Abstract
Background:
In South Africa where many patients are immunocompromised as a result of the AIDS pandemic, opportunistic fungal infections such as candidiasis caused mainly by Candida albicans are common. Arctotis arctotoides and Gasteria bicolor are two plants which are frequently and commonly used in traditional medicine in the treatment of HIV patients.
Aim:
The aim of this study was to investigate the antifungal activity of A. arctotoides and G. bicolor against opportunistic fungi common in HIV/AIDS patients.
Materials and Methods:
The agar diffusion and micro-dilution methods were used to determine the antifungal activities of the medicinal plant extracts against 10 opportunistic fungi.
Results:
All the hexane and acetone extracts were active against at least one of the fungi with zones of inhibition varying from 8 to 32 mm, while none of the aqueous extracts was active against any of the fungi. The inhibitory activity of the active extracts, based on the overall mean inhibition diameters, was in the order: A. arctotoides (hexane) > A. arctotoides (acetone) > G. bicolor (hexane) > G. bicolor (acetone). The most susceptible fungi, based on the overall mean diameter of growth inhibition, were Candida glabrata, C. krusei, and Microsporum canis, while Cyptococcus neoformans, Trycophyton tonsurans, and Microsporum gypseum were not susceptible to any of the extracts even at 5 mg/ml which was the highest concentration used.
Conclusion:
This study validates the use of these plants in traditional medicine in the treatment of secondary fungal infections in HIV/AIDS patients.
Keywords: Antifungal activity, arctotis arctotoides, gasteria bicolor, human immunodeficiency virus/acquired immunodeficiency syndrome, opportunistic fungi
INTRODUCTION
In many patients who are immunocompromised as a result of the acquired immunodeficiency syndrome (AIDS) pandemic, opportunistic fungal infections are common.[1] Some of these pathogens are: Candida albicans which is an opportunistic fungus that can cause local and systemic infections in predisposed persons, commonly affecting immunologically compromised patients and those undergoing prolonged antibiotic treatment. In human immunodeficiency virus (HIV) patients, the presence of oral candidiasis is the earliest opportunistic infection, and is displayed by two-thirds of HIV-infected individuals;[2] Cryptococcus neoformans is one of the most important opportunistic fungal pathogens that causes life-threatening meningitis of the central nervous system in both immunocompromised and apparently immunocompetent patients;[3] Aspergillus fumigatus may be associated with respiratory infections; among the pathogenic fungi, the dermatophytes have the ability to invade keratinised tissues of animals and humans and cause dermatophytosis which is the most common human contagious fungal disease;[4] Microsporum gypseum and Trichophyton mentagrophytes are dermatomycetes of which the former causes infections often referred to as ringworm.
With the increasing incidence of strains of fungi with multiple antibiotic resistance and the persistence of fungal infections in immunocompromised individuals,[5–7] it is of great importance to find effective treatments against fungal infections and other microbial pathogens. On the basis of the knowledge that plants have their own defense against fungal pathogens and are traditionally used for the treatment of human mycoses, they appear as an interesting source for the discovery of new antifungal drugs. Hence, much attention has been drawn to plant-derived fungicides in recent years.[8]
In South Africa, 60–80% of the population relies solely or partially on traditional herbal medicines to treat a variety of animal and human diseases. Many traditionally used medicinal plants are therefore sold in the market places due to increased demands for cheap medicines, high unemployment rate, and increase in HIV infections.[1] This high dependence on plants for medicinal purposes necessitates the need for the scientific validation of their therapeutic value and safety.
