Abstract
Background:
The fruits of Aronia melanocarpa (Michx.) Elliot contain large amounts of phenolic substances, mainly procyanidins, anthocyanins and other flavonoids, and phenolic acids. The ability of phenolic substances to act as antioxidants has been well established.
Objective:
In this study, we investigated the radical scavenging activity of A. melanocarpa fruit juice (AMFJ).
Materials and Methods:
The method used was electron spin resonance (ESR) spectroscopy. The galvinoxyl free radical was used as a scavenging object. AMFJ was added to the galvinoxyl free radical solution. The measure of the radical scavenging activity was the decrease of signal intensity.
Results:
AMFJ showed a potent antiradical activity causing a strong and rapid decrease of signal intensity as a function of time and juice concentration. This effect of AMFJ was probably due to the activity of its phenolic constituents.
Conclusion:
The ESR measurements in this study showed a pronounced radical scavenging effect of AMFJ, an important mechanism of its antioxidant activity.
Keywords: Aronia melanocarpa fruit juice, electron spin resonance, galvinoxyl free radical, scavenging activity
INTRODUCTION
Dietary phenolic substances have been extensively studied because of their antioxidant activity.[1] A considerable body of literature supports the role of oxidative stress in the pathogenesis of age-related human diseases and the contribution of dietary phenolics to their prevention. Berries are known as plant material very rich in phenolics.
Aronia melanocarpa (Michx) Elliot (black chokeberry) is a woody shrub of the Rosaceae family native to the eastern North America which is now commonly planted in Eastern Europe and Russia. The fruits are used for human consumption as juice, syrup, jam, and wine. Literature data show that procyanidins are the phenolic compound group with the highest concentration in chokeberry fruits,[2] higher than in the other berries.[3] The anthocyanins are the second phenolic compound group in A. melanocarpa fruits. Chokeberry fruits contain also two phenolic acids: chlorogenic and neochlorogenic,[2] and a mixture of five quercetin glycosides.[4] Anthocyanin pigments are responsible for the dark violet, and even black, color of A. melanocarpa fruits while the astringent taste of the fruits is due to the high content of procyanidins.
Up to date, there are other investigations of the antioxidant activity of A. melanocarpa fruits but there are no measurements using an ESR technique. The aim of this study was to measure the radical scavenging activity of A. melanocarpa fruit juice (AMFJ) using electron spin resonance (ESR) spectroscopy and the galvinoxyl free radical as a scavenging object.
MATERIALS AND METHODS
A. melanocarpa fruit juice preparation
AMFJ was produced from fruits of A. melanocarpa (Michx.) Elliot grown in the Balkan Mountains, Bulgaria, in the region of Troyan. The fruits were handpicked in September, crushed, and squeezed. The juice was filtered, pasteurized at 80 °C for 10 min and stored at 0 °C till the experiment.
Determination of main biologically active substances in A. melanocarpa fruit juice
Total phenolic content of AMFJ was determined according to the spectrophotometric method based on the ability of the phenolic substances to form a blue molybdenum–tungsten complex with the reagent of Folin-Ciocalteu.[5] Absorbance was measured at 760 nm (Spekol 11, Zeiss, Jena, Germany). Total phenolic content of AMFJ (mg/100 ml) was expressed as gallic acid equivalents.
Total flavonoids were measured by the colorimetric assay developed by Zhishen et al.[6] NaNO2, AlCl3, and NaOH were added to the AMFJ sample or to standard solutions of catechin in order to form a flavonoid–aluminum complex. Absorbance of the mixture, pink in color, was measured at 510 nm. Total flavonoids (mg/100 ml) were expressed as catechin equivalents.
Total anthocyanins were determined by a pH-differential spectrophotometry after the method of Guisti et al.[7] This method measures the absorbance at two different pH values (pH 1.0 and 4.5) and relies on the structural transformations of the anthocyanin chromophore as a function of pH. The colored oxonium or flavilium form predominates at pH 1.0 and the colorless hemiketal or carbinol form at pH 4.5. The difference in the absorbance between pH 1.0 and pH 4.5 is due to the anthocyanin content. Absorbance was read at 700 nm to correct for haze, and then at 520 nm for anthocyanin determination. Total anthocyanins were quantified as cyanidin-3-glucoside equivalents and were expressed as mg/100 ml.
