Abstract
Background:
Phyllanthus emblica, Camellia sinensis, Mangifera indica, Punica granatum, and Acacia catechu have been shown to possess widespread pharmacological application against multitude of diseases namely cancer, diabetes, liver disorders, and oxidative stress.
Objective:
We evaluated the hepatoprotective activity of the standardized herbal extracts against tert-butyl hydroperoxide (t-BH) induced toxicity and their mechanism of hepatoprotective action in human hepatocarcinoma cells (HepG2 cell line).
Materials and Methods:
The hepatoprotective activity was studied by observing the effect of these herbal extracts on t-BH induced reduction in cell viability of HepG2 cells. In addition, the reducing power of the extracts and their ability to scavenge free radicals were evaluated using two antioxidant assay systems: cell free [oxygen radical absorbance capacity (ORAC), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and [2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonicacid)] (ABTS)] and cell based [cellular antioxidant activity (CAA)].
Results and Discussion:
The results obtained showed that these extracts possess significant hepatoprotective activity. This may indicate that the plant extracts contain compounds, which can remove toxic metabolites following t-BH induced toxicity. The extracts exhibited significant antioxidant property as evident by the Trolox values and effective scavenging of DPPH and ABTS radicals. The extracts also demonstrated inhibition of AAPH-induced fluorescence in HepG2 cells. These results indicate the ability of the plant extracts to protect the liver cells from chemical-induced damage, which might be correlated to their radical scavenging potential.
Conclusion:
This study demonstrates that these extracts have potential hepatoprotective activity which is mainly attributed to the antioxidant potential, which might occur by reduction of lipid peroxidation and cellular damage.
Keywords: Acacia catechu, Camellia sinensis, cellular antioxidant activity assay, hepatoprotection, Mangifera indica, Phyllanthus emblica, Punica granatum
INTRODUCTION
Liver is a key organ that regulates metabolism, secretion, storage, and detoxifying functions in the body, and hepatic damage is often associated with distortion of these functions.[1] Liver cells possess a number of compensatory mechanisms to deal with reactive oxygen species (ROS) and its effects among these are the induction of antioxidant proteins such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GSHPx). Enzymatic antioxidant system [Cu–Zn, Mn–SOD, catalase, GSHPx, and GSH reductase (GR)] function by direct or sequential removal of ROS, thereby terminating their activities. An imbalance between the oxidative forces and antioxidant defense systems causes oxidative injury, which has been implicated in various diseases, such as atherosclerosis, diabetes, cancer, liver cirrhosis, etc.[2]
ROS is continuously generated in physiological conditions and effectively eliminated by several intracellular and extracellular antioxidant systems.[3] Uncontrolled production of ROS often leads to damage of cellular macromolecules (DNA, lipids, and protein) and other small antioxidant molecules. The most important ROS are the superoxide anion radical O2–, hydrogen peroxide (H2O2), alkoxyl (RO), peroxyl (ROO), hydroxyl radical (OH), and hypochlorous acid (HOCl). Other non-oxygen species existing as reactive nitrogen species (RNS), such as nitric oxide (NO) and peroxynitrite also have important bioactivity.[4] Free radical reaction is an important pathway in a wide range of unrelated biological systems. Among many ways of chemical-induced injury, the critical class of reaction is production of free radical intermediates which trigger a network of multifarious disturbances.[5]
Most of the hepatotoxic chemicals damage liver cells mainly by inducing lipid peroxidation and other oxidative damages.[6–8] Liver possesses a unique metabolism and plays a pivotal role in the removal of substances from the portal circulation due to which it is susceptible to toxicity of drugs, xenobiotics, and oxidative stress.[9] The two distinct pathways in liver metabolism occur via cytochrome p-450 and GSH-peroxidase. The current treatment for hepatotoxicity includes drugs which influence the p-450 enzyme mechanism either by inhibiting (amiodarone, cimetidine, ciprofloxacin, etc.) or inducing (rifampicin, carbamazepine, phenobarbital, phenytoin) the metabolic activity of enzymes.
Recently, much attention has been focussed on investigating the hepatoprotective function of naturally occurring compounds and their mechanisms of action. Herein, we have examined the hepatoprotective activity of selected herbal extracts based on the measurement of cytotoxicity of tert-butyl hydroperoxide (t-BH) to HepG2 cells. Chemical-induced toxicity in HepG2 cells represents a suitable in vitro model for hepatotoxicological assessment of drugs, through analysis of different cytotoxic endpoints.[10] HepG2 cells have been used to investigate the metabolism and toxicity of drugs, since these cells retain many specialized functions that are characteristic to normal human hepatocytes, including synthesis and secretion of plasma proteins.[11]
In the absence of reliable modern hepatoprotective drugs, there are a number of traditional medicines recommended for treatment of liver diseases. Many herbs such as Silybum marianum,[12] Tridax procumbens,[13] and Andrographis paniculata[14] have been reported to possess hepatoprotective activity. Plants contain wide variety of bioactive molecules including terpenoids, steroids, phenols, and flavonoids. In addition to their nutritional value these phytoconstituents exhibit a wide array of pharmacological properties such as anti-inflammatory, antiviral, anti-proliferative, and anti-carcinogenic.[15] Plant derived phenolic, flavonoid, and polyphenolic compounds are considered to contribute to the prevention of diseases associated with oxidative stress.
