Abstract
Background:
Despite the excellent anti-inflammatory and anti-rheumatic efficacy associated with kirenol generation, the content of kirenol in Siegesbeckea orientalis is quite low.
Objective:
This study was designed to establish a reliable kirenol production protocol by transformed root cultures of S. orientalis and to investigate the antimicrobial activities of kirenol, hairy root, and S. orientalis.
Materials and Methods:
Transformed root cultures of S. orientalis were established by the transformation of Agrobacterium rhizogenes A4. Transgenic status of the roots was confirmed by polymerase chain reaction (PCR) using rolB specific primers. The biomass and kirenol accumulation of hairy root clones were assessed using four different culture media: MS, MS/2, B5, and white. The antimicrobial activities of kirenol, hairy root, and S. orientalis were evaluated by the disc diffusion method.
Results:
The optimum media for kirenol synthesis was MS. The content of kirenol in transformed hairy roots made up about 80% of that observed in natural leaves of S. orientalis (1.6 mg/g dry weight). All tested samples displayed antimicrobial activity against Gram-positive pathogens including Staphylococcus epidermidis, Staphylococcus aureus, and Acinetobacter baumannii, with MIC ranging from 78 to 625 μg/mL.
Discussion and Conclusion:
The high level of kirenol contents was obtained from hairy roots of S. orientalis. Kirenol was effective against gram-positive bacteria. Interestingly, the extract from hairy roots showed a diverse antimicrobial effect from that of kirenol and S. orientalis.
Keywords: Antimicrobial activity, hairy root culture, kirenol, Siegesbeckia orientalis
INTRODUCTION
The plants of the genus Siegesbeckia (Compositae) are annual herbs widely distributed throughout tropical, subtropical, and temperate regions of the world. Siegesbeckia orientalis is a common weed in China, and its aerial part has been used traditionally to treat arthritis, hypertension, malaria, neurasthenia, and snakebite.[1] Phytochemical studies carried out with this plant revealed that ent-kaurane and ent-pimarane type diterpenoids and sesquiterpenoids were the primary components of the aerial parts.[2–4] Kirenol (8(14)-pimarene-2, 15, 16, 19-tetrol) [Figure 1] was a main and common ent-pimarane type diterpenoid in S. orientalis and had been suggested as the main anti-inflammatory and anti-rheumatic active compound in S. orientalis.[5] Moreover, kirenol was selected as the reference substance to quantify and qualify S. orientalis for Chinese Pharmacopoeia.[1]
Figure 1.
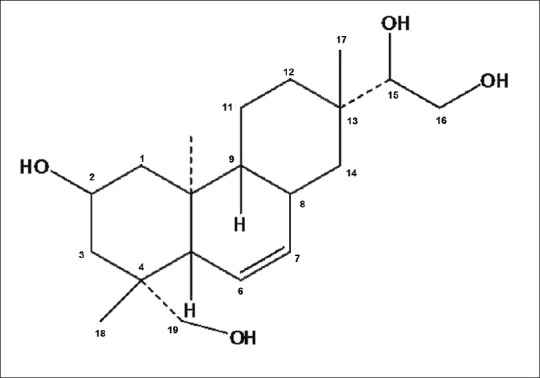
Chemical structure of kirenol
Despite the excellent efficacy associated with kirenol generation, the content of kirenol in S. orientalis is quite low (about 0.5 mg/g, dry wt); the increased production of this compound is therefore to be demonstrated.
Hairy root cultures have been proposed as an alternative method of producing plant secondary metabolites because of their genetic and biochemical stability, rapid growth rate, and their capability to synthesize secondary products at levels comparable to those of the original plants.[6] The primary objective of this study is to establish a reliable protocol of transformed root cultures of S. orientalis and to investigate their utilities in kirenol production.
In addition, our previous research confirmed the topical anti-inflammatory and wound healing activities of kirenol isolated from S. orientalis, which correlates well with their antimicrobial properties.[7,8] However, the antimicrobial activity of kirenol and S. orientalis has not been reported before. Herein, we evaluated the antimicrobial activities of kirenol, hairy root culture extract, and conventional extract of S. orientalis by the disc diffusion method.
