Abstract
Objectives:
Andrographis paniculata (Burm. f.) Nees originates from India and grows widely in many areas in Southeast Asian countries. Andrographis paniculata (Burm. f.) Nees has shown an antidiabetic effect in type 1 DM rats. The present study investigates the purified extract of the plant and its active compound andrographolide for antidiabetic and antihyperlipidemic effects in high-fructose-fat-fed rats, a model of type 2 DM rats.
Materials and Methods:
Hyperglycemia in rats was induced by high-fructose-fat diet containing 36% fructose, 15% lard, and 5% egg yolks in 0.36 g/200 gb.wt. 55 days. The rats were treated with the extract or test compound on the 50th day. Antidiabetic activity was measured by estimating mainly the pre– and postprandial blood glucose levels and other parameters such as cholesterol, LDL, triglyceride, and body weight.
Results:
The purified extract and andrographolide significantly (P<0.05) decreased the levels of blood glucose, triglyceride, and LDL compared to controls. However, no changes were observed in serum cholesterol and rat body weight. Metformin also showed similar effects on these parameters.
Conclusions:
Andrographis paniculata (Burm. f.) Nees or its active compound andrographolide showed hypoglycemic and hypolipidemic effects in high-fat-fructose-fed rat.
KEY WORDS: Andrographis paniculata (Burm. f.) Nees, andrographolide, diabetes, fructose, hyperlipidemia
Introduction
Andrographis paniculata (Burm. f.) Nees plant originates from India, and has been used for several purposes, primarily preventing diabetes mellitus (DM).[1] Ethanolic extracts of this plant can decrease the blood glucose levels in type 1 DM rats.[2] However, its antidiabetic effect in type 2 DM has not been reported well.
The anti-DM activity of Andrographis paniculata (Burm. f.) Nees has attracted many researchers to prove it scientifically and to investigate its mechanisms of actions. Zhang and Tan have reported that the ethanolic extracts are potent to decrease the blood glucose levels in streptozotocin (STZ)-induced type 1 DM rats.[2] In addition, the water soluble extract shows antioxidant activity by increasing the activities of superoxide dismutase (SOD) and catalase in type 1 DM rats.[3] An in vitro study, reported that the extract stimulates the release of insulin release Brin-BD11 cell culture.[4]
In phytochemical studies, Andrographis paniculata (Burm. f.) Nees (whole plant, leaf, and steam) contains diterpene lactone consisting of andrographolide, 14-deoxyandrographolide, neoandrographolide, andrograpin, 14-acetylandrograpolide, 14-deoxy-didehydroandrographolide, and homoandrographolide, 19-O-acetylanhydroandrographolide. The plant also contains small amount of flavonoids such as polymetoxyflavon, andrographine, panicholine, apigenin.[5] Among them, andrographolide is a major (>4%) and most active compound in a dried whole plant. Reportedly, andrographolide can decrease the blood glucose level in both normal and type 1 DM mice. The compound also increases the glucose utilization through the increase of mRNA and protein levels of GLUT-4, a transporter of glucose through the cell membrane.[6] Its antidiabetic activity is reportedly associated with antioxidant activity and inhibition of NF-kappa B.[7] The compound can also stimulate the insulin release, and inhibits the absorption of glucose through inhibition of the enzyme alpha-glucosidase and alpha-amylase.[8]
Andrographolide can be isolated from the purified extract of Andrographis paniculata (Burm. f.) Nees using gradual extraction with petroleum ether, chloroform, and methanol, respectively, and then purified by recrystallization. High dietary intake of fructose in combination with high fat aimed to stimulate insulin resistance and hyperlipidemia.[9–11] In the present study, we evaluated the antidiabetic and antihyperlipidemic effects of purified extract of Andrographis paniculata (Burm. f.) Nees and its active compound andrographolide in high-fructose-fat-fed rats.
Materials and Methods
Preparation of Purified Extract of Andrographis Paniculata (Burm. f.) Nees
Andrographis paniculata (Burm. f.) Nees was collected from the area around Yogyakarta, Indonesia. The plant was identified by a botanist at Department of Pharmaceutical Biology, Universitas Gadjah Mada, Indonesia, and the voucher specimen was stored in herbarium of the department.
