Abstract
Objective:
To evaluate ethanolic extract of leaves of Aegle marmelos in an experimental animal model of chronic fatigue syndrome for potential therapeutic benefit.
Materials and Methods:
Age/weight-matched female Wistar albino rats were grouped into five groups. (Group I- V) (n = 8). Group I served as naïve control and II served as stress control. Except for group I animals, other group animals were subjected to forced swimming every day for 15 minutes to induce a state of chronic fatigue and simultaneously treated with ethanolic extract of Aegle marmelos (EEAM) 150 and 250 mg/kg b.w. and Imipramine (20 mg.kg b.w.), respectively. Duration of immobility, anxiety level and locomotor activity were assessed on day 1, 7, 14 and 21 followed by biochemical estimation of oxidative biomarkers at the end of the study.
Results:
Treatment with EEAM (150 and 250 mg/kg b.w.) resulted in a statistically significant and dose dependent reduction (P <0.001) in the duration of immobility, reduction in anxiety and increase in locomotor activity. Dose dependent and significant reduction in LPO level and increase in CAT and SOD was observed in extract treated animals.
Conclusion:
The results are suggestive of potential protective effect of A. marmelos against experimentally induced CFS.
KEY WORDS: Aegle marmelos, chronic fatigue syndrome, duration of immobility, lipid peroxidation
Introduction
Chronic fatigue syndrome (CFS) is an illness fundamentally characterized by debilitating fatigue, complicated by various physical and mental symptoms as a result of biochemical and immunologiocal disturbances.[1] CFS affects mainly young adults, aged 20-40 year and is considered primarily an endemic disorder, but also occurs in both epidemic and in sporadic forms with higher prevalence in women than in men, apparently at a lower rate in children and adolescents.[2] The exact etiology and pathophysiology of CFS still remains unclear and several hypotheses have been put forward, which include the involvement of altered immune response against a common antigen, a neuroendocrine disturbance and specific bacterial or viral infections,[3] an abnormal activation of the T-lymphocyte subsets and high levels of proinflammatory cytokines and accompanied by metabolic disorders such as selective n-6 fatty acid depletion - suggestive of oxidative stress, more specifically, lipid peroxidation contributing to the disease process, including some of the symptoms found in the illness.[4] Malondialdehyde, a product of lipid peroxidation, was found to be significantly higher in CFS patients. Peroxidation of erythrocyte membrane lipid in CFS is also a strong evidence that suggests’ that free radicals play a part in the pathogenesis of this illness.[5] Evidence of oxidative damage of DNA and lipids in the vastus lateralis muscle of CFS patients by free radicals, and elevated levels of methemoglobin - considered a marker of oxidative stress points to oxidative stress mechanisms of CFS.[6,7]
Aegle marmelos (L.) Corr. commonly known as Bael (Family: Rutaceae) is described in the Ayurveda for its use in various illness such as fever, hyperlipidemia, hypertension, analgesic, anti-inflammatory, heart disease, etc. It is an important medicinal plant and compounds purified from Bael have proven biologically active against several major diseases in experimental animal models and have shown activities including antispermatogenic, antimicrobial, and antioxidant. More than 30 identified compounds from the leaves of Aegle marmelos have been reported. The bioactive compounds of leaves of Aegle marmelos including – skimmianine, aegelin, lupeol, cineole, citral, citronellal, marmesinin, marmelosin, aurapten, marmelide and more specifically eugenol and marmesinin are found to possess potent antioxidant property and reported to inhibit lipid peroxidation. The antioxidative phytochemicals such as flavonoids, alkaloids, sterols, tannins, phlobatannins present in the leaf extract also possess free radical scavenging activity.[8]
Ethanolic extract of leaves, is reported to possess several biological properties that includes lipid lowering activity in hyperlipidemic albino Wistar rats,[9] antibacterial and antiproliferative properties,[10,11] dose dependent, reversible antifertility effect in male albino rats[12] and analgesic, antipyretic and anti-inflammatory property.[13]
In light of proven antioxidant nature of leaves of Aegle marmelos and the definitive role of oxidative stress in the pathogenesis of CFS, the current work evaluates this herb in an experimental animal model; for potential therapeutic benefit in treatment and management of CFS.