The flavonoid quercetin was measured using a high-performance liquid chromatography (HPLC) method described by Hertog et al.,[8] by a Hewlett Packard High Pressure Liquid Chromatograph (Agilent Technologies, Inc., Santa Clara, CA). The method is based on deglycosylation of the flavonoids by acid hydrolysis for 2 h at 90 °C with 1.2 M HCl in 50% methanol. Alltima C18 column (100 × 4.6 mm i.d., 3 μm, Alltech Associates Inc., Deerfield, IL.) was employed to separate quercetin with UV detection at a wavelength of 365 nm. Quercetin concentration was expressed as mg/100 ml.
L-Ascorbic acid (mg/100 ml) was measured by HPLC with UV detection at a wavelength of 254 nm using the method of Gennaro and Bertolo[9] after extracting with 0.5% metaphosphoric acid.
Duplicate determinations of three samples were performed. Results are presented as mean ± SD.
Chemicals
Galvinoxyl free radical [2,6-di-tert-butyl-alpha-(3,5-di-tert-butyl-4-oxo-2,5-cyclohexadien-1-ylidene)-p-tolyloxy free radical] was obtained from from Sigma-Aldrich, Germany [Figure 1] and ethanol, 96%, was from Kemika, Zagreb, Croatia.
Figure 1.
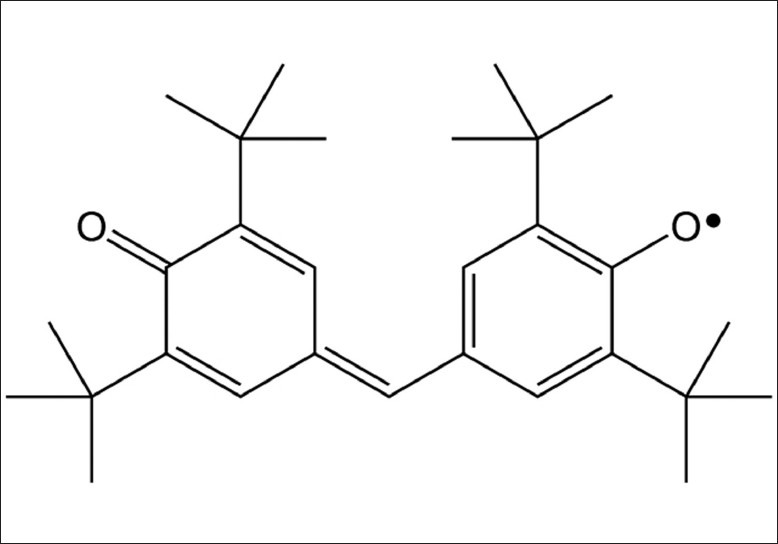
Structural formula of the galvinoxyl radical
Electron spin resonance measurements of radical scavenging activity
Electron spin resonance (ESR) measurements were performed at room temperature (24 °C) on a Varian E-109 spectrometer equipped with a Bruker ER 041 XG microwave bridge. The spectroscopic parameters were: frequency 9.27 GHz, field sweep 8 mT, microwave power 4.9 mW, and modulation amplitude 0.11 mT. The galvinoxyl free radical was used as a scavenging object in ESR radical-scavenging measurements. The stability of its freshly prepared ethanol solution was measured for 30 min, and no significant loss of signal was detected. A loss of signal of about 7% was obtained after 24 h. Therefore, the fresh radical solutions were prepared daily. AMFJ solution was added to the freshly prepared galvinoxyl solution in ethanol (c = 0.15 mmol/dm3) in order to obtain various juice concentrations (0.1, 0.2, 0.5, and 1.0 vol.%). The same final concentration of the galvinoxyl radical in all samples (c = 0.12 mmol/dm3) was obtained by addition of adequate amount of water. The juice solution was quickly mixed with the radical solution in the flask and immediately put into the capillary which was then placed in a standard ESR tube. Depending on the sample concentration, ESR spectra were recorded as a function of time starting from the juice and radical solution contact. Recording intervals were 0.5 min and 1.0 min, depending on the sample activity. The signal intensities of galvinoxyl radicals were calculated by the double integration of ESR spectra, using the EW (EPRWare) Scientific Software Service program and expressed in arbitrary units. The signal intensity of the pure 0.12 mmol/dm3 galvinoxyl solution, measured just before starting the sample measurement, was taken as the reference signal intensity (I0) for the reaction time t = 0 min. The normalized signal intensity (In) after the reaction time t, expressed as a percentage, was calculated as:
In = (I/I0) × 100,
where I is the signal intensity of galvinoxyl radicals in juice solution measured at time t. Each sample was analyzed in triplicate. The results are presented as mean values.
