Abstract
Background:
The use and investigation of natural products with antimicrobial activity from vegeral source have been reported by several researchers. Cajanus cajan (Fabaceae) is a multiple use specie mainly as human food. In popular medicine, diverse parts of the plant are used as sedative and to treat cough, hepatitis, and diabetes.
Materials and Methods:
This study shows the characterization of secondary metabolites present in ehtanolic extracts from leaves and roots of Cajanus cajan by phytochemical prospection. The evaluation of the antifungal activity was performed by the microdilution method, and from the subinhibitory concentrations (MIC 1/8) the modulatory activity of antifungical (fluconazole and ketoconazole) was analyzed by the direct contact assay against C. albicans ATCC40006, Candida krusei ATCC 6538 and Candida tropicalis ATCC 40042.
Results:
The results showed the presence of tannins, flavonoids, and alkaloids in both extracts as the clinically relevant antifungal activity. The modulatory potential is presented by the antifungal tested against yeasts.
Conclusion:
The extracts studied here have demonstrated to be a new therapeutic source to treat these microorganism-associated diseases.
Keywords: Antifungal activity, Cajanus cajan, phytochemical prospection, modulatory activity
INTRODUCTION
The increasing incidence of adverse side effects associated with conventional drugs, and the emergence of resistance to antibiotics[1,2] justifies the search for new drugs from vegetable species in order to combat the multiresistant microorganisms.[3,4] The study of natural products from vegetable origin presenting antimicrobial activity has been shown in many perspectives mainly because of the presence of tannins, alkaloids, saponins, and terpenes. This illustrates the importance of natural products as sources of new antimicrobials agents.[5]
Cajanus cajan (L.) Millsp (Fabaceae) is a annual perennial shrub largely cultivated in tropical and subtropical regions of the world, typical to dry climates.[6] Its seeds are sources of vitamins, minerals, and proteins,[7] and it is popularly known as gaundú, ervilha de pombo among others.[8] Although there are few studies regarding the phytochemical profile of the roots in comparison with ones about C. cajan seeds, these different parts of the plant are used in traditional medicine as sedative and to treat cough, hepatitis, and diabetes.[9,10]
This work is aimed to perform the phytochemical prospection and to evaluate the antifungal activity of ethanolic extracts from C. cajan fresh leaves and roots. Moreover, the possible modulator effect of fluconazole and ketoconazole was also analyzed.
MATERIALS AND METHODS
Plant material obtention
C. cajan leaves and roots were collected from Gavião farma, Missão Velha municipality (Ceatá States, Brazil) in April 2010. A voucher specimen was sent to the Herbarium Caririense Dárdano de Andrade Lima, which is deposited on the registration #5108.
Preparation of extracts and phytochemical prospection
Fresh triturated leaves (700 g) and roots (862 g) were exhaustively extracted with cold ethanol for 72 h.[11] After this, the solvent was distilled in a rota evaporator under reduced pressure to give 200 g (leaves extract) and 180 g (roots extract). Phytochemical tests to detect the presence of secondary metabolites were performed following the method described by Matos.[12] These tests were based on the visual observation of color modification or precipitate formation after the addition of specific reagents.
Antifungal activity evaluation – minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC)
The minimal inhibitory concentration (MIC) was obtained by a broth microdilution assay according to NCCLS guidelines.[13] Before the tests, fungi were activated in Sabouraud medium (48 h, 35±2°C). Three ATCC standard strains provided by Oswaldo Cruz Foundation – FIOCRUZ were used: Candida albicans ATCC40006, Candida krusei ATCC 6538, and Candida tropicalis ATCC 40042.
After the subculture, Each culture was mixed with a sufficient volume of sterile supplemented Sabouraud broth to achieve a turbidity equivalent to that of McFarland's no. 0.5 standard. To obtain the test inoculum, this suspension was further diluted with the same culture medium to approximately 1 × 106 colony-forming units (CFU)/mL immediately before use. Each dilution measuring 100 μL were distributed in 96-well plates plus extracts in different concentrations, achieving 5×105 UFC/mL as the final concentration of the inoculum.
Extracts and fractions were dissolved in distilled water and dimethyl sulfoxide (DMSO) to concentration of 1024 μg/mL. Further serial dilutions were performed by addition of the broth to reach a final concentration in the range of 512 a 8 μg/mL. All experiments were performed in triplicate, and the microdilution trays were incubated at 35±2°C for 24 h.