Arctotis arctotoides (Asteraceae) is a decumbent herb commonly found as a roadside weed in most coastal districts of South Africa.[9] The Xhosa-speaking people in the Eastern Cape Province apply the juice from the leaf as a topical paste to treat wounds.[10] The inhibitory activity of the aqueous extracts of the shoots of A. arctotoides against six fungal species, with growth inhibitions ranging from 50.7% on Aspergillus tamarii to 95.2% on Penicillium digitatum at 0.1 mg/ml, has also been reported.[10] The acetone and methanol extracts of the root of this plant demonstrated significant activity against five fungal species: Aspergillus flavus, A. niger, Fusarium oxysporium, Mucor heamalis, and Penicillium notatum. Inhibition of fungal growth ranged from 56.23% on A. flavus to 100% on P. notatum and M. heamalis at 5.0 mg/ml.[9] The antibacterial activities of A. arctotoides have previously been reported in the literature.[11] The succulent genus, Gasteria, which comprises 16 species, is endemic to South Africa and has its main centers of distribution in the savanna region in the Eastern Cape.[12] Previously, this genus was classified in the large heterogeneous Liliaceae family but is now classified under the family Asphodelaceae.[13] The selection of the medicinal plants used in this study was mainly based on their ethnomedical evidence of use for the management of opportunistic fungal infections in HIV/AIDS patients. On the basis of our previous ethnobotanical survey[14] both A. arctotoides and G. bicolor were commonly cited by HIV/AIDS patients and traditional healers for their usage in the management of symptoms of opportunistic fungal infections. The most frequently cited were symptoms of oral candidiasis; oral thrush, oral mucosal lesions, symptoms of oesopharyngeal candidiasis; pain and difficulty in swallowing, altered taste, and symptoms of dermatomycosis; ring-like patch on the skin, skin itch, and skin rash. However, the antifungal activity of these plants against opportunistic fungi in HIV/AIDS has not been investigated. In this study, the hexane, acetone, and aqueous extracts of G. bicolor and A. arctotoides were analysed for their in vitro antifungal activity against a panel of standardized opportunistic pathogenic fungi including dermatomycetes found in HIV-infected patients.
MATERIALS AND METHODS
Plant material
A. arctotoides was collected at the Agricultural Research Farm of the University of Fort Hare, South Africa, while G. bicolor was supplied by an herbalist. The plants were authenticated at the Griffin Herbarium of the Department of Botany, University of Fort Hare, where voucher specimens were deposited (A. Arctotoides: W28, G. bicolor: W31).
Extraction procedure
Leaf samples of G. bicolor and A. arctotoides were chopped, dried in an oven at 40 °C and ground into fine powder. The ground samples were put into separate conical flasks containing acetone, hexane, and water and shaken for 24 h on an orbital shaker. After filtering with a Buchner funnel and Whatman No. 1 filter paper, the hexane and acetone filtrates were concentrated to dryness under reduced pressure at a maximum of 40 °C using a rotavapor.[14] Aqueous filtrates were freeze-dried. Each extract was re-suspended in the respective solvent of extraction to yield a 20 mg/ml stock solution.[15]
Microorganisms and media
The fungi used in this study were chosen primarily on the basis of their importance as opportunistic pathogens of humans infected with HIV/AIDS. Strains from the American Type Culture Collection (ATCC) were used: C. albicans ATCC 2091, C. krusei ATCC 14243, C. glabrata ATCC 2001, C. neoformans ATCC 66031, A. fumigatus ATCC 204305, A. niger ATCC 16888, Trichophyton tonsurans ATCC 28942, T. mucoides ATCC 201382, M. gypseum ATCC 24102, and M. canis ATCC 36299. Both Sabouraud dextrose agar (SDA) and Sabouraud dextrose broth (SDB) were prepared according to the manufacturer's instructions. The fungi were maintained at 4 °C on SDA plates, and the inoculum for the assays was prepared by diluting scraped cell mass in 0.85% NaCl solution, adjusted to 0.5 McFarland standard and confirmed by spectrophotometric reading at 580 nm.[2] Cell suspensions were finally diluted to 104 CFU mL–1 for the use in the assays.
