Abstract
Context:
Reserpine-induced orofacial dyskinesia is an animal model of tardive dyskinesia which may be associated with neurodegeneration and free radical damage.
Aim:
The aim was to assess the neuroprotective potential and in vivo antioxidant status of alcoholic extract of roots and rhizomes of Nardostachys jatamansi (ANJ) and its triterpenes (TNJ) in reserpine-induced orofacial dyskinesia.
Materials and Methods:
In the present study, repeated treatment with reserpine (1.0 mg/kg) on each other day for a period of 5 days (days 1, 3, and 5) significantly induced vacuous chewing movements (VCMs) and tongue protrusions (TPs) in rats. The effect on reserpine-induced catalepsy was also studied. The effect of ANJ and TNJ on levels of superoxide dismutase (SOD), catalase (CAT), and glutathione reductase (GSH) and inhibition of lipid peroxidation (LPO) in the forebrain region was assessed.
Statistical Analysis:
All observations were expressed as mean ± SEM. Statistical analysis was performed by the one-way ANOVA followed by Dunnett's test. P<0.05 was regarded as statistically significant.
Results:
At the end of the treatment schedule, ANJ and TNJ significantly inhibited reserpine-induced VCM, TP, and catalepsy, and significantly increased the locomotion and rearing in the open-field test. Treatment with ANJ and TNJ exhibited significant elevation in the levels of superoxide dismutase (SOD), catalase (CAT), and glutathione reductase (GSH) and inhibition of lipid peroxidation (LPO) in forebrain region compared to the reserpine treated group.
Conclusions:
The study concludes that ANJ and TNJ significantly protected animals against reserpine-induced orofacial dyskinesia as well as catalepsy suggesting its potential value in the treatment of neuroleptic-induced orofacial dyskinesia and Parkinson's disease.
KEY WORDS: Nardostachys jatamansi, oxidative stress, reserpine
Introduction
Increased oxidative stress with cumulative free radical damage is a well-known feature of the aging brain.[1] It is proposed that tardive dyskinesia may be due to a neurotoxic effect of the free radical byproducts from catecholamine metabolism in the basal ganglia.[2] The neuroleptic drugs by blocking dopamine receptors cause secondary increase in turnover and metabolism of dopamine, which may lead to increased formation of dopamine quinones as well as of hydrogen peroxide through the activity of monoamine oxidase.[2] In support of this “free radical hypothesis,” studies have shown that neuroleptic drugs induce oxidative stress and cell death.[3] According to some clinical studies, levels of lipid peroxidation byproducts in blood or cerebrospinal fluid of tardive dyskinesia patients are increased compared to those in nontardive dyskinesia patients.[4] Increased lipid peroxidation in substantia nigra has been reported in Parkinson's disease also.[5]
Parkinson's disease (PD) is a serious neurological disorder accompanied by specific loss of the nigro-striatal dopamine neurons leading to extrapyramidal motor symptoms. The parkinsonian symptoms can be induced in animals by agents that block striatal dopamine D2 receptors (e.g., haloperidol) or cause dopamine deficiency (e.g., reserpine and 6-OHDA).[6] Reserpine (RE) is known to be associated with the development of tardive dyskinesia[7] and Parkinson's disease.[8] Neisweinder et al.[9] suggested that RE-induced orofacial dyskinesia may provide an animal model of tardive dyskinesia. Rats treated with this monoamine-depleting agent develop orofacial dyskinesia characterized by tongue protrusion, twitching of the facial musculature, and vacuous chewing movements.[10]
Antidepressant potential of Nardostachys jatamansi DC has been described.[11,12] We have evaluated here the effect of the ethanolic extract of Nardostachys jatamansi and terpenes isolated from its alcoholic extract on reserpine-induced oral dyskinesia, catalepsy, and hypolocomotion. We also investigated its effect on biochemical parameters such as SOD, CAT, GSH, and LPO in the forebrain region to correlate the behavioral pattern with the biochemical changes.