MATERIALS AND METHODS
Plant material, chemicals and microorganisms
S. orientalis (Compositae) was obtained in autumn 2009 from the Herbal Plant Garden of School of Pharmacy, Huazhong University of Science and Technology, China, and authenticated by Professor Jia-Chun Chen (School of Pharmacy, Huazhong University of Science and Technology, China). A voucher specimen (No. 09012-S) was preserved at the herbarium of School of Pharmacy, Huazhong University of Science and Technology. The reference substance of kirenol (batch 0774-200901, purity > 99%) was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (NICPBP), Beijing, China. The 1H and 13C NMR data are in agreement with the literature [Figure 2].
Figure 2.

The spectra of kirenol: H NMR (a), C NMR (b), MS (c)
The microbial strains used as test organisms were: Gram-positive bacteria, Bacillus subtilis (ATCC 6051), Staphylococcus aureus (ATCC 29213), Staphylococcus epidermidis (ATCC 14990), and Streptococcus oralis (ATCC 3507); Gram-negative bacteria, Acinetobacter baumannii (ATCC BAA 747), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853); and fungi, Candida albicans (ATCC 90028), and Candida glabrata (ATCC MYA 2950). All microorganism cultures were obtained from China General Microbiological Culture Collection Center (CGMCC), Beijing, China. The strains were sub-cultured on an appropriate agar plate 24 h prior to antimicrobial experiments.
Agrobacterium rhizogene
In our previous experiments, four different wild strains of Agrobacterium rhizogenes (A4, R1601, LBA9402, and ATCC15834) were used for infection, with A4 being the most efficient one.
Therefore, we chose A. rhizogenes strain A4, which was grown in a 40 mL Yeast Extract Broth (YEB) medium at 28°C while shaking at 100 rpm. When OD600 reached 1, the culture was centrifuged and the cells were resuspended in a 40 mL hormone-free Murashige and Skoog (MS) liquid medium, supplemented with 20 μM acetosyringone.[9]
Establishment of hairy root clones
Young leaves of S. orientalis were sterilized with 70% (v/v) ethanol for 30 s and 1% (w/v) NaOCl for 5–10 min, and then rinsed four times with sterile distilled water. The leaves carefully wounded with a scalpel were used as explants. The explants were submerged in the bacterial suspension for 40 min, washed with 50 mL sterilized water, and blot-dried on the sterile filter paper. The explants were then transferred to a hormone-free MS medium containing 300 mg/L of cefotaxime. Hairy roots emerging from the explants were excised. Aseptically weighed hairy roots (100 mg) were transferred to flasks containing 50 mL of four different liquid culture media: MS, half strength MS (MS/2), B5, and white.[10] The cultures were maintained in darkness at 25°C on orbital shakers (80 rpm). During a period of 49 days, following establishment of the hairy root cultures in the liquid medium, a regular subculturing routine was used to maintain the culture. Once a week, a portion of the root clump was aseptically removed and transferred to fresh culture medium.
Identification of transformed hairy roots using polymerase chain reaction
Genomic DNA was extracted and purified from both fresh transformed and native roots according to the method outlined by Thomson and Henry.[11] Approximately 200 ng genomic DNA was used for PCR. The following primers were used to amplify the TL-DNA rolB sequences: 5′-ATGTCGCAAGGACGTAAGCCGA-3′ (forward primer) and 5′-GGAGTCTTTCAGCATGGAGCAA-3′ (reverse primer). For amplification, initial denaturation was performed at 94°C for 3 min, followed by 35 cycles of amplification (each comprising 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C) and a final extension at 72°C for 5 min. The amplified products were separated on a 1.5% (w/v) agarose gel with 0.5 μg ethidium bromide/mL and detected under UV light.
Culture experiments for hairy roots
The biomass and metabolite accumulation of hairy root clones were assessed using four different culture media as described above. Four independent growth experiments were separately run, and all experiments were performed in triplicate.
For culture and time-course experiments, roots were harvested and both growth (fresh and dry weight) and kirenol contents were measured at different intervals.
To measure fresh weight, harvested hairy roots were washed twice with doubled-distilled water, then gently pressed onto filter papers to remove excess water and weighed. Dry weight was measured by drying the fresh hairy roots in an oven at 50°C until constant weight was achieved.