Antifungal susceptibility assays
The agar diffusion and microdilution methods were used to determine the antifungal activities of the medicinal plant extracts against the opportunistic fungi.[1]
Agar well diffusion assay
The agar diffusion assay was carried out with slight modifications.[16] Using the micropipette, 100 μl of 0.5 Mcfarland solution of C. albicans culture in 0.85% sterile distilled water (SDW) was placed over the surface of an agar plate, and spread using a sterile inoculation loop. The same procedure was followed for the other fungi. Using a sterile cork borer, four holes (5 mm in diameter) were punched in each of the culture plates. In the first hole, 50 μl of a positive control drug was added (Amphotericin B for C. neoformans and Aspergillus spp., Nystatin for Candida spp., and Grieseofulvin for the dermatomycetes); 50 μl of the corresponding extract solvent was added as a negative control in the second hole; and 50 μl of the plant extract was added in the third and last holes at concentrations of 25 and 50 mg/ml, respectively. Each test was duplicated. The culture plates were then incubated at 37 °C, and the results were observed after 24 h to 6 days depending on the fungi. The clear zone around each well was measured in mm, indicating the activity of the plant extract against the fungal organisms.
Microdilution assay
The microdilution method was employed to determine the minimum inhibitory concentration (MIC) of the plant extracts using 96 well microtitre plates.[16] Initially, 120 μl of SDW was added into each well of the first (A) and last (H) rows and also into all the wells of the last column.[12] Then, 120 μl of SDB was added into each well of the second row (B) and 150 μl of SDB was added into the remaining wells of the first column and 100 μl into the rest of the wells from the second column rightward. Fifty microliters of the plant extract were then added into the third well of the first column while 50 μl of the positive and negative control were separately added into the remaining wells of the first column. A two-fold serial dilution was done by mixing the contents in each well of the first column (starting from the third row) and transferring 100 μl into the second well of the same row and the procedure was repeated up to the 11th well of the same row and the last 100μl from the 11th well was discarded. Hence various concentrations of the plant extracts ranging from 5 mg/ml to 0.005 mg/ml were prepared in the wells, following the two-fold dilution method. Thereafter, 20 μl of 0.5 McFarland fungal suspension was inoculated into the wells except those which contained SDW. The growth of the fungi was measured by determining the absorbance at 620 nm with a microtitre plate reader before and after incubation. The plates were incubated at 37°C at various durations depending on the fungi (24 h for yeasts, 48 h for Aspergillus species, and 6 days for the dermatomycetes). The lowest concentration which inhibited the growth of the fungi was considered as the MIC of the extract.
Determination of the minimum fungicidal concentration
The MFC was determined by inoculating the contents from the MIC plates onto SDA plates, and the results were observed after incubation at 37°C at various durations depending on the fungi. The presence of the fungal colonies on the agar plates was an indication that the plant extract only inhibited the growth of the fungi without killing them and the absence indicated that the plant extract was able to kill the fungal organisms.[6] The smallest concentration of the plant extract that was able to kill the microorganisms was considered as the minimum fungicidal concentration (MFC).
RESULTS
Among the six plant extracts that were tested against the 10 opportunistic fungi, all the hexane and acetone extracts were active against at least one of the fungi with zones of inhibition varying from 8 to 32 mm [Table 1]. None of the aqueous extracts was active against any of the fungi. For A. arctotoides, the hexane extract was active against 6 out of the 10 fungi, while the acetone extract was active against 7 out of the 10 tested fungi. The hexane and acetone extracts of G. bicolor were active against six and five of the 10 tested fungi, respectively. The inhibitory activity of the active extracts based on the overall mean inhibition diameters was in the order: A. arctotoides (hexane) > A. arctotoides (acetone) > G. bicolor (hexane) > G. bicolor (acetone) [Figure 1]. The highest activity against the tested fungi was obtained with the hexane extract of A. arctotoides with inhibition zones diameters of 32, 25, and 22 mm against C. glabrata, T. mucoides, and M. canis, respectively [Table 1]. The lowest activity was obtained with the hexane extract of G. bicolor with inhibition zones diameter of 8 mm against A. fumigatus.
Table 1.
Inhibition zone diameters caused by the plant extracts in the tested opportunistic fungi
Figure 1.