Materials and Methods
Animals
Male Wistar rats (150-200 g), obtained from Bharat Serum and Vaccines Ltd., Thane, India were used for the study. Animals were housed in colony cages and maintained at 25°- ± 2°C, 12:12 hour light/dark cycle and 50- ± 5% relative humidity with free access to food and water ad libitum. Animals were acclimatized to laboratory conditions before test. All the experiments were carried out during the light period (08.00-16.00). The Institutional Animal Ethics Committee of MGV's Pharmacy College, Nashik, India approved the protocol of the study.
Plant Material and Extraction
Roots and rhizomes of Nardostachys jatamansi DC (Valerianaceae) were obtained from Aushadhi Bhavan, Ayurved Seva Sangh, Nashik, Maharashtra. The plant specimen was identified and authenticated by Dr. S. L. Dasari, Ayurved College, Nashik. Roots and rhizomes of Nardostachys jatamansi (2 kg) were dried, powdered, and defatted with pet ether (60-80°C). Solvent was removed and marc was successively extracted with ethanol using Soxhlet's extractor. The ethanolic extract (ANJ) was concentrated and evaporated to dryness (yield: 9.02% w/w). Terpenes were isolated from ANJ by the method described earlier.[11] ANJ was subjected to identification of phytoconstituents by the methods described earlier.[12]
Drugs and Treatment Schedule
Reserpine (Sigma-Aldrich, MO, USA) was dissolved in glacial acetic acid and then diluted in distilled water. Vehicle consisted of the same amount of acetic acid and water as used in the reserpine solution. ANJ and TNJ were suspended in distilled water. The ANJ and TNJ were given orally. Animals were divided into five groups, which received vehicle, ANJ (100 or 300 mg/ kg, p.o.), TNJ (100 mg/kg, p.o.) or vitamin E (10 mg/kg, p.o.). Reserpine (1 mg/kg, s.c.) was administered on alternate days for a period of 5 days and behavioral observations were carried out after the last dose of reserpine. In addition to these groups, one group received ANJ (300 mg/kg), without preexposure to reserpine. The vacuous chewing movements (VCM), tongue protrusions (TP), and orofacial bursts (OB) were measured in each rat on the 5th day. Biochemical estimations were carried on day 5, after completion of the behavioral assessment.
Assessment of Behavioral Parameters
Immediately after injection of reserpine, rats were placed in a plexiglas observation box (22 cm × 22 cm × 22 cm) for a 10-minute habituation period. All rats were observed for 5 minutes. An observer blinded to the treatment recorded the number of VCM, TP, and OB as described by Cousins.[13] Then the effect of ANJ and TNJ on locomotion was determined using open-field apparatus. The total number of squares traversed and the number of rearing were counted for 5 minutes. The effect on catalepsy was determined for 3 hours at 30-minute interval using the bar test.
Biochemical Estimation
Immediately after measurement of catalepsy on 5th day, the animals were sacrificed. The brains were removed; the forebrain was dissected, rinsed with isotonic saline, and weighed. Then it was homogenized with 0.1N HCl. A 10% (w/v) tissue homogenate was prepared in a 0.1 M phosphate buffer (pH 7.4); the pos nuclear fraction for catalase assay was obtained by centrifugation of the homogenate at 1000 g for 20 minutes at 4°C and for other enzyme assays, it was centrifuged at 12,000 g for 60 minutes at 4°C.
Measurement of Superoxide Dismutase Activity
The assay of SOD was based on the ability of SOD to inhibit spontaneous oxidation of adrenaline to adrenochrome.[14] To 0.05 mL supernatant, 2.0 mL of carbonate buffer and 0.5 mL of EDTA were added. The reaction was initiated by addition of 0.5 mL of epinephrine and the auto-oxidation of adrenaline (3×10-4 M) to adrenochrome at pH 10.2 was measured by following changes in optical density at 480 nm. The changes in optical density every minute were measured at 480 nm against a reagent blank. The results are expressed as units of SOD activity (milligram per protein). One unit of SOD activity induced approximately 50% inhibition of adrenaline. The results were expressed as nmol SOD U per mg wet tissue.