RESULTS
Contents of biologically active substances in A. melanocarpa fruit juice
The phenolic substances in 100 ml AMFJ were: total phenolics, 709.3 ± 28.1 mg as gallic acid equivalents; total flavonoids, 189.4 ± 8.6 mg as catechin equivalents; total anthocyanins, 106.8 ± 6.2 mg as cyanidin-3-glucoside equivalents; quercetin, 11.8 ± 0.8 mg. l-Ascorbic acid was 3.0 ± 0.08 mg in 100 ml AMFJ.
Electron spin resonance measurements of radical scavenging activity
ESR spectra of galvinoxyl radical solution (c = 0.12 mmol/dm3) before and after the addition of AMFJ at a concentration of 0.5 vol.% are shown in Figure 2. It is evident that the signal intensity strongly decreased as a function of time t. In fact, the loss of signal was about 18% and 62% for 0.5 and 3.0 min, respectively. For t = 8.0 min, the loss accounted for 85% and the signal was on the limit of detectability. The galvinoxyl free radical was previously applied in similar studies of virgin olive oil and red wine.[10–12] Comparing these results with those previously obtained, using the same radical in the study of olive oil and red wine, it can be concluded that AMFJ showed higher antioxidant activity.
Figure 2.
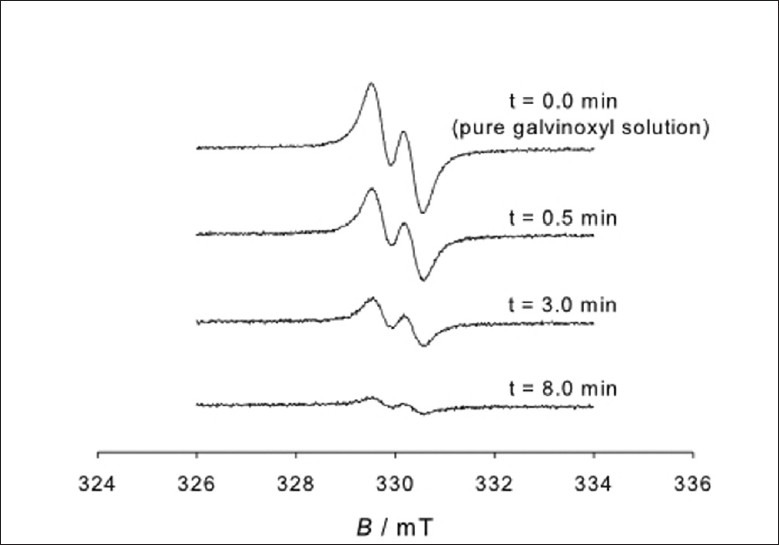
Electron spin resonance (ESR) spectra of a pure galvinoxyl radical solution (t = 0 min) and of a radical solution containing 0.5 vol.% of Aronia melanocarpa fruit juice (AMFJ) measured for various reaction times t. The concentration of the galvinoxyl radical in the pure solution and the starting solution with juice was the same (c = 0.12 mmol/dm3). B denotes the magnetic field
As evident from Figure 3, very small amount of AMFJ (0.1 vol.%) was sufficient to reduce the ESR signal in a relatively short time (26 min), the effect being most prominent during the first 15 min. Higher juice concentrations (0.2, 0.5, and 1.0 vol.%) lead to a radical scavenging effect, which resulted in faster signal loss. As expected, the fastest signal loss (8 min) was caused by the 1 vol.% concentration of AMFJ [Figure 3]. However, it seems that the signal loss is not simply proportional to the AMFJ concentration. The lower AMFJ concentrations (0.1% and 0.2%) were not sufficient to react with the whole amount of radical, while from the slope of two higher concentrations (0.5% and 1.0%) at t = 8 min and t = 10 min, respectively, it is evident that these juice solutions had still radical scavenging potential. This residual radical scavenging potential could not be measured by ESR because of an unfavorable signal-to-noise ratio.