The plates containing the fungal strains were analyzed and the turbidity of the broth as the indication of growth of microorganisms.[14] MIC was defined as the minimal concentration able to inhibit the yeast growth in comparison to positive control. The fungal culture that did not present growth was used to inoculate plates containing solid medium in dextrose potato agar (PDA) in order to obtain the minimal fungicidal concentration (MFC).[15,16]
Fluconazole and ketoconazole modifying activity
The evaluation of ethanolic extracts as antifungal activity modifiers was performed using fluconazole and ketoconazole MICs obtained in the presence and absence of the microdilution method modified according to CLSI/NCCLS M7-A6 guideline.[13] Inhibitory concentration (MIC 1/8, 10% Sabouraud broth) were used against all strains previously described. Fungi were exposed to extracts that presented MICs ≤ 512 μg/mL.
The antifungal solutions (1024 μg/mL) were prepared in distillated water for use on the same day. A total of 100 μL of the antifungal solution, using serial dilutions (1 : 2), was added to the wells containing 10% sabouraud and the diluted fungal suspension (1 : 10). Final antifungal concentrations were 512, 256, 128, 64, 32, 16, 8, 4, 2, and 1 μg/mL. Microplates were incubated for 24 h at room temperature and the modulatory activity was determined by visualization of turbidity.
RESULTS AND DISCUSSION
Phytochemical prospection
Phytochemical prospection of C. cajan leaves and roots ethanolic extract indicated the presence of tannins, flavonoids and alkaloids. These results are in concordance with other studies performed with C. cajan leaves extracts too, where these same secondary metabolites classes were found.[17,18] Other reports quantified and isolated flavonoids in leaves extract (quercetin, luteolin, apigenin, and isorhamnetin)[19,20] and roots of this specie.[21,10]
Biological activities are described for different secondary metabolites classis. Although the antifungal propriety of flavonoids is well known, flavonoids also possess antibacterial, antilipoperoxidative and antiparasitic activities.[22,23] Tannins are reported to exhibit bactericidal and fungicidal properties[24] and alkaloids are antitumor, antiinflammatory and antimicrogial.[25]
Evaluation of antifungal activity (MIC) and minimal fungicidal conncentration (MFC)
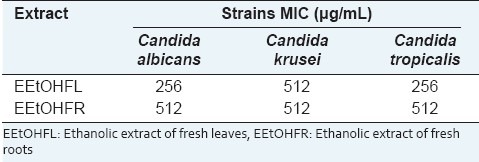
As shown in Table 1, the ethanolic extracts obtained from C. cajan inhibited the growth of yeasts strains. The leaves extracts presented a MIC of 256 μg/mL against C. albicans and C. tropicallis.
Table 1.
Minimal inhibitory concentration of C. cajan leaves and roots extracts
Leaves and seeds from C. cajan have been extensively studied because of the ethnopharmacological properties. Diverse activities have been elucidated as the anticandidal. In the study performed by Runyoro et al.[26] using the methanolic extract of leaves, a moderate activity against C. albicans was verified by the bioautography method. However, according to Bolle et al.[27] this method may be not accurate because it can induce the compound decomposition.
Ethanolic extracts from C. cajan leaves were very active against C. albicans[28] using the cavity and disk diffusion methods. This result corroborates our findings that can be considered clinically relevant[29] as the extract presented MIC ≤ 1000 μg/mL against all strains tested.
When Braga et al.[17] tested methanolic extracts of leaves and seeds were tested against C. albicans e Cryptococcus neoformans, the inhibition halos were considered to be very active, but a MIC value of 2500 μg/mL was obtained against C. albicans. A value much higher than that one found here. This can be explained by extracts of this species in different regions of Brazil can lead to extracts with different chemical composition from that previously reported.
The extracts studied here presented MFC ≥ 1024 μg/mL against all Candida species suggesting that the extracts can be fungistatic too.
Evaluation of fluoconazole and ketoconazole modifying activity
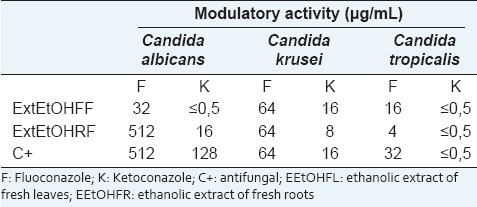
The modifier effect of fluoconazole and ketoconazole activity are shown in Table 2. It is possible to see a strong synergism between both antifungal and C. cajan extracts (MIC 1/8) against Candida species.
Table 2.