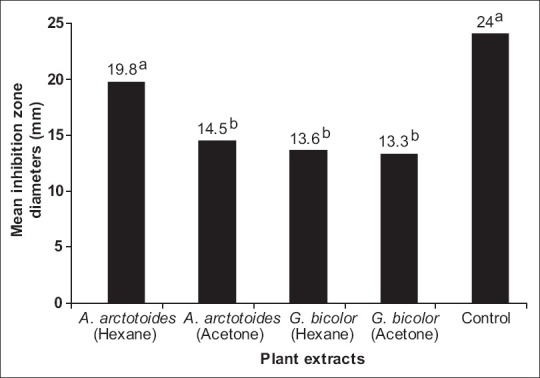
Inhibitory activity of the plant extracts against the tested opportunistic fungi. Mean inhibition zone diameters with the same superscript letters are not significantly different from each other (P > 0.05)
The most susceptible fungi based on the overall mean diameter of growth inhibition were C. glabrata, C. krusei, and M. canis, while C. neoformans, T. tonsurans, and M. gypseum were not susceptible to any of the extracts even at 5 mg/ml which was the highest concentration used [Figure 2]. T. mucoides was susceptible to A. arctotoides, but not to G. bicolor extracts.
Figure 2.
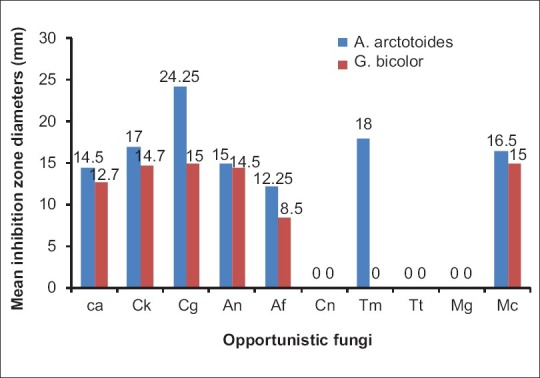
Susceptibility of the tested opportunistic fungi to the plant extracts, shown by their mean inhibition zone diameters. Reproduction size: At column width. Ca = C. albicans, Ck = C. krusei, Cg = C. glabrata, Cn = Cryptococcus neoformans, Af = A. fumigatus, An = A. niger, Tt = T. tonsurans, Tm = T. mucoides, Mg = M. gypseum, Mc =M. canis
The varying concentrations between 5 and 0.005 mg/ml of the plant extracts were tested in order to determine their MICs. The MICs and MFCs of the plant extracts against the 10 tested fungi are presented in Table 2. For A. arctotoides, the lowest MICs were obtained with the hexane extract (0.005 mg/ml against T. mucoides and 0.04 mg/ml against A. niger) and the acetone extract (0.04 mg/ml against A. fumigatus). For G. bicolor, the lowest MICs were obtained with the hexane extract: 0.04 mg/ml against A. fumigatus and 1.25 mg/ml against C. glabrata. The acetone extract of G. bicolor was inactive against A. niger and A. fumigatus at the concentrations tested.
Table 2.
Minimum inhibitory concentrations (MIC) and minimum fungicidal concentration (MFC) of the extracts against the tested opportunistic fungi
DISCUSSION
Both A. arctotoides and G. bicolor were chosen for this study because they are widely used in traditional medicine and are readily available in the Eastern Cape Province of South Africa. Since the hexane and acetone extracts of both plants were active against at least one of the yeasts (especially Candida spp.) and moulds (including the dermatomycetes: M. canis and T. mucoides) and considering that the mean inhibition zone diameters of A. arctotoides (hexane) and the positive controls were not significantly different (P > 0.05) [Figure 2], this suggest that both A. arctotoides and G. bicolor appear to have high efficacy with a broad spectrum of antifungal activity. The overall antifungal activity evaluated by the diameter of the inhibition zone of fungal growth indicates that among the four active extracts, the hexane extract of A. arctotoides exhibited the highest antifungal activity, comparable to that of the reference drug (Amphotericin B). This highlights the medicinal value of A. arctotoides as a potential source for drug development amidst the obvious dearth of effective and safe antifungal drugs. On the other hand, the absence of any significant inhibition with any of the remaining three extracts when compared to the control agent was attributed the fact that crude plant extracts are generally mixtures of active and nonactive compounds.