Measurement of Catalase Activity
The CAT activity assay was carried out as described by Beers and Sizer.[15] The reaction mixture consisted of 2 mL phosphate buffer (pH 7.0), 0.95 mL of hydrogen peroxide (0.019 M), and 0.05 mL supernatant in final volume of 3 mL. Absorbance was recorded at 240 nm every 10 seconds for 1 minute. One unit of CAT was defined as the amount of enzyme required to decompose 1 mmol of peroxide per minute, at 25°C and pH 7.0. The results were expressed as units of CAT activity (milligram per protein). Units of activity were determined from the standard graph of H2O2. The results were expressed as catalase U per mg wet tissue.
Estimation of Reduced Glutathione
GSH was determined by the method of Ellman.[16] To the homogenate 10% trichloroacetic acid was added and centrifuged, followed by addition of 1.0 mL Ellman's reagent [19.8 mg of 5, 5-0-dithiobisnitro benzoic acid in 100 mL of 1.0% sodium citrate and 3 mL of phosphate buffer (pH 8.0)]. The color that developed was measured at 412 nm. The results were expressed as nanomole GSH per milligram wet tissue.
Estimation of Lipid Peroxidative Indices
Lipid peroxidation as evidenced by the formation of thiobarbituric acid reactive substances (TBARS) was measured by the method of Niehaus and Samuelsson.[17] In brief, 0.1 mL of homogenate (Tris-HCl buffer, pH 7.5) was treated with 2 mL of (1: 1: 1 ratio) TBA-TCA-HCl reagent (thiobarbituric acid 0.37%, 0.25N HCl, and 15% TCA) and placed in water bath for 15 minutes, cooled, and centrifuged at room temperature for 10 minutes at 1000 g. The absorbance of clear supernatant was measured against a reference blank at 535 nm. The results were expressed as LPO nanomole per milligram wet tissue.
Statistical Analysis
Observations have been shown as mean ± SEM. Statistical analysis was performed by the one-way ANOVA followed by Dunnett's test. P<0.05 was regarded as statistically significant.
Results
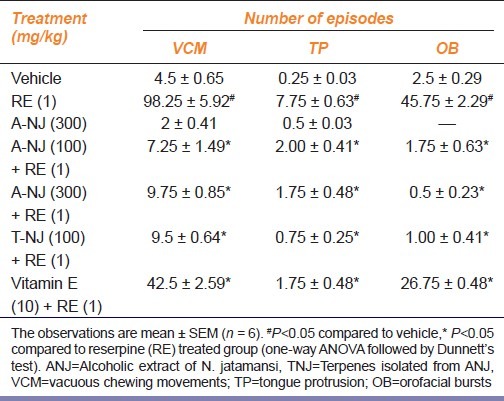
Reserpine (1 mg/kg, s.c.) treatment on alternate days for a period of 5 days significantly (P<0.05) increased vacuous chewing movements (VCM), tongue protrusion (TP), and orofacial bursts (OB). ANJ (100, 300 mg/kg), TNJ (100 mg/ kg), and vitamin E (10 mg/kg) for a period of 5 days dose-dependently and significantly inhibited reserpine-induced VCMs, TPs, and OBs [Table 1]. ANJ (300 mg/kg) administered per se did not produce any significant change in VCMs, TPs, and OBs when compared with the vehicle.
Table 1.
Effect of ethanolic extract of Nardostachys jatamansi and its triterpenes on reserpine-induced orofacial dyskinesia in rats
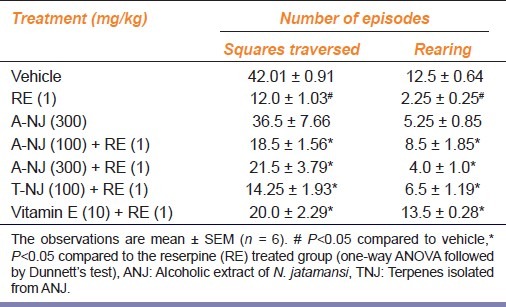
Reserpine administration on alternate days for a period of five days significantly (P<0.05) decreased locomotor activity. Pretreatment with ANJ (100, 300 mg/kg), TNJ (100 mg/kg) and Vitamin E (10 mg/kg) prevented attenuation of reserpine-induced hypolocomotion significantly. ANJ (300 mg/kg) administered alone did not produce any significant change in the total locomotor activity when compared with the vehicle treated group [Table 2].