To measure kirenol content, slightly modified methods of PCCh[1] were used. In brief, 100 mg powdered dry hairy roots were extracted with 50 mL of methanol using a reflux condenser. A HPLC system consisting of a Hypersil ODS-2 column (ThermoElectron Corporation, USA; 250 mm × 4.6 mm, particle size 5 μm) and a SPD-10A variable wavelength programmable UV/Vis detector was used. The mobile phase was acetonitrile–water (25:75 v/v). The temperature of the column was 30°C. The detective wavelength was set at 215 nm. The flow rate was 1.0 mL/min. Calibration tests were performed, and a suitable calibration curve was determined. A correlation coefficient value higher than 0.9997 indicated a linearity for the tested concentrations. Samples from hairy roots, native roots, and leaves (10 μL) were injected and their peak areas were compared against the calibration curves.
Antimicrobial activity
The hairy roots were cultured in MS medium for 49 days. Samples of S. orientalis (hairy roots, leaves, roots, and stem) were dried, powdered, and then extracted with methanol (50 mL) by using a reflux condenser. The resulting extracts were dried, dissolved in DMSO, and used for estimation of minimum inhibitory concentration (MIC). Assays were carried out as described previously.[12] The MIC of an extract was defined as the lowest concentration where microbial growth was inhibited.[13] All experiments were performed in triplicate, and MIC values were expressed in μg/mL.
Statistical analysis
Analysis of variance (one-way ANOVA) was performed to test the significance of differences between means obtained among the treatments in each experiment at the 5% level of significance (P < 0.05).
RESULTS AND DISCUSSION
Hairy root cultures of S. orientalis were initiated by inoculation of leaf discs with A. rhizogenes A4 and grown in the dark in liquid MS, B5, white, and MS/2 culture media. These roots exhibited typical characteristics of transformed roots: rapid growth, extensive lateral branch, and a lack of geotropism[14] [Figure 3]. The transformed roots were confirmed by PCR to determine the presence of a T-DNA sequence in their genomes [Figure 4]. The PCR products from the hairy roots for rolB regions but not from untransformed roots gave the expected 540 bp PCR product. This finding indicated that the rolB genes from the Ri plasmid of A. rhizogenes were integrated into the genome of S. orientalis hairy roots.
Figure 3.
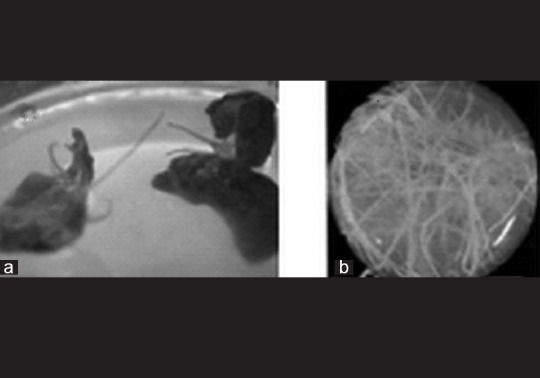
Hairy root cultures of S. orientalis. Hairy roots emerging from the leaves after A. rhizogenes A4 infection (a) and exhibiting a normal phenotype with a large percentage of branched fine roots and an intermediate growth rate when cultured in liquid media (b)
Figure 4.

Electrophoretogram of rol genes PCR identification of hairy roots. Lane 1: molecular weight marker (100 bp ladder), lane 2 and 4: DNA from non-transformed roots (negative control), lane 3: Agrobacterium rhizogenes DNA (positive control), lane 5–10: DNA from different lines of hairy roots
The hairy roots grown in MS medium showed a higher biomass increase, expressed either in fresh weight or dry weight when compared with those grown in White and MS/2 media. Fresh weight growth curves of the hairy roots grown in the B5 medium were similar to those of the hairy roots grown in the MS medium [Figure 5a and b].
Figure 5.
