
EORTC-QLQ C-30 is a 30 item questionnaire composed of multi-item scales that reflects the multidimensionality of the QOL construct. It incorporates five functional scales (physical, role, cognitive, emotional and social), three symptom scales – fatigue, pain, nausea and vomiting, global health scale and additional symptoms commonly reported by cancer patients as well as the perceived financial impact of disease and treatment. Score ranges from 0 to 100. A higher score represents a higher level of functioning or a greater degree of symptoms. Similarly esophageal cancer specific module includes 18 items. There are four scales and six items for each scale. High score for an item means worse QOL or more problems.
RESULTS
Thirty patients of esophageal carcinoma were enrolled in the study. Seventeen were male and thirteen were female. Mean age of the patient was 52 years (range 35-70 years). Neoadjuvant chemotherapy was administered in 15 patients and 15 patients were directly subjected to surgery. THE was performed in all patients.
Comparison of QOL sores using EORTC-QLQ C-30 [Table 1]
Table 1.
Comparison of global, functional and symptom scales by using EORTC-QLQ C-30
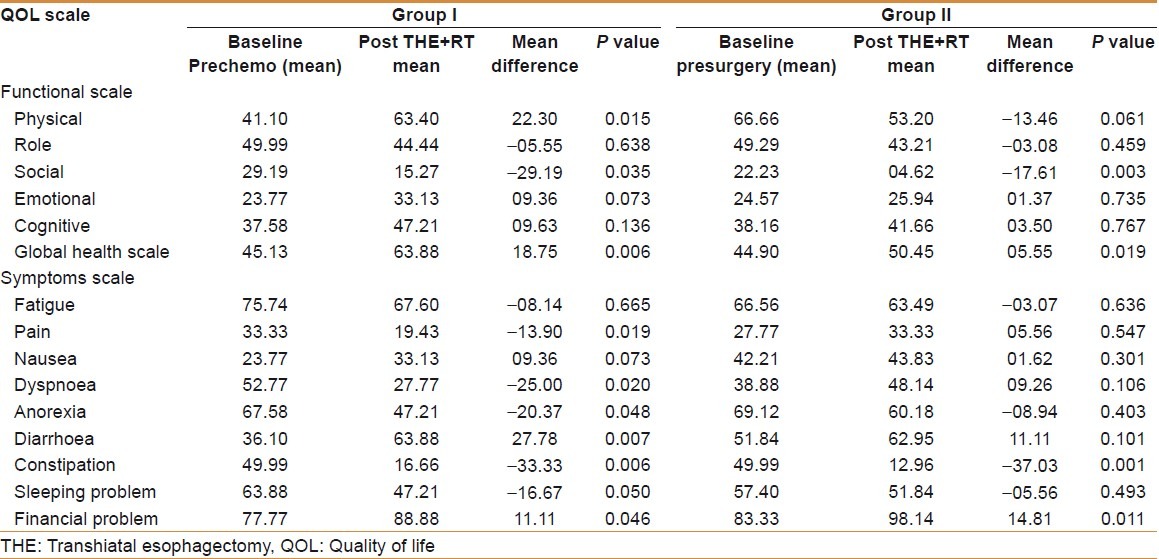
Functional scales [Figure 1]
Figure 1.
Comparison of functional scales between two groups by using EORTC-QLQ C-30
There was significant improvement in physical functional scale in group I patients by 22.30 (P=0.015), whereas it deteriorated by 13.46 in group II (P=0.061) at the end of treatment. The social and role functional scales showed worsening in both groups. Worsening in social functional scale was significant (P=0.035 and P=0.003), while worsening in role functional scale in both groups was not significant (P=0.638 and P=0.459). Similarly emotional and cognitive functional scales improved in both groups of patients but this improvement was not significant
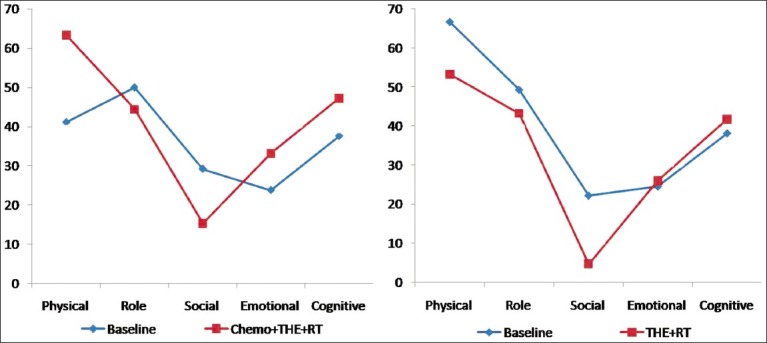
Global health scale [Figure 2]
Figure 2.