Table 2.
Effect of ethanolic extract of Nardostachys jatamansi and its triterpenes on locomotor activity in reserpine treated rats
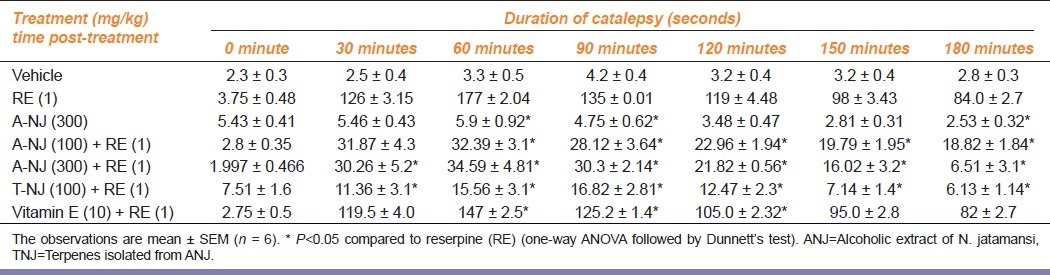
Reserpine induced a time-dependent catalepsy in rats. Pretreatment with ANJ (100, 300 mg/kg), TNJ (100 mg/kg), and Vitamin E (10 mg/kg) significantly inhibited reserpine-induced catalepsy [Table 3].
Table 3.
Effect of ethanolic extract of Nardostachys jatamansi and its triterpenes on reserpine-induced catalepsy in rats
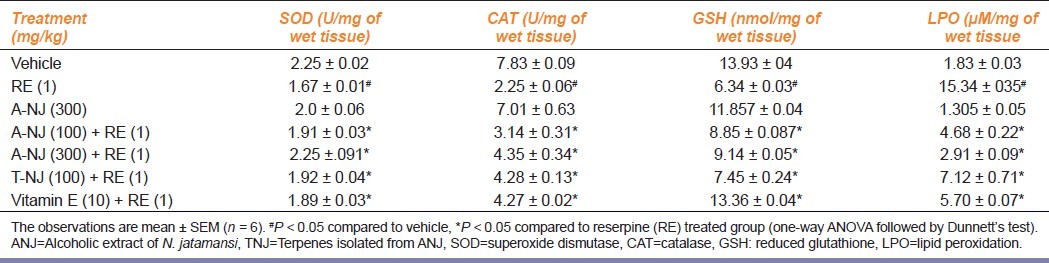
Reserpine-treated animals exhibited an increase in the levels of lipid peroxidation, and decreased levels of GSH and protective antioxidant enzymes such as SOD and CAT, suggesting a possible free radicals generation. Treatment with ANJ (100, 300 mg/kg), TNJ (100 mg/kg), and vitamin E (10 mg/kg) attenuated these increased levels of lipid peroxidation. It also increased levels of GSH and protective enzymes such as SOD and CAT, suggesting its possible antioxidant action [Table 4].
Table 4.