Materials and Methods
Experimental Animals
Adult, female, healthy albino Wistar rats in the weight range of 130-150 g were procured from registered breeder and were acclimatized for a week. The protocol of animal experimentation was cleared by Institutional Animal Ethics Committee of this instituition. Prior to and during study, the test animals were maintained under standard animal house conditions. [temperature (18-25°C), relative humidity (50-70%)], 12:12light: dark cycle and other micro and macroenvironment conditions as suggested by CPCSEA). Naive and test animals were housed (not more than 4 in a single cage) in a polypropylene cage covered with a stainless steel wire mesh and a paddy husk bed, with adequate provision for feed and water. Test animals were maintained on commercial feed (M/s Amrut feeds, Mumbai.) and water ad libitum.
Drugs and Chemicals
Solvent for extraction and chemicals for biochemical assays were procured from local supplier and were of analytical grade. Ethanol from bonded excise warehouse of state department, bovine serum albumin, copper sulfate, Folin-Ciocalteau reagent from M/s Central Drug House, New Delhi, NAD (P)H, thiobarbituric acid from M/s. Spectrochem Pvt. Ltd., Mumbai, nitroblue tetrazolium chloride, phenazine methosulfate, potassium dichromate, potassium sodium tartrate, potassium dihydrogen phosphate, sodium bicarbonate, hydrogen peroxide and trichloroacetic acid from M/s SD Fine Chem, Mumbai, glacial acetic acid from M/s Merck, Mumbai, sodium hydroxide from M/s Qualigens, Mumbai, n- Butanol from M/s Ranchem, New Delhi.
Collection, Authentication and Extraction
Fresh leaves of Aegle marmelos (L.) Corr. was collected locally in the month of March and was authenticated by competent scientists from Botanical Survey of India (BSI), Western circle, Pune and Voucher specimen of the same was deposited in BSI, Pune (PGSAM1) and in the department of pharmacology. The extract of collected leaves was prepared by cold maceration process. Coarse powdered, shade dried leaves of Aegle marmelos (around 270 g) was soaked in ethanol (90%) and then set aside for 72 hours with occasional shaking. The ethanolic extract of Aegle marmelos (EEAM) was concentrated by distilling off solvent and stored in an airtight container till further use. The EEAM, so obtained was dark green in color, pleasant and aromatic in odor and was of semi-solid consistency. The final yield was around 5%.
Phytochemical Analysis
EEAM was subjected qualitative phytochemical analysis for flavonoids, alkaloids, sterols, tannins and others.[14]
Acute (oral) Toxicity Study
EEAM was subjected to acute oral toxicity test following OECD test guidelines 425 (Up and Down method)[15] and was found to be safe up to 2000 mg/kg body weight.
Experimental Protocol
Grouping, treatment and induction of CFS[16]
Weight-matched test animals were randomly assigned to five groups of eight animals (n = 8) each.
Group I: Naive animals, which are neither subjected to stress nor given any drug/extract. (Naïve)
Group II: Subjected to forced swimming (to induce CFS), for 21 days, but without drug/extract treatment (Stress control).
Group III: Subjected to forced swimming + treated with Dose I (150 mg/kg b.w.) of extract, for 21 days
Group IV: Subjected to forced swimming + treated with Dose II (250 mg.kg b.w.) of extract, for 21 days
Group V: Subjected to forced swimming + treated with Imipramine (20 mg/kg b.w), for 21 days
Extract and Imipramine were also administered orally each day, 1 hour prior to exposure to fatigue/forced swimming. The experimental animals were subjected to force swimming (except group I) individually in glass jar (42 × 14 cm) containing water at ambient temperature for a period of 15 minutes, consecutively for 21 days. The water level was adjusted and kept constant throughout the experiment.
Assessment of Duration of Immobility[16]
The experimental animals were exposed to force swimming to induce chronic fatigue where animals were forced to swim for 15 minutes every day for 21 days in a glass gar. After swimming for 10 minutes the immobility period for next 5 minutes was then observed on day 1, day 7, day 14 and day 21 and noted. Immobility is defined as when the animal ceases to make any struggling movements of their limbs to keep their head above water.[17]
Assesment of Anxiety and Ambulatory Activity
Elevated plus maze (EPM) fabricated according to dimensions suggested by Kulkarni et al.[18] was used to assess the magnitude of anxiety of all groups of test animals. Assessment of anxiety was made 24 hrs, after the last forced swim test on day 1,7,14 and 21 during investigations. Briefly, test animals were placed individually at the center of the EPM with their heads facing toward an open arm.[19] The test was conducted for five minutes and during this period, latency to enter open arm and time spent in open arm were recorded. The ambulatory activity was assessed using an actophotometer.[20] Each animal was placed individually in the central arena for 3 minutes as a habituation period and the locomotor activity was recorded for the next 5 minutes. The ambulatory activity was assessed on day 1,7,14 and 21 of all groups of test animals. Assessment of anxiety and ambulatory activity of test animals were made by an observer blind to the treatment protocol.