In this study, we aimed to evaluate the hepatoprotective activity of five standardized herbal extracts namely Phyllanthus emblica Linn. (Euphorbiaceae), Camellia sinensis Linn. (Theaceae), Punica granatum Linn. (Punicaceae), Mangifera indica Linn. (Anacardiaceae), and Acacia catechu Linn. (Mimosaceae) on t-BH induced liver toxicity using HepG2 cells. Further, in order to elucidate the possible mechanism of action, we screened these extracts for their antioxidant activity using two assay systems: cell free [oxygen radical absorbance capacity (ORAC), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and {2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonicacid)} (ABTS) radical scavenging tests] and cell based [cellular antioxidant activity (CAA) assay].
MATERIALS AND METHODS
Chemicals
2’,7’-dichlorofluorescein diacetate (DCFH-DA), quercetin dihydrate, fluorescein sodium salt, Trolox (6-hydroxy-2,5,7,8-tetramethyl-2-carboxylic acid), ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonicacid)], AAPH (2,2′-azo-bis[2-methyl propionamidine] dihydrochloride), and t-BH were purchased from Sigma-Aldrich (St. Louis, MO, USA). 2,2-Diphenyl-1-picryl hydrazyl (DPPH) was purchased from Himedia (Mumbai, India). Gibco Life Technologies (Grand Island, NY) supplied Earle's minimum essential media (EMEM). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, USA).
Preparation of standardized herbal extracts
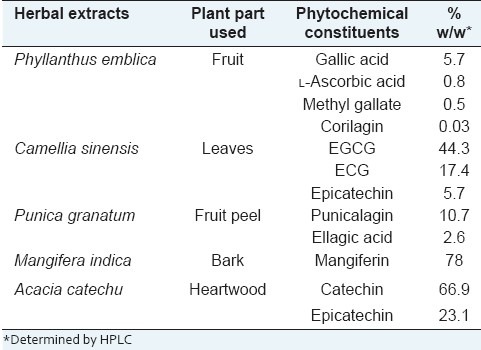
The herbal extracts were prepared in the industrial processing plant of our organization (Natural Remedies Pvt. Ltd., Bangalore). Dried fruits of P. emblica were refluxed with water, and the liquid extract was subjected to distillation under vacuum and spray dried. Dried leaves of C. sinensis were refluxed with 70% methanol in water, and the liquid extract was subjected to distillation under vacuum and partitioned with ethyl acetate. The ethyl acetate layer was concentrated under vacuum, dispersed in water, and spray dried. The dried peels of P. granatum were refluxed with methanol, and the liquid extract was subjected to distillation under vacuum to a thick paste and dried under vacuum. The dried bark of M. indica was refluxed with methanol, and the extract was subjected to distillation under vacuum to a thick paste and processed with methanol and acetone. The final paste was dried under vacuum. The dried heartwood of A. catechu was boiled with water. The extract was then concentrated to a thick paste and subjected for repeated processing with alcohol and water. The final paste was dried under vacuum. The extracts were quantified for the marker compounds using HPLC. The phytochemical constituents of each herbal extract are tabulated in Table 1.
Table 1.
Phytochemical composition of the herbal extracts
Cell line and culture condition
HepG2 cell line (hepatocellular carcinoma) (# HB-8065™) was procured from American Type Culture Collection (ATCC) (Rockville, MD, USA). The cells were cultured in EMEM containing 10% FBS, 1 mM sodium pyruvate, and 2 g/l sodium bicarbonate under an atmosphere of 5% CO2 at 37°C.
Cell viability assay
The viability of HepG2 cells was determined by a colorimetric MTT assay as described by Mosmann.[16] The assay is based upon the ability of mitochondria to catalyze the reduction of MTT bromide to insoluble formazan, the concentration of which is measured spectrophotometrically. HepG2 cells were first cultured in 96-well plates at a density of 4 × 104 cells/well for 24 h. After incubation the cells were washed with PBS and treated with different concentrations of P. emblica (15.6–125 μg/ml), C. sinensis (20–50 μg/ml), M. indica (31.25–250 μg/ml), P. granatum (12.5–75 μg/ml) and A. catechu (15.6–125 μg/ml), silymarin (10–50 μg/ml), and quercetin (0.68–3.38 μg/ml) for 24 h. Thereafter, the cells were washed and further incubated for 1 h with MTT (500 μg/ml). After 1 h, the formazan crystals were dissolved using DMSO (200 μl/well). The absorbance was measured colorimetrically at 570 nm. Consequently, the noncytotoxic concentrations were chosen for conducting the hepatoprotection and CAA studies.
Hepatoprotection activity
The hepatoprotective activity of the extracts was evaluated using well maintained HepG2 cells as per the method described in our previous publication.[17] t-BH was used as a hepatotoxicant and silymarin was used as a standard hepatoprotective drug. Confluent HepG2 cells were cultured in growth media (EMEM + 10% FBS) at a density of 5 × 104 cells/well in a 96-well tissue culture plate and incubated overnight. Postincubation, cells were treated with varying concentrations of extracts and incubated for 2 h, thereafter; t-BH (1 mM) was added and allowed for further 2 h incubation. Postincubation, the treated cells were washed with DPBS and incubated with MTT containing growth media. Finally, the medium was removed, and the formazan crystals were dissolved using DMSO. The optical density was measured at 570 nm.