Figure 3.
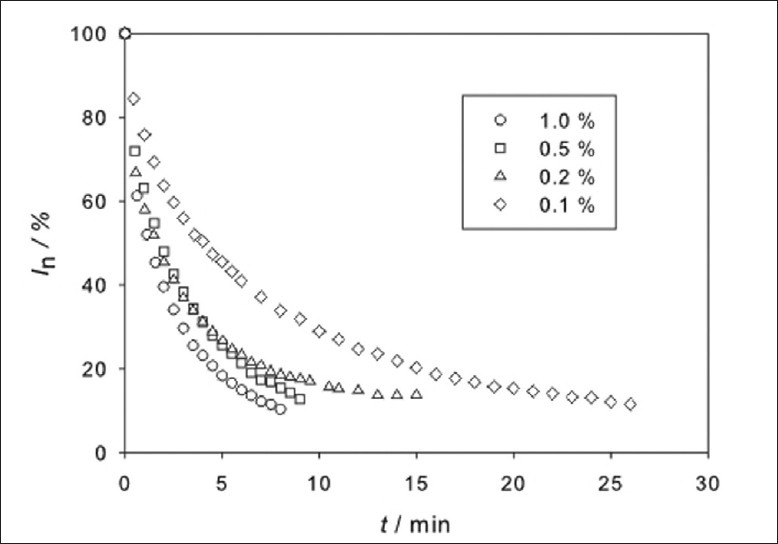
The normalized ESR signal intensity (In) of the galvinoxyl radical measured as a function of the reaction time t for the various concentrations (vol.%) of Aronia melanocarpa fruit juice (AMFJ)
DISCUSSION
Many methods have been introduced to study the antioxidant activity of different foodstuffs. More recently, ESR spectroscopy has been used.[13,14] ESR spectroscopy applied on the galvinoxyl free radical was proposed by Quiles et al.,[15] as a rapid and very sensitive method.
The present results indicate a high antioxidant activity of AMFJ. They are in accordance with other investigations measuring the radical scavenging activity of A. melanocarpa fruits. An investigation of Nakajima et al.[16] demonstrated potent antiradical activities of a chokeberry extract against the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical. Jakobek et al.[17] applied the DPPH test to investigate the antiradical activity of individual phenolic fractions isolated from chokeberry as well as the influence of the interactions between phenolic compounds on the antiradical activity. Using methanolic fruit extracts of particular cultivars of A. melanocarpa (Michx.) Elliot, Rop et al.[18] showed their high scavenging activity for reactive oxygen species (superoxide anion, hydroxyl radical, and nitric oxide).
The antiradical activity of AMFJ demonstrated in this study is probably due mainly to the phenolic ingredients of the juice (procyanidins, flavonoids mainly from the subclass of anthocyanins, and phenolic acids). Previous studies have demonstrated a high degree of positive correlation between the antioxidant capacity and the total phenolic content of AMFJ. As is generally well known, berries contain a large amount of phenolic compounds that act as antioxidants besides anthocyanins.[19,20] The ability of small phenolics including flavonoids and phenolic acids, to act as antioxidants has been well established but the high molecular weight phenolics (procyanidins) have been neglected.[2] However, there are data that procyanidins possess potent antioxidant capacity and possible protective effects on human health.[21]
CONCLUSION
The ESR measurements in this study showed a pronounced radical scavenging effect of AMFJ which is an important mechanism of its antioxidant activity. ESR spectroscopy has proven to be a powerful and precise tool in the analysis of this type of activity.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Fresco P, Borges F, Diniz C, Marques MP. New insights on the anticancer properties of dietary polyphenols. Med Res Rev. 2006;22:747–66. doi: 10.1002/med.20060. [DOI] [PubMed] [Google Scholar]
- 2.Oszmianski J, Wojdylo A. Aronia melanocarpa phenolics and their antioxidant activity. Eur Food Res Technol. 2005;221:809–13. [Google Scholar]
- 3.Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, et al. Procyanidin content and variation in some commonly consumed foods. J Nutr. 2004;134:613–7. [Google Scholar]
- 4.Slimestad R, Torskangerpoll K, Nateland HS, Johannessen T, Giske NH. Flavonoids from black chokeberries, Aronia melanocarpa. J Food Compost Anal. 2004;18:61–8. [Google Scholar]
- 5.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Emol Viticult. 1965;16:144–58. [Google Scholar]
- 6.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–9. [Google Scholar]