Minimal inhibitory concentration of antifungal alone or associated to C. cajan extracts against Candida species
According to studies performed by Giordani et al,[30] essential oil from Thymus vulgaris and Origanum vulgare were strongly active against Candida albicans. Besides this, the essential oil from T. vulgaris potentialized the action of amphotericin B leading to the complete inhibition of fungal growth.
Table 2 shows that both antifungal had their activity potentialized by leaves extracts against C. albicans. Roots extract, in combination with the fluoconazole and ketoconazole, presented better effects against the other Candida species. This is the first report of a natural product potentializing the fluoconazole and ketoconazole effects.
CONCLUSION
The phytochemical prospection of C. cajan leaves and roots extracts have demonstrated the presence of diverse class of compound and these compounds are related to known biological activities (as antifungal). The leaves extract presented best activity against C. albicans and C. tropicalis. However, the evaluation of antifungal modifying activity suggest that the extracts studied here demonstrated to be a new therapeutic source to treat diseases associated to the yeasts resistant to conventional antifungal. The results are promising and they can stimulate future researches regarding the phytochemical, toxicological, and pharmacological aspects of natural products from Cajanus cajan.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shepherd GM. Hypersensitivity reactions to drugs: evaluation and management. Mt Sinai J Med. 2003;70:113–25. [PubMed] [Google Scholar]
- 2.Keith CT, Borisy AA, Stockwell BR. Multicomponent therapeutics for networked systems. Nat Rev Drug Discov. 2005;4:71–8. doi: 10.1038/nrd1609. [DOI] [PubMed] [Google Scholar]
- 3.Costa JG, Rodrigues FF, Angélico EC, Pereira CK, Sousa EO, Caldas GF, et al. Chemical composition and evaluation antibacterial activity and toxicity of essential oil of. Croton zehntneri (variedade estragol) Braz J Pharmacogn. 2008;18:583–6. [Google Scholar]
- 4.Coutinho HD, Costa JG, Siqueira-Júnior JP, Lima EO. In vitro anti-staphylococcal activity of Hyptis martiusii Benth against methicillin-resistant Staphylococcus aureus-MRSA strains. Braz J Pharmacogn. 2008;8:670–5. [Google Scholar]
- 5.Kuete V, Tangmouo JG, Penlap Beng V, Ngounou FN, Lontsi D. Antimicrobial activity of the methanolic extract from the stem bark of Tridesmostemon omphalocarpoides (Sapotaceae) J Ethnopharmacol. 2006;104:5–11. doi: 10.1016/j.jep.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Odeny DA, Jayasshree B, Fergusow M, Woisington D, Cry LJ, Gebhardt C. Development, characterization and utilization of microsatellite markers in Pigeonpea. Plant Breed. 2007;126:130–6. [Google Scholar]
- 7.Fujita K, Kai Y, Takayanagi M, El-Shemy H, Adu-Gyamfi JJ, Mohapatra PK. Genotypic variability of pigeonpea in distribution of photosynthetic carbon at low phosphorus level. Plant Sci. 2004;166:641–9. [Google Scholar]
- 8.Beltrame TP, Rodrigues E. Comparing different densities of pigeon pea (Cajanus cajan (L.) Millsp.) for restoration of forest reserves in Pontal do Paranapanema, SP. Sci Forum. 2008;36:317–27. [Google Scholar]
- 9.Duker-Eshun G, Jaroszewski JW, Asomaning WA, Oppong-Boachie F, Christensen S Brogger. Antiplasmodial constituents of Cajanus cajan. Phytother Res. 2004;18:128–30. doi: 10.1002/ptr.1375. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D, Zhang S, Zu Y, Fu Y, Kong Y, Gao Y, et al. Negative pressure cavitation extraction and antioxidant activity of genistein and genistin from the roots of pigeon pea [Cajanus cajan (L.) Millsp.] Sep Purif Technol. 2010;74:261–70. [Google Scholar]
- 11.Matos FJ. Introduction to Experimental Phytochemistry. Fortaleza: Editora UFC; 1998. [Google Scholar]
- 12.Matos FJ. Introduction to Experimental Phytochemistry. 2nd ed. Fortaleza: Editora UFC; 1997. [Google Scholar]
- 13.Methods for Dilution Antimicrobial Susceptibility Tests for bacteria that grow aerobically, Approved Standard M7-A6. 6th ed. Wayne: NCCLS; 2003. National Committee for Clinical Laboratory Standards. [Google Scholar]