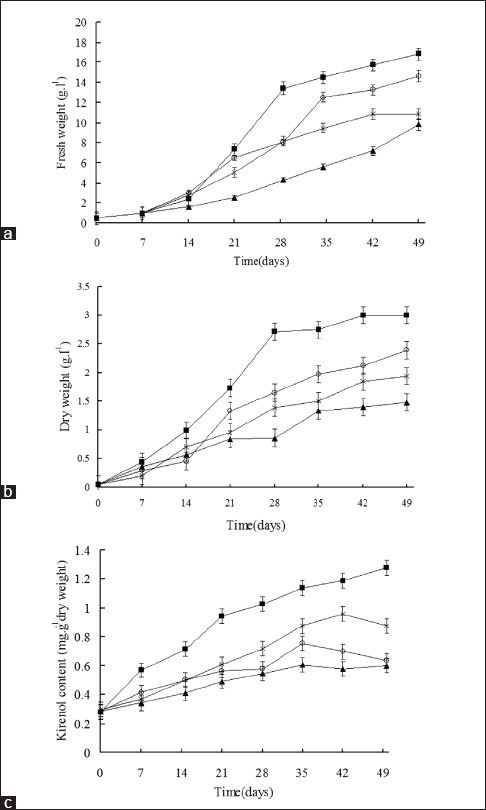
Fresh weight (a), dry weight (b) and kirenol content of hairy root clones (c) growth curves of hairy roots grown in MS (■), B5 (○), White (▲) and MS/2 (×) liquid media
Following 49 days of cultivation, time course of kirenol production in different hairy root culture media was achieved. The different hairy root clones showed variation in kirenol contents, with the white medium culture having the lowest and MS medium culture having the highest kirenol content [Figure 5c].
With the objective to achieve high biomass and kirenol production, kirenol production increased with biomass on four different media like MS, MS/2, B5, and white. Hairy roots grown on MS medium accumulated the maximum amount of kirenol content followed by MS/2, B5 and White. It suggested that MS medium contains higher ionic concentrations of inorganic salts compared to MS/2, B5, and White, favoring growth and kirenol production. Altering inorganic nutrients of the cultured medium are known to influence the production of bioactive molecules from cultured tissues.[15,16]
The kirenol content was detected in methanol extracts of nontransformed roots, stem, and leaves as well as hairy roots using HPLC analysis. Retention time of the peak obtained with the kirenol standard was used to identify the corresponding peaks in extracts [Figure 6].
Figure 6.
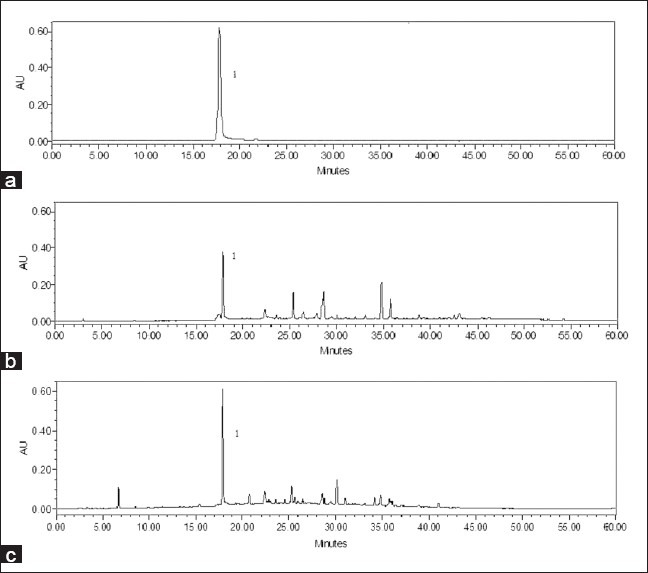
HPLC chromatograms of kirenol standard (a), methanol extract from hairy roots (b) and methanol extract from leaves of S. orientalis (c)
The presence of kirenol was detected in all tested samples. The kirenol content of hairy roots cultured in MS medium for 49 days was compared with those of various origins of S. orientalis [Table 1]. The medium was free of kirenol throughout the cultivation process. The kirenol content of 1.6 mg/g dry wt was obtained using S. orientalis hairy root cultures, which was comparable with amounts present in the leaves and roots of the parent plant (2.1 and 0.3 mg/g dry wt, respectively). Much higher level of the kirenol content was obtained from hairy roots than the native roots of S. orientalis.
Table 1.
Kirenol contents from various origins of S. orientalis and hairy roots

Although the hairy roots under these conditions produced approximately 20% less kirenol than the leaves of S. orientalis, the growing time was much shorter when compared to field-grown plants. With hairy root cultures, product quality and quantity are easy to control because natural variances in seasonal climates and geographical environments are excluded and culture conditions and process variables are easily optimized.[17] Several strategies for the enhancement of biomass and kirenol content have been adopted in which the effects of medium compositions, culture conditions, and elicitations on root growth and kirenol accumulation are currently under further investigation.