- 7.Guisti MM, Rodrigues-Saona LE, Wrolstad RE. Specrtral characteristics, molar absorptivity and color of pelargonidin derivatives. J Agric Food Chem. 1999;47:4631–7. doi: 10.1021/jf981271k. [DOI] [PubMed] [Google Scholar]
- 8.Hertog MG, Hollman PC, Vanema DP. Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits. J Agric Food Chem. 1992;40:1591–8. [Google Scholar]
- 9.Gennaro MC, Bertolo PL. L-ascorbic acid determination in fruits and medical formulation by ion interaction reagent reverse phase HPLC technique. J Liq Chrom. 1990;13:1419–34. [Google Scholar]
- 10.Papadimitriou V, Sotiroudis TG, Xenakis A, Sofikiti N, Stavyiannoudaki V, Chaniotakis NA. Oxidative stability and radical scavenging activity of extra virgin olive oils: An electron paramagnetic resonance spectroscopy study. Anal Chim Acta. 2006;573:453–8. doi: 10.1016/j.aca.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Koprivnjak O, Şkevin D, Valić S, Majetić V, Petričević S, Ljubenkov I. The antioxidant capacity and oxidative stability of virgin olive oil enriched with phospholipids. Food Chem. 2008;111:121–6. [Google Scholar]
- 12.Espinoza M, Olea-Azar C, Speisky H, Rodriguez J. Determination of reactions between free radicals and selected Chilean wines and transition metals by ESR and UV-vis technique. Spectrochim Acta A Mol Biomol Spectrosc. 2009;71:1638–43. doi: 10.1016/j.saa.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen JA. On the application of electron paramagnetic resonance in the study of naturally occurring quinones and quinols. Spectrochim Acta A. 2002;58:1257–70. doi: 10.1016/s1386-1425(01)00715-6. [DOI] [PubMed] [Google Scholar]
- 14.Polovka M, Brezová V, Stasko A. Antioxidant properties of tea investigated by EPR. Biophys Chem. 2003;106:39–56. doi: 10.1016/s0301-4622(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 15.Quiles L, Ramirez-Tortosa MC, Gomez JA, Huertas JR, Mataix J. Role of vitamin E and phenolic compounds in the antioxidant capacity, measured by ESR, of virgin olive, olive and sunflower oils after frying. Food Chem. 2002;76:461–8. [Google Scholar]
- 16.Nakajima JI, Tanaka I, Seo S, Yamasaki M, Saito K. LC/PDA/ESI-MS profiling and radical scavenging activity of anthocyanins in various berries. J Biomed Biotechnol. 2004;5:241–7. doi: 10.1155/S1110724304404045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakobek L, Seruga M, Krivak P. The influence of interactions among phenolic compounds on the antiradical activity of chokeberries (Aronia melanocarpa) Int J Food Sci Nutr. 2011;62:345–52. doi: 10.3109/09637486.2010.534438. [DOI] [PubMed] [Google Scholar]
- 18.Rop O, Mlcek J, Jurikova T, Valsikova M, Sochor J, Reznicek V, et al. Phenolic content, antioxidant capacity, radical oxygen species scavenging and lipid peroxidation inhibiting activities of extracts of five black chokeberry (Aronia melanocarpa (Michx.) Elliot) cultivars. J Med Plant Res. 2010;4:2431–7. [Google Scholar]
- 19.Zheng W, Wang SY. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J Agric Food Chem. 2003;51:502–9. doi: 10.1021/jf020728u. [DOI] [PubMed] [Google Scholar]
- 20.Vinson JA, Su X, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem. 2001;49:5315–21. doi: 10.1021/jf0009293. [DOI] [PubMed] [Google Scholar]
- 21.Santos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds: Nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agric. 2000;80:1094–117. [Google Scholar]