Antioxidant activity studies
CAA assay
Cultured HepG2 cells were seeded at a density of 6 × 104 cells/well and incubated overnight. Postincubation cells were pretreated with the extracts at varying concentrations prior to addition of DCFH-DA (25 μM) and incubated for 1 h. Thereafter, AAPH (600 μM) was added, and the fluorescence kinetics was measured for 1 h using Fluostar Optima (BMG Labtech, Germany) at 37 °C, excitation 485 nm, emission 540 nm with a cycle time of 300 s. Quercetin was used as a reference standard. The median effective dose (EC50) was calculated from the median effect plot of two independent trials. EC50 values were determined from the X-axis intersection value, where log (Fa/Fu) = 0.[18]
ORAC assay
The assay was performed as per the method described by Alberto et al.[19] The reaction was carried out in 75 mM phosphate buffer (pH 7.4), and the final reaction mixture was 200 μl. The extracts at different concentrations and sodium fluorescein (67 μM) solutions were added in the well of the microplate. The mixture was preincubated for 10 min at 37 °C. AAPH (12 mM) was added rapidly using a multichannel pipette. The microplate was immediately placed in the reader, and the fluorescence kinetics was measured at 485 nm excitation and 520 nm emission wavelengths for 90 min using Fluostar Optima (BMG Labtech, Germany). Trolox, a vitamin E analogue, was used as a reference standard. Sample curves (fluorescence versus time) were first normalized to the curve of the blank corresponding to the same assay. From the normalized curves, the area under the fluorescence decay curve (AUC) was calculated. The net AUC corresponding to a sample and the regression equations between net AUC and sample concentration were calculated. ORAC values were expressed as μmoles of Trolox equivalent/g (TE/g).
ABTS radical scavenging assay
The ABTS radical scavenging activity was determined by the method of Auddy et al.[20] In brief, the total reaction mixture containing 10 mM PBS (pH 7.4), various concentrations of samples, and ABTS radical solution (0.238 mM) were mixed and immediately read at 734 nm using a VersaMax micro plate reader (Molecular Devices, USA). Gallic acid was used as a reference standard. The experiment was carried out in triplicates, half-maximal inhibitory concentration (IC50) was determined using Finney software, and the values were expressed in μg/mL.
DPPH radical scavenging activity assay
The stable DPPH radical scavenging activity was determined by the method of Vani et al.[21] Reaction volume containing methanol, various concentrations of extracts, and DPPH (0.659 mM) were incubated at 25 °C for 20 min, following which the absorbance was read at 510 nm using a VersaMax micro-well plate reader (Molecular Devices, USA). Gallic acid was employed as the reference standard. Assay was performed in triplicates, and the IC50 values were calculated using Finney software, the values were expressed in μg/ml.
Statistical analysis
Data are expressed as mean ± standard deviation (SD) of two independent experiments. Each experiment was performed in triplicates. Statistical differences between the treatments and the control were evaluated by one-way analysis of variance (ANOVA) and Dunnett's multiple comparison tests using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA). The criterion for statistical significance was *P < 0.05 and **P < 0.01.
RESULTS
Cytotoxicity of the herbal extracts in HepG2 cells
All the herbal extracts were checked for MTT reducing potential. The results demonstrated that the extracts did not reduce MTT on their own and not cytotoxic up to the maximum tested concentration. Hereafter, we selected the noncytotoxic concentrations for further studies.
Effect of the herbal extracts on t-BH-induced hepatotoxicity
In this study, we examined the possible hepatoprotective effects of five standardized herbal extracts against t-BH-induced cytotoxicity, by preincubating the cells with or without the extracts or silymarin. A significant decrease in cell viability was observed upon treatment of HepG2 cells with t-BH (1 mM). Treatment with extracts demonstrated a significant dose-dependent protection toward cell toxicity resulting from t-BH exposure. As shown in Figure 1, P. emblica (12.5–75 μg/ml), C. sinensis (30–50 μg/ml), M. indica (62.5–250 μg/ml), P. granatum (12.5–75 μg/ml) and A. catechu (62.5–125 μg/ml) exhibited a significant dose-dependent increase in cell viability at the indicated concentration. Based on the IC50 values, the extracts showed their potency in the following order: P. emblica (IC50 = 32.4 μg/ml) ≥ C. sinensis (IC50 = 34.7 μg/ml) ≥ P. granatum (IC50 = 42.6 μg/ml) > A. catechu (IC50 = 114.8 μg/ml) > M. indica (IC50 = 190.5 μg/ml). The reference standard, silymarin demonstrated a dose-dependent increase in cell viability against t-BH-induced toxicity at concentrations ranging from 40 to 50 μg/ml [Figure 1], with 50% protection obtained at 49.0 μg/ml. Further, on comparing the IC50 values of the extracts with silymarin, P. emblica, C. sinensis, and P. granatum showed equipotent hepatoprotective activity against t-BH-induced cytotoxicity in HepG2 cells.
Figure 1.
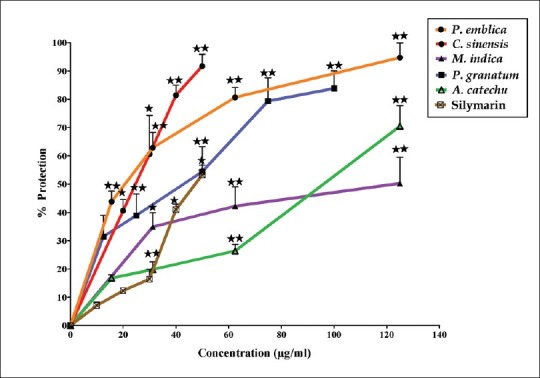
Effect of P. emblica, C. sinensis, M. indica, P. granatum, A. catechu, and silymarin on t-BH (1 mM) induced cytotoxicity in HepG2 cells. The values are expressed as mean ± SD. The criterion for statistical significance was *P < 0.05 and **P < 0.01
Determination of antioxidant potential of the herbal extracts
CAA assay
The antioxidant capacity of the extracts was estimated in terms of degree of inhibition on AAPH-induced fluorescence. At a concentration of 600 μM AAPH yielded a time-dependent increase of fluorescence. The reference standard quercetin exhibited dose-dependent inhibition of AAPH induced fluorescence at concentrations ranging from 0.68 to 3.38 μg/ml [Figure 2a], with an EC50 value of 4.65 μM (1.5 μg/ml). The increase in fluorescence from DCF formation was inhibited by the herbal extracts in a dose-dependent manner, as evident from the curves generated from cells treated with the extracts [Figure 2b–f]. Among all the extracts, P. emblica (7.81–125 μg/ml) exhibited maximum inhibition in fluorescence at the indicated concentrations with an EC50 value of 39.50 ± 0.70 μg/ml. The EC50 values of the extracts are listed in Table 2; based on which the inhibition of DCF formation was in the following order: P. emblica > C. sinensis ≥ P. granatum ≥ M.indica > A. catechu.