Assessment of Oxidative Stress
Twenty-four hours after the last forced swimming, experimental animals were sacrificed by decapitation. The whole brain was removed and a 10% (w/v) tissue homogenate was prepared in 0.1 M phosphate buffer (pH 7.4). The postnuclear fraction obtained by centrifugation of the homogenate at 1000 g for 15 min. was used for catalase assay. For other enzyme assays, the homogenate was centrifuged at 10,000 g for 20 min.
Biochemical assessment of brain homogenate included following:
Protein estimation: Amount of protein was measured according to the method of Lowry et al. modified by Pomory, using bovine serum albumin as standard.[21]
Assessment of lipid peroxidation: Malondialdehyde (MDA) levels were measured by the double heating method[22] and the method based on spectrophotometeric measurement of the purple color generated by the reaction of thiobarbituric acid with MDA.
Catalase assay: Catalase activity was measured spectrophotometrically according to the method of Sinha et al.[23]
Superoxide dismutase assay: Superoxide dismutase activity was measured in brain tissue was according to method of Kakker et al.[24]
Statistical Analysis
All values are expressed as mean ± SEM of n=8, and analyzed by one way ANOVA followed by Student-Newman keul multiple comparison test. P value≤0.05 and were considered significant.
Results
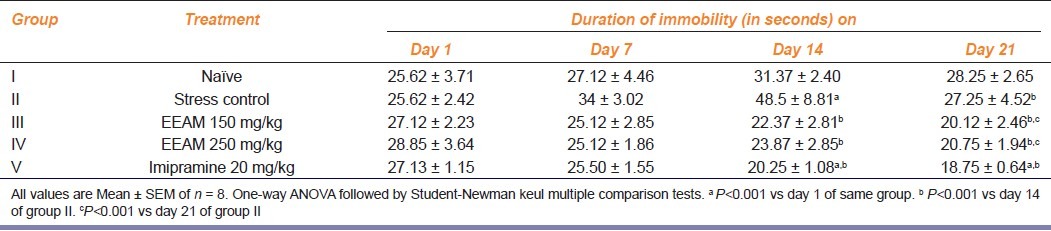
Effect of extract treatment on duration of immobility in test animals exposed to chronic stress
Result of extract treatment on duration of immobility in test animals exposed to chronic stress is as shown in Table 1 Group I (naive group) animals recorded an insignificant rise in duration of immobility during three weeks of study while group II (stress group), recorded a gradual and significant increase in duration of immobility. On day 14, stressed animals recorded peak (P<0.001) in duration of immobility, after which there was a fall. Dose-dependent and significant (P<0.001) reduction in duration of immobility (compared to stress group) was observed in test animals treated with extract by 14th day and continued till the end day 21 (compared to 14th day of stress group). Similar significant reduction in duration of immobility was observed in imipramine treated animals and duration of immobility did not significantly differ between extract and imipramine-treated group of animals.
Table 1.
Effect of extract treatment on the duration of immobility of chronically fatigued animals at different intervals of time during 3-week study
Effect of extract treatment on the level of anxiety in test animals exposed to chronic stress
Results of extract treatment on the level of anxiety in test animals exposed to chronic stress is as shown in Table 2. By day 14, group II animals recorded statistically significant (P<0.001) increase in latency to enter open arm compared to group I animals and extract treatment resulted in dose-dependent and significant (P<0.001) reduction in latency to enter the open arm compared to group I and II during similar interval of time. Change in latency to enter open arm continues to remain significant (P<0.001), by day 21, but did not improve.
Table 2.