For the isolation and purification of kirenol from the cultured hairy roots, the methods of Wang et al,[18] with small modifications were adopted. Briefly, the hairy roots were separated from culture media and soaked in MeOH overnight. Cells were destroyed using Ultra Turrax for 10 min, followed by filtration and exhaustive extraction. The MeOH extract was mixed with H2O to form a suspension and then partitioned successively with petroleum ether, EtOAc, and n-BuOH. The EtOAc-soluble part was subjected to silica gel column chromatography (CC) eluted with petroleum ether–acetone (30:1, 15:1, 8:1, 4:1, 2:1, 1:1, 0:100, and MeOH) to give eight fractions (A–H). Fraction E was separated by silica gel CC eluted with CHCl3–MeOH (10:1) to give subfraction E′, which was recrystallized with acetone to yield kirenol.
The results of the antimicrobial assays have been reported [Table 2]. All tested samples were found to be active on S. epidermidis, S. aureus, and A. baumannii, and the MIC ranged from 78 to 625 μg/mL. Kirenol showed the greatest antibacterial activity against B. subtilis and S. oralis with a MIC of 39 μg/mL. MIC of kirenol on bacteria ranged from 39 μg/mL to 625 μg/mL, though it showed almost no activity on the two studied fungi species. Antimicrobial activity seemed to be higher for methanolic extracts from leaves of S. orientalis compared to extracts from roots and stem of S. orientalis. Previous results from our laboratory showed that kirenol production was tissue-specific with highest amounts present in leaves.
Table 2.
MICs (μg/ml) of kirenol, hairy root culture extract, and conventional extract of Siegesbeckia orientalis against microorganism
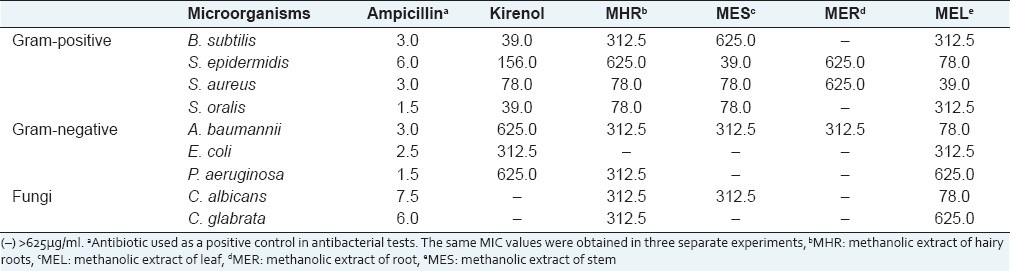
The extract from hairy roots showed a very different antimicrobial effect from that of kirenol and S. orientalis. The transformed roots showed distinguished HPLC chromatograms, implying their possible contribution to synthesize and store significant quantities of secondary metabolites. This suggests either an interaction between compounds from hairy roots or the synthesis of new constituents in hairy roots, which might be responsible for this activity. Interestingly, kirenol, hairy root extract, and S. orientalis extract were more effective against gram-positive bacteria (such as Stypholococcus and Streptococcus) than Gram-negative bacteria (Escherichia, Pseudomonas). This phenomenon could be explained by the fact that the structures of cell envelope differ substantially between gram-positive and gram-negative bacteria. Gram-negative bacteria possess an outer membrane surrounding the cell wall, which restricts diffusion of hydrophobic compounds through its lipopolysaccharide covering. The cell wall of gram-positive bacteria without outer membrane can be permeated more easily, and the constituents of hairy root extract and S. orientalis extract, including kirenol, can disturb the various molecular targets such as the cytoplasmic membrane, proton motive force (PMF), electron flow, active transport, and coagulation of cell contents.[19]
ACKNOWLEDGMENTS
This work was supported by the Fundamental Research Funds for the Central Universities (2011QN243). Jian-Ping Wang is grateful to Daniela Ackermann (Institut für Pharmazeutische Biologie und Biotechnologie, Heinrich-Heine-Universität Düsseldorf, Germany) for linguistic revision.
Footnotes
Source of Support: Fundamental Research Funds for the Central Universities (2011QN243)
Conflict of Interest: None declared.