Figure 2.
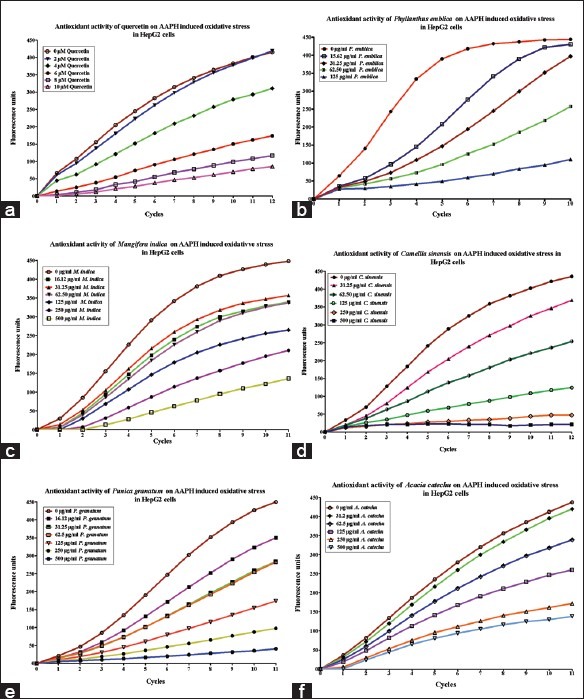
In vitro antioxidant activity of the (a) Quercetin, (b) P. emblica, (c) M. indica, (d) C. sinensis, (e) P. granatum, and (f) A. catechu at various concentrations as carried out by the CAA assay. The curves shown in the graph are from a single experiment which demonstrates the inhibition of increased fluorescence from DCF formation in a dose-dependent manner
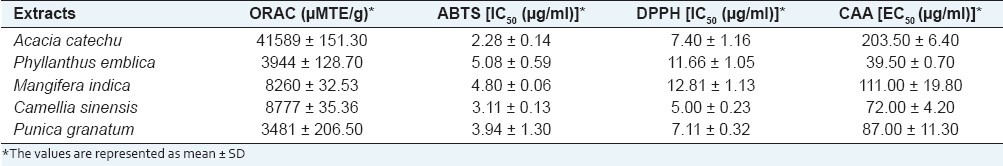
Table 2.
ORAC, ABTS, DPPH, and CAA values of the herbal extracts
ORAC assay
The peroxyl radical scavenging activity of the extracts was determined by ORAC assay. This assay is based on the susceptibility of sodium fluorescein to AAPH, with concomitant loss of its fluorescence.[22] All the extracts showed significant antioxidant potential, and the values represent ORACROO+ activities of the tested extracts equivalent to Trolox. The ORACROO+ values obtained for the extracts are shown in Table 2, based on which following degree of potency was obtained: A. catechu > C. sinensis ≥ M. indica > P. emblica ≥ P. granatum. The results showed that A. catechu with the ORACROO+ value of 41589 ± 151.30 TE/g had the maximum antioxidant activity in comparison to other extracts [Table 2].
ABTS assay
All the extracts demonstrated ABTS free radical scavenging activity. Taking 0% inhibition in control with the absence of extract, regression equations were prepared from the concentrations of the extracts and the percentage inhibitions of free radical formation were calculated. IC50 values were calculated from these regression equations. Based on the IC50 values [Table 2], the following order of free radical scavenging activity was depicted: A. catechu > C. sinensis > P. granatum > M. indica > P. emblica. Further, in comparison to the reference standard gallic acid (IC50 = 1.32 ± 0.07 μg/ml), A. catechu (IC50 = 2.28 ± 0.14 μg/ml) showed lesser potency in scavenging ABTS free radicals [Table 2].
DPPH assay
The herbal extracts were evaluated by their reactivity towards a stable free radical, 2,2-diphenyl-1-picrylhydrazyl (DPPH•). All the extracts examined were found to possess good DPPH radical scavenging activity. The IC50 values as depicted in Table 2 showed the following order of radical scavenging activity: C. sinensis > P. granatum > A. catechu > P. emblica ≥ M. indica. Further, on comparing the 50% scavenging activity of C. sinensis with the reference standard gallic acid, we observed that C. sinensis (IC50 = 5.00 μg/ml) was less potent than gallic acid (IC50 = 1.32 ± 0.07 μg/ml).