Effect of extract treatment on the latency to enter the open arm of elevated plus maze in chronically fatigued animals at different intervals of time
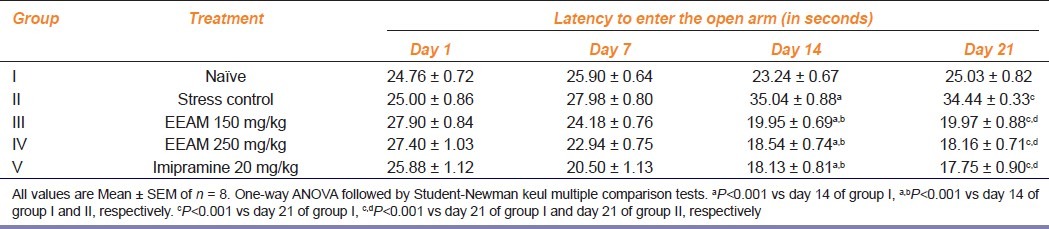
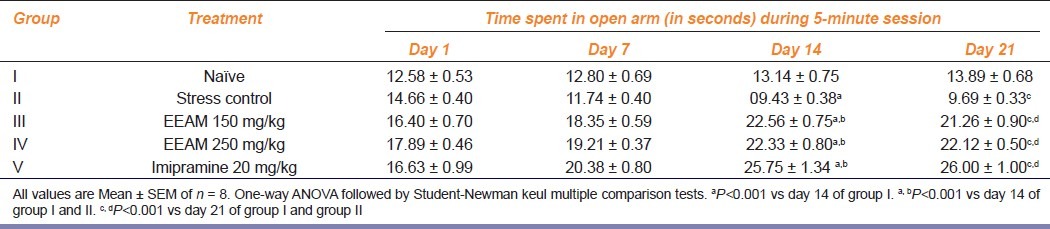
Table 3 summarizes the changes in the time spent in open arm by various groups of animals. Significant (P<0.001) reduction in time spent in open arm was recorded by group II animals by day 14 and 21, compared to group I animals. On the contrary, extract treatment and imipramine treatment resulted in increased duration of time spent in open arms by day 14 and 21, compared to group I and II animals during similar interval of time.
Table 3.
Effect of extract treatment on time spent in open arm of elevated plus maze in chronically fatigued animals at different intervals of time
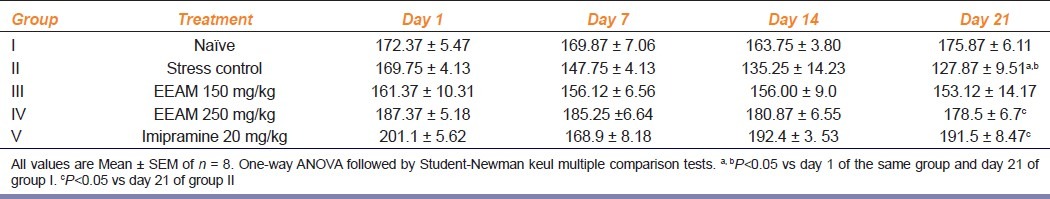
Effect of extract treatment on ambulatory activity in test animals exposed to chronic stress
Results of extract treatment on ambulatory activity in test animals exposed to chronic stress is as shown in Table 4. Group II animals recorded significant reduction in (P<0.05) in locomotor activity score, compared to its day 1 score and day 21 score of group I animals. Although, no significant change was recorded by extract treated animals by day 14, only group IV animals recorded a marginal, but significantly elevated score (P<0.05) compared to group II animals.
Table 4.
Effect of extract treatment on the locomotor activity on chronically fatigued animals at different intervals of time during 3-week of study
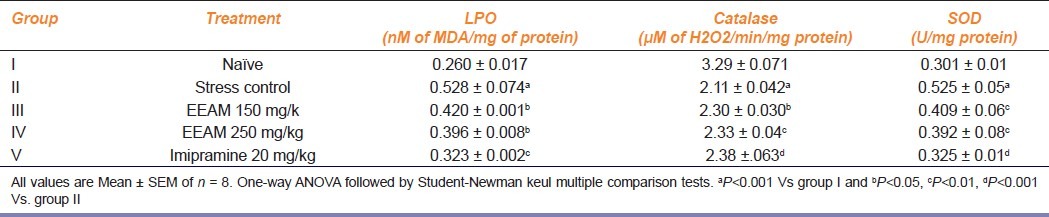
Effect of extract treatment on antioxidant levels in brain homogenate of test animals exposed to chronic stress.