For a crude extract to be considered active, it should have a MIC value of 0.1 mg/ml or less.[17] Considering the above criterion, both A. arctotoides and G. bicolor are worthy of further investigation. Hence, these extracts are being subjected to bioassay-guided fractionations in order to isolate the compound/s responsible for the activity. Previous reports have shown that the aqueous extract of A. arctotoides was inhibitory (though not fungicidal) against Aspergillus tamari and Penicillum digitatum.[10] However, this study has shown that the extracts of A. arctotoides were fungicidal against some of the opportunistic fungi common in HIV infections. This discrepancy could be attributed to the fact that the concentration of active compounds in a plant may vary depending on the time of collection.[18]
Scientific studies on the antifungal activity of A. arctotoides and G. bicolor on opportunistic fungi in HIV/AIDS patients are scarce. Candidiasis is the most common opportunistic fungal infection found in HIV/AIDS patients.[19] Oral candidiasis is common in the elderly, HIV-infected patients, and also in patients with the long-term use of broad-spectrum antibiotics, corticosteroids, and immunosuppressants.[19] The results of this in vitro study showing the fungicidal effect of the hexane and acetone extracts of A. arctotoides and G. bicolor against Candida spp. suggests that these plants possess a strong potential therapeutic value against candidiasis. Therefore, the extracts with strong fungicidal activity against these opportunistic fungi might produce encouraging clinical outcomes. Notwithstanding, future research is warranted to identify the active compounds that inhibit candida growth as well as the appropriate dosages and formulations for the clinical use.
Human infections, particularly those involving the skin and mucosal surfaces, constitute a serious problem, especially in tropical and subtropical developing countries, dermatomycetes and Candida spp. being the most frequent pathogens.[5] Hence, the fact that the hexane and acetone extracts of the test plants were fungicidal and inhibitory against T. mucoides and M. canis, respectively, further justifies the ethnopharmacological use of these species against mycotic infections and also highlights the possibility of using them as sources of antifungal compounds against fungi in dermatomycoses.
The results of this study have shown that the hexane and acetone extracts obtained from both G. bicolor and A. arctotoides were active against at least one of the opportunistic fungi. This highlights the potential of both G. bicolor and A. arctotoides as antifungal agents, and this increases the likelihood for the discovery of new phytomedicines and active principles employed in the treatment of mycoses associated with HIV/AIDS. Generally, the hexane extract of both plants exhibited a stronger fungicidal activity than the acetone extracts, while the aqueous extracts were inactive. Although this study validates the use of these plants in traditional medicine in the treatment of secondary fungal infections in HIV/AIDS patients, further research is warranted in the isolation and identification of the active principles.
ACKNOWLEDGMENTS
We greatly appreciate the Govan Mbeki Research and Development Centre (GMRDC) of the University of Fort Hare and the National Research Foundation (NRF), South Africa, for financing this project.
Footnotes
Source of Support: Govan Mbeki Research and Development Centre (GMRDC) of the University of Fort Hare and the National Research Foundation (NRF), South Africa
Conflict of Interest: None declared.