DISCUSSION
P. emblica is considered beneficial against various diseases namely cancer, diabetes, liver treatment, and various other diseases. Other useful properties of P. emblica include anti-tumor, anti-inflammatory, anti-bacterial, antioxidant, immunomodulatory, analgesic, etc.[23] In this study, we evaluated the protective effect of P. emblica against t-BH-induced cytotoxicity. Incubating HepG2 cells with 1 mM t-BH for 2 h caused a significant loss in the cell viability. Pretreatment with the extract resulted in a dose-dependent increase in cell viability at concentrations ranging from 15.6 to 125 μg/ml. Then, 50% protection was attained at a concentration of 32.36 μg/ml. Further, on comparing with silymarin, P. emblica was found equipotent in protecting the HepG2 cells against t-BH-induced oxidative damage. In order to elucidate the possible mechanism of hepatoprotection, we further evaluated the antioxidant potential of the extract. The results depicted that in the chemical-based assays, i.e. ORAC, ABTS, and DPPH, and the cell based assay, CAA the extract showed significant antioxidant and radical quenching potential [Table 2]. However, P. emblica (EC50 = 39.50 μg/ml) displayed a greater degree of free radical scavenging ability in CAA assay, although with a lesser potency than quercetin (EC50 = 1.50 μg/ml). Previous studies have shown the hepatoprotective activity of the P. emblica fruit extract against a variety of toxins namely carbon tetrachloride (CCl4), paracetamol, thioacetamide (TAA), alcohol, cyclophosphomide, and anti-TB drugs (rifampicin, isonizid, etc.).[24–30] Hence, the overall results indicate that the extract of P. emblica possesses a potent protective effect against t-BH-induced hepatic damage, and the main mechanism involved in the protection could be associated with its strong capability to reduce the intracellular level of ROS. The effective components which might act against oxidative damage are mainly phenolic compounds, flavonoids, tannins, phyllambic compounds, vitamin C, and others.[30]
C. sinensis commonly called ‘Green tea’ is rich in flavanol monomers known as catechins,[31] which have beneficial effects in cardiovascular diseases including LDL oxidative susceptibility, inflammation, abdominal disorders, etc.[32,33] In this study, we examined the in vitro hepatoprotective activity of leaves extract of C. sinensis on t-BH-induced toxicity in HepG2 cells. The extract demonstrated a significant protective effect against t-BH elicited cell death at a concentration range of 30–50 μg/ml, as evident by an increase in cell viability. As apparent from the IC50 values, C. sinensis displayed equipotent activity in comparison to the reference standard, silymarin. Further, to assess the mechanism of hepatoprotection, the extract was tested for its antioxidant activity. The ORAC, ABTS, and DPPH values indicated that C. sinensis possesses significant radical quenching property [Table 2]. However, among all the assays it demonstrated highest scavenging activity towards DPPH free radicals. In CAA assay, the extract exhibited 50% decrease in fluorescence at a concentration of 72 μg/ml; however, the radical quenching ability was less potent than the reference standard quercetin (EC50 = 1.50 μg/ml). Previous reports have stated the hepatoprotective activity of C. sinensis against acute liver injury induced by tamoxifen,[34] CCl4,[35] 2-nitropropane,[36] lipopolysaccharide (LPS), and d-galactosamine.[37] Hence, it is possible that the mechanism of hepatoprotection of C. sinensis is due to its antioxidant effect, which is mainly influenced by polyphenols, epigallocatechin gallate, and epicatechin gallate.
In this study, the fruit peel extract of P. granatum exhibited significant hepatoprotective activity. The extract demonstrated a dose-dependent increase in cell viability at concentrations ranging from 12.5 to 75 μg/ml, with an IC50 value of 42.6 μg/ml. In comparison to silymarin (IC50 = 49.0 μg/ml), it showed nearly equipotent protective activity. Moreover, P. granatum showed good antioxidant property as evident from the Trolox values (3481 ± 206.50 μMTE/g) and scavenging of free radicals ABTS and DPPH. In CAA assay, AAPH-induced fluorescence was significantly reduced by P. granatum, with an EC50 value of 87.00 μg/ml. However, in comparison to quercetin (EC50 = 1.50 μg/ml) it showed less potent activity against free radical scavenging. Previous in vivo studies have been reported similar hepatoprotective properties of fruit peel and flowers’ extracts of P. granatum against CCl4, ferric nitrilotriacetate (Fe-NTA), and TCA-induced hepatotoxicity.[38–40] The extract showed protection against hepatic lipid peroxidation and preserved GSH levels and activities of antioxidant enzymes namely, catalase (CAT), glutathione peroxidase (GPX), glutathione reductase (GR), and glutathione-S-transferase (GST). In total, these results suggest that hepatoprotection shown by the P. granatum extract may be due to its antioxidant properties.