Results of extract treatment on antioxidant levels in brain homogenate of test animals exposed to chronic stress is as shown in Table 5. Level of product of lipid peroxidation (measured as MDA), catalase and superoxide dismutase was measured in brain homogenate of all groups of animals. Group II animals recorded significantly elevated levels (P<0.001) of MDA, compared to group I animals and a dose-dependent significant reduction (P<0.05) in MDA was recorded in extract-treated animals, as well by imipramine-treated animals. Group II animals recorded a significantly lower level of catalase enzyme activity (P<0.001) compared to naive group and a marginal, yet, a significant elevation (P<0.05, 0.01) was recorded in extract-treated group III, IV and V animals treated with imipramine (P<0.001). Group II animals recorded a significantly elevated level of SOD (P<0.001) compared to naive group of animals and significant (P<0.01) reduction in SOD was recorded by extract-treated group III, IV and group V animals treated with imipramine (P<0.001).
Table 5.
Effect of extract treatment on the level of brain antioxidant enzymes of chronically fatigued animals at the end of the study
Discussion
The current investigation is based on proven antioxidant nature of Aegle marmelos extracts and some evidence suggesting that CFS is accompanied by increased oxidative stress in the pathophysiology of CFS and stress-induced depression.[25] Chronic swimming-induced stress increases the permeability of the blood-brain barrier in experimental animals and abnormalities of the hypothalamic-pituitary-adrenal axis in CFS patients have been observed.[26] CFS patients often suffer from sleep disturbances and anxiety, presence of depressive symptoms - probably due to dysregulation of 5-HT and other monoamine receptors.[27]
In the present study, chronic forced swimming for 21 successive days in animals resulted in significant changes in behavioral parameters such as increased immobility period, elevation of anxiety level and reduced locomotor activity in the experimental animals and there was also significantly depressed levels of oxidative stress biomarkers like CAT and elevation in SOD and LPO level.
Chronic exposure to an aversion situation from which there is no possibility of escape causes, animals to cease to struggle, thereby assuming a typical immobile posture indicating a behavioral depression.[12] This experimental model in rats is useful for assessing the behavioral alterations caused by chronic exposure to physical and mental stresses which produce CFS. It helps to evaluate the drugs that would be useful in counteracting CFS. Chronic exposure to forced swimming for 21 successive days produced a state of fatigue with significant increase in anxiety and depression levels when assessed on day 7, 14, 21 as compared to day1 in untreated stressed (Group II) animals. However, these alterations found in the stress animals were reversed by EEAM treatment – signifying the potent protective effect on chronic fatigue-induced manifestations.
Immobility period was found to be gradually decreased indicating that chronic exposure to repeated swimming might have induced CFS and was reversed by extract treatment suggesting that the extracts may be acting by altering the hypothalamic-pituitary-adrenal axis (HPA) function, the same way in which an anti-stress drug are acting.[28]
Chronic forced swimming for 21 successive days also produced a significant anxiety-like behavior in the stressed group of animals, indicated by the gradual increase in the latency to enter open arm and lesser and lesser time spent in open arm of elevated plus maze suggesting behavioral alterations. However, treatment with EEAM in the dose of 150 and 250 mg/kg b.w. reversed this state as evidenced by gradual decrease in these parameters. Locomotor activity was also found to gradually decrease in the stress animals as observed on day 7, day 14 and day 21 when compared to day 1, but in the EEAM-treated group of animals it was found that these stress-induced reduction of locomotor activity have been reversed.
The use of an antioxidant in CFS in its management may play an effective role in counteracting the oxidative stress involved. It was found that there was a significant increase in the lipid peroxidation of brain tissues of stressed group of animals when compared to normal unstressed animals. This report has been supported by the findings from the enzymatic estimation of SOD and CAT. These primary antioxidant enzymes including SOD and CAT defense mechanisms become weaker during chronic fatigue and other disease conditions. So, improvement in the defense mechanisms can help fight against fatigue.