Comparison of Global health scale between two groups by using EORTC-QLQ C-30
There was significant improvement in global health scale in both groups of patients at the end of treatment. It improved by 18.75 in group I (P=0.006) and 05.55 in group II (P=0.019).
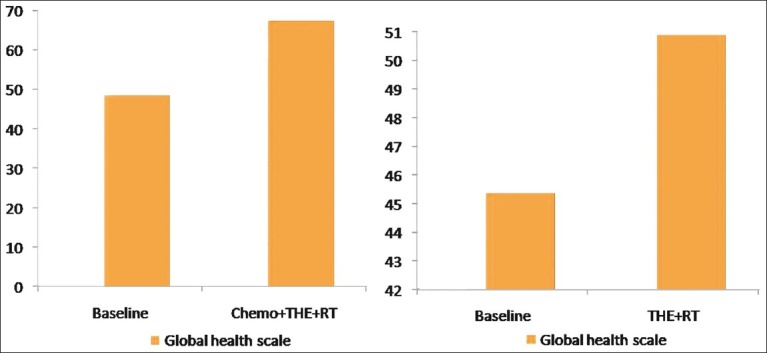
Symptoms scale [Figure 3]
Figure 3.
Comparison of symptoms scale between two groups by using EORTC-QLQ C-30
There was significant improvement in pain and dyspnea scores in group 1 patients (P=0.019 and P=0.020), whereas both symptoms deteriorated in group II patients. But this deterioration was not significant (P=0.547 and P=0.106). Similarly anorexia score also showed improvement and this improvement was significant in group I (P=0.048 and P=0.403). Diarrhea scores showed significant worsening in group I (P=0.007) and nonsignificant worsening in group II (P=0.101). Constipation score improved significantly in both groups of patients (P=0.006 and P=0.001). Although fatigue and sleeping problem scores improved in both groups but this improvement was not significant. Nausea score showed nonsignificant worsening in both groups of patients. Financial impact was significant in both groups of patients. It worsened by 21.43% in group I (P=0.046) and 17.77% in group II (P=0.011).
Comparison of QOL sores using EORTC OES-18 [Table 2 and Figure 4]
Table 2.
Comparison of symptoms between two groups by using EORTC OES-18
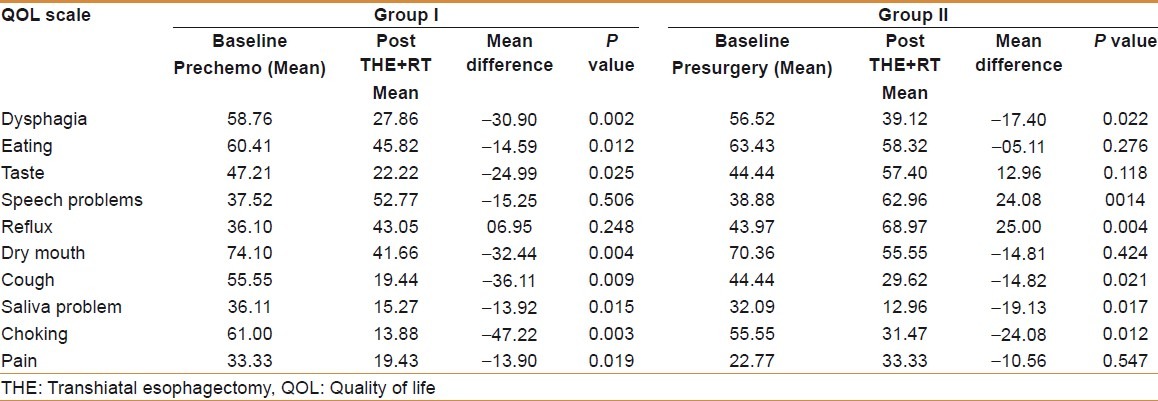
Figure 4.