REFERENCES
- 1.Shai LJ, McGaw LJ, Masoko P, Eloff JN. Antifungal and antibacterial activity of seven traditionally used South African plant species active against Candida albicans. S Afr J Bot. 2008;74:677–84. [Google Scholar]
- 2.Duarte MC, Figueira GM, Sartoratto A, Rehder LG, Delarmelina C. Anti-Candida activity of Brazilian medicinal plants. J Ethnopharmacol. 2005;97:305–11. doi: 10.1016/j.jep.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Hamza OJ, van den Beukel CJ, Matee MI, Moshi MJ, Mikx FH, Selemani HO, et al. Antifungal activity of some Tanzanian plants used traditionally for the treatment of fungal infections. J Ethnopharmacol. 2006;108:124–32. doi: 10.1016/j.jep.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Cruz MC, Santos PO, Barbosa AM, Jr, de Mélo DL, Alviano CS, Antoniolli AR, et al. Antifungal activity of Brazilian medicinal plants involved in popular treatment of mycoses. J Ethnopharmacol. 2007;111:409–12. doi: 10.1016/j.jep.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Gurgel LA, Sidrim JJ, Martins DT, Cechinel Filho V, Rao VS. In vitro antifungal activity of dragon's blood from Croton urucurana against dermatophytes. J Ethnopharmacol. 2005;97:409–12. doi: 10.1016/j.jep.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Jones NP, Arnason JT, Abou-Zaid M, Akpagana K, Sanchez-Vindas P, Smith ML. Antifungal activity of extracts from medicinal plants used by First Nations Peoples of Eastern Canada. J Ethnopharmacol. 2000;73:191–8. doi: 10.1016/s0378-8741(00)00306-8. [DOI] [PubMed] [Google Scholar]
- 7.Kelly SL, Lamb DC, Kelly DE, Manning NJ, Loeffler J, Hebart H, et al. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol D3,5-desaturation. FEBS Lett. 1997;400:80–2. doi: 10.1016/s0014-5793(96)01360-9. [DOI] [PubMed] [Google Scholar]
- 8.Stein AC, Alvarez SS, Avancini C, Zacchino S, von Poser G. Antifungal activity of some coumarins obtained from species of Pterocaulon (Asteraceae) J Ethnopharmacol. 2006;107:95–8. doi: 10.1016/j.jep.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Afolayan AJ, Jimoh FO, Sofidiya MO, Koduru S, Lewu FB. Medicinal Potential of the root of Arctotis arctotoides. Pharm Biol. 2007;45:486–93. [Google Scholar]
- 10.Afolayan AJ. Extracts from the shoots of Arctotis arctotoides inhibit the growth of bacteria and fungi. Pharm Biol. 2003;41:22–5. [Google Scholar]
- 11.Sultana N, Afolayan AJ, Bhuiyan RF. Antimicrobial compounds from the shoots of Arctotisa arctotoides. Bangladesh J Sci Ind Res. 2008;43:89–96. [Google Scholar]
- 12.Dagne E, Van Wyk BE, Mueller M, Steglich W. Three dihydroanthracenones from Gasteria bicolor. Phytochemistry. 1996;41:795–9. doi: 10.1016/0031-9422(95)00704-0. [DOI] [PubMed] [Google Scholar]
- 13.Sultana N, Afolayan AJ. Bioactive sesquiterpene lactones isolated from the shoots of Arctotis arctotoides. S Afr J Bot. 2003;69:1–3. [Google Scholar]
- 14.Otang WM, Grierson DS, Ndip RN. Ethnobotanical survey of medicinal plants used in the management of opportunistic fungal infections in HIV/AIDS patients in the Amathole District of the Eastern Cape Province, South Africa. J Med Pl Res. 2012;6:2071–80. [Google Scholar]
- 15.Koduru S, Grierson DS, Afolayan AJ. Antimicrobial Activity of Solanum aculeastrum. Pharm Biol. 2006;44:283–6. [Google Scholar]
- 16.Samie, Tambani T, Harshfield E, Green E, Ramalivhana JE, Bessong PO. Antifungal activities of selected Venda medicinal plants against Candida albicans, Candida krusei and Cryptococcus neoformans isolated from South African AIDS patients. Afr J Biotechnol. 2010;9:2965–6. [Google Scholar]
- 17.Aligiannis N, Kalpotzakis E, Mitaku S, Chinou IB. Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem. 2001;40:4168–70. doi: 10.1021/jf001494m. [DOI] [PubMed] [Google Scholar]
- 18.Jainkittivong A, Butsarakamruha T, Langlais RP. Antifungal activity of Morinda fruit extract against Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:394–8. doi: 10.1016/j.tripleo.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 19.WHO, World Health Organisation. Regional office for South East Asia. Laboratory manual for diagnosis of fungal opportunistic infections in HIV/AIDS patients. 2009. [Last accessed on 2011 Jul 26]. Available from: http://www.searo.who.int .