Effect of ethanolic extract of Nardostachys jatamansi and its triterpenes on biochemical alterations in reserpine treated rats
Discussion
Dopamine and noradrenaline are involved in the control of motor activity.[18] Free radicals are involved in the development of orofacial dyskinesia in rats. We observed that reserpine significantly increased VCM, TP, and OB in rats. ANJ (100, 300 mg/kg), TNJ (100 mg/kg), and Vitamin E (10 mg/kg) significantly inhibited these changes. Reserpine administration also decreased the total locomotor activity and ANJ, TNJ, and vitamin E prevented this. Reserpine induced a time-dependent catalepsy which was significantly inhibited by ANJ, TNJ, and vitamin E, indicative of a dopaminergic action of the ethanolic extract of Nardostachys jatamansi. The antipsychotic-induced catalepsy is a sign of the extrapyramidal side effect which is brought about through inhibition of the nigro-striatal dopaminergic neurotransmission.[6]
It is reported that reserpine-induced oral dyskinesia is inhibited by the GABA mimetic drug valproic acid[19] as the reserpine-induced persistent neuropathological changes may involve GABAergic efferent to striatal dopaminergic neurons.[20] Alcohol extract of Nardostachys jatamansi has been reported to cause an overall increase in the levels of biogenic amines and inhibitory amino acids including a change in the levels of serotonin, 5-hydroxyindole acetic acid, gamma amino butyric acid, and taurine in rat brain.[21]
In the present study, reserpine-treated animals exhibited an increase in the levels of lipid peroxidation, and decreased levels of GSH and protective antioxidant enzymes such as SOD and CAT, suggesting a possible free radicals generation. Previous studies have also demonstrated the reserpine-induced oral dyskinesia to be closely associated with the oxidative stress.[22] Treatment with ANJ (100, 300 mg/kg), TNJ (100 mg/ kg), and vitamin E (10 mg/ kg) attenuated these increased levels of lipid peroxidation. It also increased levels of GSH and protective enzymes such as SOD, CAT, suggesting its possible antioxidant action. As antidepressants are useful in treatment of Parkinsonism, findings of present study support the earlier observations of antidepressant activity of Nardostachys jatamansi.[23]
The ANJ revealed the presence of terpenes, saponins, glycosides, flavonoids, tannins, and phenolic compounds. Terpenes are known to have antioxidant action and they also inhibit mono-amine oxidase (MAO) enzymes.[24] Flavonoids also possess MAO inhibitory activity.[25] This activity may be the result of the synergistic effect produced by different constituents of the extract. It is therefore concluded that the triterpenes of Nardostachys jatamansi inhibit reserpine-induced orofacial dyskinesia and catalepsy and may be investigated further as a putative antiparkinsonian agent.
Acknowledgment
The authors are grateful to the management and Prof V. M. Aurangabadkar, Principal, MGV's Pharmacy College, Nashik for providing research facilities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Benzi G, Moretti A. Age – and peroxidative stress-related modifications of the cerebral enzymatic activities linked to mitochondria and the glutathione system. Free Radic Biol Med. 1995;19:77–101. doi: 10.1016/0891-5849(94)00244-e. [DOI] [PubMed] [Google Scholar]
- 2.Lohr JB. Oxygen free radicals and neuropsychiatric illness. Arch Gen Psychiatry. 1991;48:1097–106. doi: 10.1001/archpsyc.1991.01810360061009. [DOI] [PubMed] [Google Scholar]
- 3.Behl C, Rupprecht R, Skutella T, Holsboer F. Haloperidol-induced cell-death mechanism and protection with vitamin E in vitro. Neuro Rep. 1995;7:360–4. [PubMed] [Google Scholar]
- 4.Lohr JB, Kucenski R, Bracha HS, Moir M, Jeste DV. Increased indices of free radical activity in the cerebrospinal fluid of patients with tardive dyskinesia. Biol Psychiatry. 1990;28:535–9. doi: 10.1016/0006-3223(90)90490-s. [DOI] [PubMed] [Google Scholar]