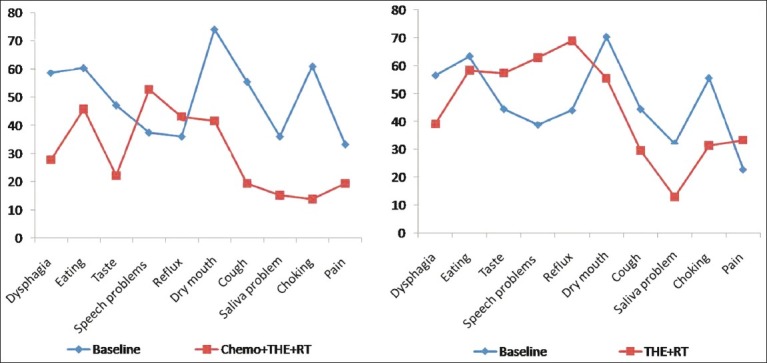
Comparison of symptoms between two groups by using EORTC OES-18
There was significant improvement in dysphagia, cough, choking and saliva problems in both groups of patients. Eating and dry mouth score showed significant improvement in group I patients and nonsignificant improvement in group II patients. There was significant worsening in reflux score in both groups of patients but worsening of speech score was significant in group I only. Pain score showed significant improvement in group I, while group II patients showed nonsignificant deterioration. Taste score showed significant improvement in group 1 patients (P=0.025) and a nonsignificant deterioration in group II (P=0.118).
DISCUSSION
Disease as well as their treatment affects human life in a profound manner. Disease causes structural and functional limitations that may seriously affect the well being of the individual i.e., QOL.[6] The issue of QOL is especially relevant to chronic illnesses like cancer, autoimmune disease, diabetes, hypertension, etc., therefore, in the past two decades, QOL has become an important determinant of demand for care, compliance with treatment regimen and overall treatment satisfaction.[7] There is no consensus as yet regarding the definition, concept and ways to measure the QOL of an individual. Studies on this subject have equated QOL with concepts like “life satisfaction”, “subjective well being”, “health-related QOL”, etc. Various definitions have also been given which are either global[6,8] or specifically related to health.[9] However, most of the researchers agree that QOL is a multidimensional[10] and dynamic concept.[11] It includes both objective and subjective components but controversy exists as to which is more important.[12,13] Recent opinion suggests a shift of attention towards the subjective perception of QOL. To assess and quantify the QOL of a person, a number of instruments have been created and adopted for use with different patient populations.[14] There are two basic types of instruments, disease specific and generic. Generic instruments are applicable to a wide range of health problems. For cancer modular format, the instruments have recently been developed, which comprise a core of general purpose QOL items together with more specific instruments design for each main type of cancers. Gelfand and Finley[15] in 1994 had observed that out of the 7569 publications on esophageal carcinoma, only 44 (0.58%) dealt with QOL in patients with esophageal carcinoma using valid multidimensional tools.[4,5] Accurate data indicating as to how different treatments affect the short and long-term QOL of patients can assist the doctors in customizing the treatment protocols.
Surgery has been the treatment of choice for localized esophageal cancer. A number of studies have investigated whether preoperative chemotherapy followed by surgery leads to an improvement in cure rates, but the individual reports have been conflicting. Several randomized trials have compared preoperative chemotherapy and surgery with surgery alone for localized esophageal carcinoma. There appears to be a survival advantage for chemotherapy although this is associated with increased treatment toxicity and mortality.[7] None has included a measure of self reported QOL and it is recommended that future studies must include validated QOL questionnaires. For patients with esophageal carcinoma, QOL measures may guide treatment decisions because of the magnitude of negative impact that potentially curative treatment has on QOL and fairly small survival benefits related to combination treatment.
A number of studies, assessing the QOL in esophageal cancer have also reported the benefits of neoadjuvant chemotherapy with acceptable morbidity. Besides the advantages of better QOL, neoadjuvant chemotherapy has been reported to have a survival advantage as compared to patients of surgery alone.[11] Although inter-trial group by Kelson et al.[16] did not report the benefit of neoadjuvant chemotherapy, a study published by Blazeby et al.[17] supported the use of neoadjuvant chemotherapy before surgery. In this study also, patients with chemotherapy in general showed improvement in most of the scales. Dysphagia, the most distressing symptom, improved along with other symptoms and they were in a better physical and psychological frame of mind before surgery. This may be the reason why this group of patients showed better QOL following surgery in comparison to those who were directly subjected to surgery. The role and social aspects of functional scales deteriorated after completion of treatment in both groups. This was primarily due to the after effects of major surgery. However, they were better from an emotional and cognitive point of view, after surgery and radiotherapy. It may be concluded that there was overall improvement in functional, global health and symptom scales following esophagectomy in patients with or without chemotherapy. However, the improvement was significant in majority of scales in the patients who received neoadjuvant chemotherapy. This group of patients also showed significant improvement in physical scale. Fourteen out of 30 patients (43.75%) experienced vomiting and diarrhea due to radiotherapy. Apparently it was transient and self limiting. It may be inferred that neoadjuvant chemotherapy in esophageal carcinoma not only improves the short-term QOL but also has potential to increase the long-term survival.