REFERENCES
- 1.Pharmacopoeia of People's Republic of China (PCh) Vol. 1. Beijing: Chemical Industry; 2010. Pharmacopoeia Commission of Peoples Republic of China (PCCh) pp. 255–6. [Google Scholar]
- 2.Zdero C, Bohlmann F, King RM, Robinson H. Sesquiterpene lactones and other constituents from Siegesbeckia orientalis and Guizotia scabra. Phytochemistry. 1991;30:1579–84. [Google Scholar]
- 3.Wang LL, Hu LH. Chemical constituents of Siegesbeckia orientalis L. J Integr Plant Biol. 2006;48:991–5. [Google Scholar]
- 4.Wang JP, Cai YL, Wu YX. Antiinflammatory and analgesic activity of topical administration of Siegesbeckia pubescens. Pak J Pharm Sci. 2008;21:89–91. [PubMed] [Google Scholar]
- 5.Hu HH, Tang LX, Li XM. Experimental research of effect of crude and processed Herba Siegesbeckiae on anti-inflammation and anti-rheumatism. Chin J Chin Mat Med. 2004;29:542–5. [PubMed] [Google Scholar]
- 6.Georgiev M, Pavlov A, Bley T. Hairy root type plant in vitro systems as sources of bioactive substances. Appl Microbiol Biotechnol. 2007;74:1175–85. doi: 10.1007/s00253-007-0856-5. [DOI] [PubMed] [Google Scholar]
- 7.Devib P, Meeraa R. Study of antioxdant, antiinflammatory and wound healing activity of extracts of Litsea glutinosa. J Pharm Sci Res. 2010;2:55–163. [Google Scholar]
- 8.Dickson RA, Fleischer TC, Ekuadzi E, Mensah AY, Annan K, Woode E. Antibacterial, antioxidant and anti-inflammatory properties of Margaritaria discoidea, a wound healing remedy from Ghana. Pharmacognosy J. 2010;17:29–32. [Google Scholar]
- 9.Rahnama H, Hasanloo T, Shams MR, Sepehrifar R. Silymarin production by hairy root culture of Silybum marianum (L.) Gaertn. Iran J Biotechnol. 2008;6:113–9. [Google Scholar]
- 10.Owen HR, Miller RA. An examination and correction of plant tissue culture basal medium formulations. Plant Cell Tissue Organ Cult. 1992;28:147–50. [Google Scholar]
- 11.Thomson D, Henry R. Single-step protocol for preparation of plant tissue for analysis by PCR. Biotechniques. 1995;19:394–400. [PubMed] [Google Scholar]
- 12.Teeyapant R, Woerdenbag HJ, Kreis P, Hacker J, Wray V, Witte L, et al. Antibiotic and cytotoxic activity of brominated compounds from the marine sponge Verongia aerophoba. Z Naturforsch C. 1993;48:939–45. doi: 10.1515/znc-1993-11-1218. [DOI] [PubMed] [Google Scholar]
- 13.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 14.Alpizar E, Dechamp E, Lapeyre-Montes F, Guilhaumon C, Bertrand B, Jourdan C, et al. Agrobacterium rhizogenes-transformed roots of coffee (Coffea arabica): Conditions for long-term proliferation, and morphological and molecular characterization. Ann Bot. 2008;101:929–40. doi: 10.1093/aob/mcn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan XW, Xu HH, Liu X, Gao X, Lu YT. Improvement of growth and camptothecin yield by altering nitrogen source supply in cell suspension cultures of Camptotheca acuminata. Biotechnol Lett. 2004;26:1745–8. doi: 10.1007/s10529-004-4580-2. [DOI] [PubMed] [Google Scholar]
- 16.Sivakumar G, Yu KW, Hahn EJ, Paek KY. Optimisation of organic nutrients for ginseng hairy roots production in large scale bioreactors. Curr Sci. 2005;89:641–9. [Google Scholar]
- 17.Payne GF, Bringi V, Prince C, Shuler ML. Plant Cell and Tissue Culture in Liquid Systems. New York: Hanser; 1992. pp. 225–79. [Google Scholar]
- 18.Wang JP, Zhou YM, Ye YJ, Shang XM, Cai YL, Xiong CM, et al. Topical anti-inflammatory and analgesic activity of kirenol isolated from Siegesbeckia orientalis. J Ethnopharmacol. 2011;137:1089–94. doi: 10.1016/j.jep.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Tian F, Li B, Ji BP, Yang JH, Zhang GZ, Chen Y, et al. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis: The polarity affects the bioactivities. Food Chem. 2009;113:173–9. [Google Scholar]