M. indica and its components are commonly used in folk medicine for many curative effects. It possesses antioxidant,[41] immunomodulatory,[42] anti-mutagenic,[43] and anticancer[44] properties. In this study, we examined the possible hepatoprotective property of the bark extract of M. indica against t-BH-induced cytotoxicity. According to the results, M. indica at concentration ranging from 62.5 to 250 μg/ml resulted in concentration-dependent protection of HepG2 cells, thereby indicating the opposing actions against toxic stimuli [Figure 1]. However, on comparing with silymarin (IC50 = 49.0 μg/ml), it showed lesser efficiency, with 50% protection achieved at a concentration of 190.5 μg/ml. Further, we examined the antioxidant property of the extract in two different assay systems. In ORAC assay, the extract displayed a good activity with 8260 ± 32.53 μMTE/g. It showed efficient scavenging of ABTS and DPPH radicals with IC50 values of 4.80 μg/ml and 12.81 μg/ml, respectively. In CAA assay, M. indica showed moderate inhibition of AAPH-induced fluorescence, with an EC50 value of 111.00 μg/ml. Further, on comparing with quercetin (EC50 = 1.50 μg/ml) it showed less potent activity against free radical scavenging. These results are in good agreement with previous studies showing antioxidant and hepatoprotective activities of M. indica.[45–47] Rodeiro et al.[48] reported the hepatoprotective and antioxidant activity of the stem bark extract of M. indica against t-BH, ethanol, CCl4, and LPS-induced cytotoxicity in rat primary hepatocytes. The extract was reported to prevent lipid peroxidation and GSH depletion produced by t-BH, apparently, mangiferin was identified as the main bioactive component responsible for scavenging ROS and free radicals involved in initiation of lipid peroxidation and in inhibiting the activity of CYP2E1.[49] Previous studies have also reported the hepatoprotective activity of M. indica against cumene hydroperoxide-induced toxicity.[50]
A. catechu and its phytoconstituents possess widespread pharmacological properties namely hypoglycaemic, hepatoprotective, antipyretic, digestive, etc. In this study, we evaluated the hepatoprotective property of heartwood extract of A. catechu against t-BH-induced cytotoxicity. The extract exhibited dose-dependent protection at concentrations ranging from 62.5 to 125 μg/ml, 50% inhibition in t-BH induced toxicity was observed at 114.8 μg/ml. However, in comparison to silymarin (IC50 = 49.0 μg/ml) the extract showed very less protection. Further, the extract was tested for its antioxidant property, where it showed maximum activity in ORAC assay with the highest Trolox value (41589 ± 151.30 μMTE/g). It also demonstrated a significant effect toward ABTS and DPPH free radicals. A. catechu at the tested concentrations exhibited a marked decrease in AAPH-induced fluorescence, with an EC50 value of 203.50 μg/ml; although, in comparison to quercetin it showed very less radical quenching property. Prior in vivo studies have stated the hepatoprotective activity of A. catechu extract against CCl4-induced liver damage.[51–53] These studies indicated that the protective effect resulted due to increase in the serum level of GOT, GPT, alkaline phosphates, improved serum lipid profile, restoration of structural integrity of hepatocyte cell membrane, and regeneration of damaged liver cells. Additionally, the hepatoprotective role of A. catechu was thought to be due to the presence of tannins, cyanidanol, and quercetin.[54,55] Hence, the probable mechanism by which A. catechu exerts its protective action is by minimizing the effects of free radicals, its antioxidant activity in association with the inhibition of lipid peroxidation.
In summary, it is well established that t-BH is metabolized by two distinct pathways in hepatocytes; one via cytochrome p-450, and other by glutathione (GSH) peroxidase converting t-BH to t-butanol and oxidized GSH.[56] These metabolic pathways increase cellular reactive metabolites which attack the membrane phospholipids, proteins, and nucleic acids. Thus, antioxidants that can inhibit free radical generation are important in terms of protecting the liver from chemical-induced damage by stabilizing the antioxidant systems in the cell. Our study clearly demonstrates that P. emblica, C. sinensis, M. indica, P. granatum, and A. catechu possess a significant protective effect against t-BH-induced cytoxicity. In conclusion, the results of the present investigation infer that these plant extracts possess potent antioxidant and hepatoprotective property, the former being probably responsible for the latter. Thus, the extracts can be beneficial in treating liver damages caused due to chemical or xenobiotic exposure.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wolf PL. Biochemical diagnosis of liver diseases. Indian J Clin Biochem. 1999;14:59–90. doi: 10.1007/BF02869152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge JM. Free radicals in Biology and Medicine. 3rd ed. Oxford: University Press; 1999. p. 936. [Google Scholar]
- 4.Jakus V. The role of free radicals, oxidative stress and antioxidant systems in diabetic vascular disease. Bratisl Lek Listy. 2000;101:541–51. [PubMed] [Google Scholar]
- 5.Roberfroid MB, Viehe HG, Remacle J. Advances in drug research. Vol. 16. London: Academic Press Ltd; 1984. Free radicals in drug research; pp. 1–84. [Google Scholar]
- 6.Recknagel RO. A new direction in the study of carbon tetrachloride hepatotoxicity. Life Sci. 1983;33:401–8. doi: 10.1016/0024-3205(83)90787-7. [DOI] [PubMed] [Google Scholar]