The fact that changes in QOL score have been well reflected in both proformas (EORTC – OES 18 and EORTC – QLQ-30), validates the usefulness of these proformas in the assessment of the QOL in esophageal carcinoma. This would allow assessment of benefits as well as detrimental effects experienced during the treatment. Results from this randomized cohort undergoing potentially curative treatment for localized esophageal carcinoma support the use of neoadjuvant treatment before surgery. QOL scores would help to guide therapy for esophageal carcinoma by allowing patients and clinicians to make more informed treatment decisions.
CONCLUSIONS
THE in carcinoma esophagus improves global health scales and majority of symptom scales in all patients. QOL improvement in general is better in patients who were administered neoadjuvant chemotherapy along with Surgery. Nausea, vomiting and financial problems are the factors that worsen following THE.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Salazar JD, Doty JR, Lin JW, Dyke MC, Roberts J, Heitmiller ES, et al. Does cell type influence postesophagectomy survival in patients with esophageal cancer. Dis Esophagus. 1998;11:168–71. doi: 10.1093/dote/11.3.168. [DOI] [PubMed] [Google Scholar]
- 2.Muller JM, Erasmi H, Stelzner M, Zieren U, Pichlmaier H. Surgical therapy of oesophageal carcinoma. Br J Surg. 1990;77:845–57. doi: 10.1002/bjs.1800770804. [DOI] [PubMed] [Google Scholar]
- 3.Blazeby JM, Brooks ST, Alderson D. The prognostic value of quality of life scores during treatment for oesophageal cancer. Gut. 2001;49:227–30. doi: 10.1136/gut.49.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seenu V, Pal H, Chattopadhayay TK. Quality of life after total esophagectomy for esophageal cancer. Trop Gastroenterol. 2001;22:7–13. [PubMed] [Google Scholar]
- 5.Blazeby JM, Williams MH, Brookes ST, Alderson D, Farndon JR. Quality of life measurement in patients with oesophageal cancer. Gut. 1995;37:505–8. doi: 10.1136/gut.37.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy DM. Quality of life, a critical assessment. Scand J Gastroenterol Suppl. 1995;208:141–6. doi: 10.3109/00365529509107777. [DOI] [PubMed] [Google Scholar]
- 7.Leplege A, Hunt S. The problem of quality of life in medicine. JAMA. 1997;278:47–50. doi: 10.1001/jama.1997.03550010061041. [DOI] [PubMed] [Google Scholar]
- 8.Calman KC. Quality of life in cancer patients - a hypothesis. J Med Ethics. 1984;10:124–7. doi: 10.1136/jme.10.3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schipper H. Guidelines and caveats for quality of life measurement in clinical practice and research. Oncology (Williston Park) 1990;4:51–7. discussion 70. [PubMed] [Google Scholar]
- 10.Rice S, Miller D. Quality of life issues. South Med J. 1990;83:942. doi: 10.1097/00007611-199008000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Medical Research Council esophageal cancer working Group. Surgical resection with or without preoperative chemotherapy in esophageal cancer: A randomized controlled trial. Lancet. 2002;359:1727–33. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 12.Campbell A. Subjective measure of well being. Am Psychologist. 1976;31:117–24. doi: 10.1037//0003-066x.31.2.117. [DOI] [PubMed] [Google Scholar]
- 13.Spilker B. In: Quality of life assessment in clinical trials. Spilker B, editor. New York: Raven Press; 1990. pp. 3–9. [Google Scholar]
- 14.Gladis MM, Gosch EA, Dishuk NM, Critis-Christoph C. Quality of life: expanding the scope of clinical significance. J Consult Clin Psychol. 1999;67:320–31. doi: 10.1037//0022-006x.67.3.320. [DOI] [PubMed] [Google Scholar]
- 15.Gelfand GA, Finley RJ. Quality of life with carcinoma of the esophagus. World J Surg. 1994;18:399–405. doi: 10.1007/BF00316820. [DOI] [PubMed] [Google Scholar]
- 16.Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–84. doi: 10.1056/NEJM199812313392704. [DOI] [PubMed] [Google Scholar]
- 17.Blazeby JM, Sanford E, Falk SJ, Alderson D, Donovan JL. Health related quality of life during Neoadjuvant treatment and surgery for localized esophageal carcinoma. Cancer. 2005;103:1791–9. doi: 10.1002/cncr.20980. [DOI] [PubMed] [Google Scholar]