- 14.Lennete EH, Hansler JR, Shadomy HJ. Manual of Clinical Microbiology. 4th ed. Washington, D.C: American Society for Microbiology Press; 1985. pp. 282–301. [Google Scholar]
- 15.Food and Drugs Administration Code. Fed Regul. 1991;21:300. [Google Scholar]
- 16.Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and others plants extracts. J Appl Microbiol. 1999;86:985–90. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 17.Braga FG, Bouzada ML, Fabri RL, de O Matos M, Moreira FO, Scio E, et al. Antileishmanial and antifungal activity of plants used in traditional medicine in Brazil. J Ethnopharmacol. 2007;111:396–402. doi: 10.1016/j.jep.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Nwachukwu E, Uzoeto HO. Antimicrobial activities of leaf of Vitex doniana and Cajanus cajan on some bacteria. Researcher. 2010;2:37–47. [Google Scholar]
- 19.Zu YG, Fu YJ, Liu W, Hou CL, Key YK. Simultaneous Determination of Four Flavonoids in Pigeonpea [Cajanus cajan (L.) Millsp.] Leaves Using RP-LC-DAD. Chromatographia. 2006;63:499–505. [Google Scholar]
- 20.Wu N, Fu K, Fu Y, Zu Y, Chang F, Chen Y, et al. Antioxidant Activities of Extracts and Main Components of Pigeonpea [Cajanus cajan (L.) Millsp.] Leaves. Molecules. 2009;14:1032–43. doi: 10.3390/molecules14031032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu LM, Zu Y, Fu Y, Zhang S, Yao L, Efferth T. Cajanol, a novel anticancer agent from Pigeonpea [Cajanus cajan (L.) Millsp.] roots, induces apoptosis in human breast cancer cells through a ROS-mediated mitochondrial pathway. Chem Biol Interact. 2010;188:151–60. doi: 10.1016/j.cbi.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Sen G, Mandal S, Saha Roy S, Mukhopadhyay S, Biswas T. Therapeutic use of quercetine in the control of infection and anemia associated with visceral leishmaniasis. Free Radic Biol Med. 2005;38:1257–64. doi: 10.1016/j.freeradbiomed.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Simões CM, Schenkel EP, Gosmann G, Mello JC, Mentz LA, Petrovick PR. Porto Alegre/ Florianópolis, Ed. Universidade/ UFRGS/ Ed. da UFSC. 4th ed 2002. Farmacognosia: Da planta ao medicamento. [Google Scholar]
- 24.Ho KY, Tsai CC, Huang JS, Chen CP, Lin TC, Lin CC. Antimicrobial activity of tannin components from Vaccinium viti-sidaea L. J Pharm Pharmacol. 2001;53:187–91. doi: 10.1211/0022357011775389. [DOI] [PubMed] [Google Scholar]
- 25.Castilhos TS, Giordani RB, Henriques AT, Menezes FS, Zuanazzi JA. In vitro evaluation of the antioxidant, anti-inflammatory and antimicrobial activities of the montanine alkaloid. Braz J Pharmacogn. 2007;17:209–14. [Google Scholar]
- 26.Runyoro DK, Matee MI, Ngassapa OD, Joseph CC, Mbwambo ZH. Screening of Tanzanian medicinal plants for anti-Candida activity. BMC Complement Altern Med. 2006;6:11. doi: 10.1186/1472-6882-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Bolle MF, Goderis IJ, Terras FR, Cammue BP, Broekaert WF. A technique for detecting antifungal activity of proteins separated by polyacrylamide gel electrophoresis. Electrophoresis. 1991;12:442–4. doi: 10.1002/elps.1150120612. [DOI] [PubMed] [Google Scholar]
- 28.Eizefeka GO, Orji MU, Mbata TI, Patrick AO. Antimicrobial activies of Cajanus cajan, Garcinia kolal and Xilopia aehtiopica Ion Pathogenic Microorganism. Biothecnology. 2004;3:41–3. [Google Scholar]
- 29.Houghton P, Fang R, Techatanawat I, Steventon G, Hylands PJ, Lee CC. The sulphordamine (SRB) assay and other approaches to testing plant extracts ans derived compounds for activies related to reputed anticancer activity. Methods. 2007;42:377–87. doi: 10.1016/j.ymeth.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Giordani R, Regli P, Kaloustian J, Mikaïl C, Abou L, Portugal H. Antifungal effect of various essential oils against Candida albicans. Potentiation of antifungal action of amphotericin B by essential oil from Thymus vulgaris. Phytother Res. 2004;18:990–5. doi: 10.1002/ptr.1594. [DOI] [PubMed] [Google Scholar]