An increased concentration of end products of lipid peroxidation is the evidence most frequently quoted for the involvement of free radicals in human disease[29] and these free radicals are produced during peroxide formation from fatty acids containing methylene-interrupted double bonds, i.e., those found in the naturally occurring polyunsaturated fatty acids. Lipid peroxidation is a chain reaction providing a continuous supply of free radicals that initiate further peroxidation and further leads to breakdown of erythrocyte membranes and oxidation of proteins and DNA. SOD is an important enzyme, formed in the red blood cell by the auto-oxidation of hemoglobin to methemoglobin and plays essential role in protecting aerobic cells against oxidative cells. It catalyzes O2 radicals to H2O2. SOD causes an increase in the conversion of superoxide to hydrogen peroxide.[30]
CAT is tetrameric hemoprotein found in blood, bone marrow, mucous membranes, kidney, and liver. The hydrogen peroxide formed by superoxide dismutase and by other processes is scavenged by catalase, a ubiquitous heme protein that catalyzes the dismutation of hydrogen peroxide into water and molecular oxygen.[31]
These observations demonstrated an increased in oxidative stress in chronically stressed animals, suggestive of involvement of oxidative stress in the pathogenesis of CFS. Treatment with EEAM caused a decrease in lipid peroxidation and SOD level and increased CAT level which protected the test animals from chronic swim stress-induced oxidative stress.
Aegle marmelos has been an important herb in the Ayurveda and indigenous medical systems for over 3000 years. Clinical trials and animal research studies have shown that this plant preparation has anti-inflammatory, anticancer, antistress and immunomodulatory properties. Phytochemical analysis has reported that this plant contains antioxidative constituents such as flavonoids, alkaloids, sterols, tannins, phlobatannins which may be responsible for its antioxidant property. An in vivo study on artificially induced diabetic animals have shown that leaf extract of Aegle marmelos has a potent antioxidant property as it scavenges the free radicals which are produced.[8] However, the involvement of its antioxidant property could not be ruled out as the main reason as there are also other mechanisms that operate during stress or anxiety.[32] The present study identified the protective effect of ethanolic extract of leaves of Aegle marmelos likely to be due to antioxidant nature of extract and established its therapeutic potential in the management of CFS.
Acknowledgment
Authors sincerely thank Prof. (Dr.) Desai BG, Principal, KLE University's College of Pharmacy, Bangalore for providing facilities to the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Fakuda K, Straus SE, Hickle I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: A comprehensive approach to its definition and study.International Chronic Fatigue syndrome study group. Ann Intern Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Afari N, Buchwald D. Chronic Fatigue Syndrome: A Review. Am J Psychiatry. 2003;160:221–36. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 3.Carruthers M, Jain AK, DeMeirleir KL, Peterson DL, Klimas NG. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. J Chronic Fatigue Syndr. 2003;11:7–97. [Google Scholar]
- 4.Pall ML. Elevated, sustained peroxynitrite levels as the cause of chronic fatigue syndrome. Med Hypotheses. 2000;54:115–25. doi: 10.1054/mehy.1998.0825. [DOI] [PubMed] [Google Scholar]
- 5.Ross SR, Wang L. Erythrocyte damage in chronic fatigue syndrome. Arch Med Res. 2007;38:92–8. doi: 10.1016/j.arcmed.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy G, Spence VA, McLaren M, Hill A, Underwood C, Belch JF. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med. 2005;39:584–9. doi: 10.1016/j.freeradbiomed.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Richards RS, Roberts TK, McGregor NR. Blood parameters indicative of oxidative stress are associated with symptom expression in chronic fatigue syndrome. Redox Rep. 2000;5:35–41. doi: 10.1179/rer.2000.5.1.35. [DOI] [PubMed] [Google Scholar]
- 8.Maity P, Hansda D, Bandyopadhyay U, Mishra DK. Biological activities of crude extracts and chemical constituents of Bael. Indian J Exp Biol. 2009;47:849–61. [PubMed] [Google Scholar]