- 5.Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, et al. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989;52:381–9. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 6.Jolicoeur FB, Rivest R. Neuromethods. Animal Models of Neurological Disease. Vol. 21. New Jersey USA: The Humana Press; 1992. Rodent models of Parkinson's disease; pp. 135–58. [Google Scholar]
- 7.Uhrbrand L, Faurbye A. Reversible and irreversible dyskinesia after treatment with perphenazine, chlorpromazine, reserpine, and electroconvulsive therapy. Psychopharmacology (Berl) 1960;1:408–18. [Google Scholar]
- 8.Ishibashi T, Ohno Y. Antiparkinsonian actions of a selective 5-HT1A agonist, tandospirone, in rats. Biogen Amines. 2004;18:329–38. [Google Scholar]
- 9.Neisweinder JL, Castaneda E, Davis DA. Dose-dependant differences in the development of reserpine-induced oral dyskinesia in rats: Support for the model of tardive dyskinesia. Psychopharmacology (Berl) 1994;116:79–84. doi: 10.1007/BF02244874. [DOI] [PubMed] [Google Scholar]
- 10.Calvente PR, Araujo CC, Bergao M, Abilio VC, D’Almeida V, Ribeiro R de A, et al. The mitochondrial toxin 3-nitropropionic acid aggravated reserpine-induced oral dysskinesia in rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2002;26:1–5. doi: 10.1016/s0278-5846(01)00255-x. [DOI] [PubMed] [Google Scholar]
- 11.Harborne JB. Phytochemical Methods. A guide to modern techniques of plant analysis. New Delhi: Springer Publication; 2007. p. 119. [Google Scholar]
- 12.Evans WC. Trease and Evans’ Pharmacognosy. New Delhi, India: Elsevier; 2005. p. 233. (230, 247, 289, 339). [Google Scholar]
- 13.Cousins MS, Carriero DL, Salamone JD. Tremulous Jaw Movements induced by the acetylcholinesterase inhibitor tacrine: Effect of anti-parkinsonian drugs. Eur J Pharmacol. 1997;322:137–45. doi: 10.1016/s0014-2999(97)00008-3. [DOI] [PubMed] [Google Scholar]
- 14.Misra H, Fridovich I. Role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170. [PubMed] [Google Scholar]
- 15.Beers R, Sizer I. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133. [PubMed] [Google Scholar]
- 16.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 17.Niehaus WG, Samuelsson B. Formation of malondialdehyde from phospholipids arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 18.Carlsson A. Drugs which block the storage of 5-HT and related amines. In: Eichler O, Farah A, Erspamer V, editors. Handbook Exp. Pharmacol. Berlin Heidelberg-NewYork: Springer-Verlag; 1966. pp. 529–92. [Google Scholar]
- 19.Peixoto MF, Araujo NP, Silva RH, Castro JP, Fukushiro DF, Faria RR, et al. Effects of gabaergic drugs on reserpine-induced oral dyskinesia. Behav Brain Res. 2005;160:51–9. doi: 10.1016/j.bbr.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Sussman AN, Traan-Nguyen LT, Neisewander JL. Acute reserpine administration elicits long-term spontaneous oral dyskinesia. Eur J Pharmacol. 1997;37:157–62. doi: 10.1016/s0014-2999(97)01271-5. [DOI] [PubMed] [Google Scholar]
- 21.Prabhu VM, Karanth SK, Rao A, Vidya PM, Sudhakar K. Effect of Nardostachys jatamansi on biogenic amines and inhibitory amino acids on rat brain. Planta Med. 1994;60:114–7. doi: 10.1055/s-2006-959429. [DOI] [PubMed] [Google Scholar]
- 22.Naidu PS, Singh A, Kulkarni SK. Carvedilol attenuates neuroleptic induced orofacial dyskinesia: Possible antioxidant mechanism. Br J Pharmacol. 2002;136:193–200. doi: 10.1038/sj.bjp.0704717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rektorova I, Rektor I, Bares M. Pramipexole and pergolide in the treatment of depression in Parkinson's disease: A national multicentre prospective randomized study. Eur J Neurol. 2003;1:399–406. doi: 10.1046/j.1468-1331.2003.00612.x. [DOI] [PubMed] [Google Scholar]
- 24.Youdim MB, Weinstock M. Therapeutic applications of selective and non-selective inhibitors of monoamine oxidase a and b that do not cause significant tyramine potentiation. Neurotoxicology. 2004;25:243–50. doi: 10.1016/S0161-813X(03)00103-7. [DOI] [PubMed] [Google Scholar]
- 25.Jager AK, Saaby L. Flavonoids and the CNS. Molecules. 2011;16:1471–85. doi: 10.3390/molecules16021471. [DOI] [PMC free article] [PubMed] [Google Scholar]