- 7.Wendel A, Feurensteins S, Konz KH. Acute paracetamol intoxication of starved mice leads to lipid peroxidation in vivo. Biochem Pharmacol. 1979;28:2051–5. doi: 10.1016/0006-2952(79)90223-5. [DOI] [PubMed] [Google Scholar]
- 8.Dianzani MU, Muzio G, Biocca ME, Canuto RA. Lipid peroxidation in fatty liver induced by caffeine in rats. Int J Tissue React. 1991;13:79–85. [PubMed] [Google Scholar]
- 9.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–76. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Ma M, Purcell WM. Characterization of some cytotoxic endpoints using rat liver and HepG2 spheroids as in vitro models and their application in hepatotoxicity studies. I. Glucose metabolism and enzyme release as cytotoxic markers. Toxicol App Pharmacol. 2003;189:100–11. doi: 10.1016/s0041-008x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 11.Thabrew MI, Hughes RD, McFarlane IG. Screening of hepatoprotective plant constituents using a HepG2 cell cytotoxicity assay. J Pharm Pharmacol. 1997;49:1132–5. doi: 10.1111/j.2042-7158.1997.tb06055.x. [DOI] [PubMed] [Google Scholar]
- 12.Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139–43. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 13.Ravikumar V, Shivashangari KS, Devaki T. Hepatoprotective activity of Tridax procumbens against D-galactosamine/lipopolysaccharide-induced hepatitis in rats. J Ethnopharmacol. 2006;101:55–60. doi: 10.1016/j.jep.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Pramyothin P, Udomuksorn W, Poungshompoo S, Chaichantipyuth C. Hepatoprotective effect of Andrographis paniculata and its constituent andrographolide on ethanol hepatotoxicity in rats. Asia Pac J Pharmacol. 1994;9:73–8. [Google Scholar]
- 15.Eastwood MA. Interaction of dietary antioxidant in vivo: how fruits and vegetables prevent diseases. QJM. 1999;92:527–30. doi: 10.1093/qjmed/92.9.527. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Chandrasekaran CV, Sundarajan K, David K, Agarwal A. In vitro efficacy and safety of poly-herbal formulations. Toxicol In Vitro. 2010;24:885–97. doi: 10.1016/j.tiv.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe KL, Liu RH. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods and dietary supplements. J Agric Food Chem. 2007;55:8896–907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- 19.Dávalos A, Gómez-Cordovés C, Bartolomé B. Extending applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) assay. J Agric Food Chem. 2004;52:48–54. doi: 10.1021/jf0305231. [DOI] [PubMed] [Google Scholar]
- 20.Auddy B, Ferreira M, Blasina F, Lafon L, Arredondo F, Dajas F, et al. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J Ethnopharmacol. 2003;84:131–8. doi: 10.1016/s0378-8741(02)00322-7. [DOI] [PubMed] [Google Scholar]
- 21.Vani T, Rajini M, Sarkar S, Shishoo CJ. Antioxidant properties of the Ayurvedic Formulation-Triphala and its constituents. Int J Pharmacogn. 1997;35:313–7. [Google Scholar]
- 22.Ou B, Hampsch-Woodill M, Prior RL. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J Agric Food Chem. 2001;49:4619–26. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 23.Dhir H, Roy AK, Sharma A, Talukder G. Modification of clastogenicity of lead and aluminium in mouse bone marrow cells by dietary ingestion of Phyllanthus emblica fruit extract. Mutat Res. 1990;241:305–12. doi: 10.1016/0165-1218(90)90029-2. [DOI] [PubMed] [Google Scholar]
- 24.Malar HL, Bai SM. Hepatoprotective activity of Phyllanthus emblica against paracetamol induced hepatic damage in Wister Albino Rats. Afr J Basic Appl Sci. 2009;1:21–5. [Google Scholar]
- 25.Gulati RK, Agarwal S, Agarwal SS. Hepatoprotective studies of Phyllanthus emblica Linn. and quercetin. Indian J Exp Biol. 1995;33:261–8. [PubMed] [Google Scholar]
- 26.Bhattacharya A, Kumar M, Ghosal S, Bhattacharya SK. Effect of bioactive tannoid principles of Emblica officinalis on iron-induced hepatic toxicity in rats. Phytomedicine. 2000;7:173–5. doi: 10.1016/S0944-7113(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 27.Tasduq SA, Kaisar P, Gupta DK, Kapahi BK, Maheshwari HS, Jyotsna S, et al. Protective effects of 50% hydroalcoholic fruit extract of Emblica officinalis against anti-tuberculosis drugs induced liver-toxicity. Phytother Res. 2005;19:193–7. doi: 10.1002/ptr.1631. [DOI] [PubMed] [Google Scholar]
- 28.Sultana S, Ahmad S, Khan N, Jehangir T. Effect of Emblica officinalis (Gaertn) on CCl4 induced hepatic toxicity and DNA synthesis in Wistar rats. Indian J Exp Biol. 2005;43:430–6. [PubMed] [Google Scholar]
- 29.Pramyothin P, Samosorn P, Poungshompoo S, Caichantipyuth C. The protective effects of Phyllanthus emblica extract on ethanol induced rat hepatic injury. J Ethnopharmacol. 2006;107:361–4. doi: 10.1016/j.jep.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 30.Haque R, Bin-Hafeez B, Ahmad I, Parvez S, Pandey S, Raisuddin S. Protective effect of Emblica officinalis Gaertn. in cyclophosphamide-treated mice. Hum Exp Toxicol. 2001;20:643–50. doi: 10.1191/096032701718890568. [DOI] [PubMed] [Google Scholar]
- 31.Graham HN. Green tea composition, consumption and polyphenol chemistry. Prev Med. 1992;21:334–50. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 32.Wan Y, Vinson JA, Etherton TD, Proch J, Lazarus SA, Kris-Etherton PM. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am J Clin Nutr. 2001;74:596–602. doi: 10.1093/ajcn/74.5.596. [DOI] [PubMed] [Google Scholar]
- 33.Benelli R, Vene R, Bisacchi D, Garbisa S, Albini A. Anti-invasive effects of green tea polyphenol epigallocatechin-3-gallate (EGCG), a natural inhibitor of metallo and serine proteases. Biol Chem. 2002;383:101–5. doi: 10.1515/BC.2002.010. [DOI] [PubMed] [Google Scholar]