- 9.Vijaya C, Ramanathan M, Suresh B. Lipid lowering activity of ethanolic extract of leaves of Aegle marmelos (Linn.) in hyperlipedemic model of wistar albino rats. Indian J Exp Biol. 2009;47:182–5. [PubMed] [Google Scholar]
- 10.Prema P. Antibacterial activiy of selected medicinal plants. J Ecobiol. 2004;16:333–7. [Google Scholar]
- 11.Premchanien M, Kosam N, Luanratana O, Jongsomboonkusos S, Ponipon N. Anti proliferative activity of Thai medicinal plant extracts on human breast adrenocarcinoma cell line. Fitoterapia. 2004;75:375–7. doi: 10.1016/j.fitote.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Chauhan A, Agarwal S, Kwhwaha S, Mutresa A. Suppression of fertility in nmale albino rats following the administration of 50% ethanolic extract of Aegle marmelos. Contraception. 2007;76:474–81. doi: 10.1016/j.contraception.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Arul V, Miyazaki S, Dhanjayan R. Studies on antiinflammatory, antipyretic and analgesic properties of leaves of Aegle marmelos Corr. J Ethnopharmacol. 2005;96:159–63. doi: 10.1016/j.jep.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. 28th ed. Pune: Nirali Prakashan; 2008. pp. 6.16–6.17. [Google Scholar]
- 15.OECD Enviornment, Health and safety publication, series on testing and assesment No.24, Guidance document on Acute oral toxicity testing, Enviornment Directorate, Organisation for Economic Co-operation and Development, Paris. 2001 [Google Scholar]
- 16.Lyle N, Sur T, Munshi S, Paul S, Chatterjee S, Bhattacharyya D. The role of antioxidant properties of Nardostachys jatamansi in alleviation of the symptoms of the chronic fatigue syndrome. Behav Brain Res. 2009;202:285–90. doi: 10.1016/j.bbr.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Garg R, Kumar P. Nitric oxide modulation mediates the protective role of trazodone in a mouse model of chronic fatigue syndrome. Pharmacol Rep. 2008;60:664–72. [PubMed] [Google Scholar]
- 18.Kulkarni SK. Handbook of experimental pharmacology. New Delhi: Vallabh Prakashan; 2005. pp. 135–7. [Google Scholar]
- 19.Kulkarni SK, Singh K, Bishnoi M. Elevated Zero Maze: A Paradigm to evaluate antianxiety effects of drugs. Methods Find Exp Clin Pharmacol. 2007;29:343–8. doi: 10.1358/mf.2007.29.5.1117557. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Garg R. Protective effects of antidepressants against chronic fatigue syndrome-induced behavioral changes and biochemical alterations. Fundam Clin Pharmacol. 2009;23:89–95. doi: 10.1111/j.1472-8206.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 21.Pomory CM. Color development time of the Lowry protein assay. Anal Biochem. 2008;378:216–7. doi: 10.1016/j.ab.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Yazdanparast R, Bahramikia S, Ardestani A. Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hyperchloresterolaemic rats. Chem Biol Interact. 2008;172:176–84. doi: 10.1016/j.cbi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Sinha KA. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 24.Kakker P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 25.Alan C, Logan ND, Wong C. Chronic fatigue syndrome: Oxidative stress and dietary modifications. Altern Med Rev. 2001;6:450–9. [PubMed] [Google Scholar]
- 26.Kalenova LF, Sukhovei YG, Fisher TA. Specific and non-specific reactions of mouse immune system under the effect of short term exposure in warm and/or cold water. Bull Biol Med. 2005;6:23–34. doi: 10.1007/s10517-006-0065-8. [DOI] [PubMed] [Google Scholar]
- 27.Demitrack MA, Gold PW, Dale JK, Krahn DD. Plasma and cerebrospinal fluid monoaminemetabolism in patients with chronic fatigue syndrome: Preliminary findings. Biol Psychiatry. 1992;32:1065–77. doi: 10.1016/0006-3223(92)90187-5. [DOI] [PubMed] [Google Scholar]
- 28.Giorgio D, Hudson A, Jerjes M, Cleare W. 24-hour pituitary and adrenal hormone profiles in chronic fatigue syndrome. Psychol Med. 2005;67:433–40. doi: 10.1097/01.psy.0000161206.55324.8a. [DOI] [PubMed] [Google Scholar]
- 29.Halliwell B, Chirico S. Lipid peroxidation: Its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57:15–25. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 30.Murray KM, Granner DK, Mayes PA, Rodwell VW. Harper's Illustrated Biochemistry. 26th ed. McGraw-Hill Publication; 2003. pp. 90–128. chap. 11. [Google Scholar]
- 31.Saltsman K, Berg JM, Tomaselli G. Biochemistry. 5th ed. WH Freeman and Company Publication; 2005. pp. 748–9. chap. 18. [Google Scholar]
- 32.Hugh SB, Deacon RM, Rawlins JNP, Bannerman DM. Amygdala and ventral hippocampuscontribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]