- 34.El-Beshbishy AH. Hepatoprotective effect of green tea (Camellia sinensis) extract against tamoxifen-induced liver injury in rats. J Biochem Mol Biol. 2005;38:563–70. doi: 10.5483/bmbrep.2005.38.5.563. [DOI] [PubMed] [Google Scholar]
- 35.Sengottuvelu S, Duraisami S, Nandhakumar J, Duraisami R, Vasudevan M. Hepatoprotective Activity of Camellia sinensis and its 3 Possible Mechanism of Action. Iran J Pharmacol Ther. 2008;7:9–14. [Google Scholar]
- 36.Sai K, Kai S, Umemura T, Tanimura A, Hasegawa R, Inoue T, et al. Protective effect of green tea on hepatotoxicity, oxidative DNA damage and cell proliferation in the rat liver induced by repeated oral administration of 2-nitropropane. Food Chem Toxicol. 1998;36:1043–51. doi: 10.1016/s0278-6915(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 37.He P, Noda Y, Sugiyama K. Green Tea suppresses lipopolysaccharide-induced liver injury in d-galactosamine-sensitized rats. J Nutr. 2001;131:1560–7. doi: 10.1093/jn/131.5.1560. [DOI] [PubMed] [Google Scholar]
- 38.Chidambara Murthy KN, Jayaprakasha GK, Singh RP. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem. 2002;50:4791–5. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- 39.Kaur G, Jabbar Z, Athar M, Alam MS. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem Toxicol. 2006;44:984–93. doi: 10.1016/j.fct.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Celik I, Temur A, Isik I. Hepatoprotective role and antioxidant capacity of pomegranate (Punica granatum) flowers infusion against trichloroacetic acid-exposed in rats. Food Chem Toxicol. 2009;47:145–9. doi: 10.1016/j.fct.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Ajila CM, Rao UJ Prasada. Protection against hydrogen peroxide induced oxidative damage in rat erythrocytes by Mangifera indica L. peel extract. Food Chem Toxicol. 2008;46:303–9. doi: 10.1016/j.fct.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 42.Makare N, Bodhankar S, Rangari V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J Ethnopharmacol. 2001;78:133–7. doi: 10.1016/s0378-8741(01)00326-9. [DOI] [PubMed] [Google Scholar]
- 43.Prasad S, Kalra N, Shukla Y. Hepatoprotective effects of lupeol and mango pulp extract of carcinogen induced alteration in Swiss albino mice. Mol Nutr Food Res. 2007;51:352–9. doi: 10.1002/mnfr.200600113. [DOI] [PubMed] [Google Scholar]
- 44.Percival SS, Talcott ST, Chin ST, Mallak AC, Lounds-Singleton A, Pettit-Moore J. Neoplastic transformation of BALB/3T3 cells and cell cycle of HL-60 cells are inhibited by mango (Mangifera indica L.) juice and mango juice extracts. J Nutr. 2006;136:1300–4. doi: 10.1093/jn/136.5.1300. [DOI] [PubMed] [Google Scholar]
- 45.Martinez SG, Jalil EC, Giuliani A, Leon FOS, Ram S, Delgado HR, Nunez AJ. Mangifera indica L. (Vimang) reduces ischemia induced neuronal loss and oxidative damage in Gerbil brain. Free Radic Res. 2002;32:1–7. doi: 10.1080/10715760100301481. [DOI] [PubMed] [Google Scholar]
- 46.Remirez D, Tafazoli S, Delgado R, Harandi AA, O’Brien PJ. Preventing hepatocyte oxidative stress cytotoxicity with Mangifera indica L. extract (Vimang) Drug Metabol Drug Interact. 2005;21:19–29. doi: 10.1515/dmdi.2005.21.1.19. [DOI] [PubMed] [Google Scholar]
- 47.Rodeiro I, Donato MT, Jiménez N, Garrido G, Delgado R, Gómez-Lechón MJ. Effects of Mangifera indica L. aqueous extract (Vimang) on primary culture of rat hepatocytes. Food Chem Toxicol. 2007;45:2506–12. doi: 10.1016/j.fct.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 48.Rodeiro I, Donato MT, Martínez I, Hernández I, Garrido G, González-Lavaut JA, et al. Potential hepatoprotective effects of new Cuban natural products in rat hepatocytes culture. Toxicol Vitro. 2008a;5:1242–9. doi: 10.1016/j.tiv.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Rodeiro I, Donato MT, Lahoz A, González-Lavaut JA, Laguna A, Castell JV, et al. Modulation of P450 enzymes by Cuban natural products rich in polyphenolic compounds in rat hepatocytes. Chem Biol Interact. 2008;172:1–10. doi: 10.1016/j.cbi.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Pourahmad J, Eskandari MR, Shakibaei R, Kamalinejad M. A search for hepatoprotective activity of fruit extract of Mangifera indica L. against oxidative stress cytotoxicity. Plants Foods Hum Nutr. 2010;65:83–9. doi: 10.1007/s11130-010-0161-9. [DOI] [PubMed] [Google Scholar]
- 51.Jayasekhar P, Mohanan PV, Rathinam K. Hepatoprotective activity of ethyl acetate extract of Acacia catechu. Indian J Pharmacol. 1997;29:426–8. [Google Scholar]
- 52.Ray D, Sharatchandra Kh, Thokchom IS. Antipyretic, antidiarrhoeal, hypoglycemic and hepatoprotective activities of ethyl acetate extract of Acacia catechu Wild in Albino rats. Indian J Pharmacol. 2006;6:408–13. [Google Scholar]
- 53.Pingale SS. Hepatoprotection by Acacia catechu in CCl4 induced liver dysfunction. Int J Pharm Sci Rev Res. 2010;1:150–4. [Google Scholar]
- 54.Rage N, Dahanukar S, Karandikar SM. Hepatoprotective effect of cyanidanol against carbon tetrachloride induced liver damage. Indian Drugs. 1984;22:556–560. [Google Scholar]
- 55.Rajnarayana K, Reddy MS, Chaluvadi MR, Krishna DR. Bioflavonoid classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharmacol. 2001;33:2–16. [Google Scholar]
- 56.Rush GF, Gorski JR, Ripple MG, Sowinski J, Bugelski P, Hewitt WR. Organic hydroperoxide-induced lipid peroxidation and cell death in isolated hepatocytes. Toxicol Appl Pharmacol. 1985;78:473–83. doi: 10.1016/0041-008x(85)90255-8. [DOI] [PubMed] [Google Scholar]