In brief, a dried ground powder of Andrographis paniculata (Burm. f.) Nees was extracted with 90% ethanol for 24 hours. After filtration, the filtrate was obtained, while the sediment was re-extracted using the same solvents for 24 hours. The re-extraction was done twice. The extract was then collected, and evaporated under reduced pressure for viscous extract. The extract was fractionated with n-hexane at a ratio of 1:20 (extract: n-hexane) yielding two fractions of hexane soluble fraction and insoluble fraction. The insoluble fraction of n-hexane was then fractionated using ethyl acetate at a ratio of 1:10 (insoluble fraction of n-hexane: ethyl acetate) yielding the ethyl acetate soluble fraction and insoluble fraction of ethyl acetate. The insoluble fraction was collected, and then concentrated by rotary vacuum evaporator to obtain viscous extract. The insoluble fraction was then washed with hot water, and diluted with ethanol 90% to yield a purified extract of 1.71%, with levels of 16.31% andrografolide.
Isolation of Andrographolide
The purified extract of Andrographis paniculata (Burm. f.) Nees was chromatographed over silica gel GF 254 in a column chromatography, and eluted with a mixture of solvents consisting of chloroform and methanol (9:1) as the mobile phase. The clear solution was collected, and then allowed to stand for 24 hours at 4°C to yield crystals of andrographolide. The crystals were collected, and washed with cold methanol and then with cold n-hexane, respectively
Animals
Wistar rats weighing 100–150 g were used. They were housed at a constant temperature (22 ± 2°C) with a constant relative humidity (55 ± 10%) on an automatically controlled 12:12 hour light-dark cycle (light on at 7:00 a.m.) and had free access to food and water. The animal handling protocols of this study were in accordance with the guidelines of the animal care of the Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Gadjah Mada, Indonesia.
Experimental Design
The rats were acclimatized for 1 week. Hyperglycemia in the rat was induced by high-fructose fat.[9–11] This diet contained 36% fructose, 15% lard, and 5% egg yolks in 0.36 g/200 g BW. Control rats were fed with standard chow diet (normal rats). The composition was based on our preliminary study. The diet was maintained for the total period of 55 days. The rats were treated with either extract or test compound at day 50. Body weight was monitored regularly during the study (55 days). The high-fructose-fat-fed rats were divided into six groups consisting of six rats each as follows:
Group 1: the rats received oral saline 10 ml/kg BW (control group).
Group 2: the rats received puried extract of Andrographis paniculata (Burm. f.) Nees dose 434.6 mg/kg BW orally, twice daily.
Group 3: the rats received puried extract of Andrographis paniculata (Burm. f.) Nees dose 1303.8 mg/kg BW orally, twice daily.
Group 4: the rats received andrographolide dose 1.5 mg/kg BW orally, twice daily.
Group 5: the rats received andrographolide dose 4.5 mg/kg BW orally, twice daily.
Group 6: the rats received metformin dose 45 mg/kg BW orally, twice daily.
The purified extract of Andrographis paniculata (Burm. f.) Nees, andrographolide or metformin (positive control) was administered from day 50 onward for the next 5 days. At days 0 (basal value), 20, 30, 50, and 55, the blood samples were collected from retro-orbital plexus after 8 – to 10-hour fasting. The samples were centrifuged at 1000 rpm for 10 minutes. Serum was removed for determination of glucose (preprandial), triglyceride, LDL, and cholesterol levels. Two hours after feeding, blood was collected for determination of postprandial blood glucose levels.
Statistical Analysis
All data were presented as mean ± the standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test was used for statistical analysis to compare more than two groups, while the unpaired t-test was used to compare the mean of two groups. P values of less than 0.05 were considered significant.
Results
High Fructose Diet and Body Weight
High-fructose-fat diet significantly increased (P<0.05) the body weight in comparison to the control group (normal rats). Administration of high-fructose-fat significantly increased the body weight (P<0.05) after 20 days of the treatment compared to the control group. At the 55th day, the diet could increase the rat body weight by 26.73 ± 4.59%.
High-fructose-fat diet for 55 days significantly increased (P<0.05) the blood glucose level in comparison to the control group (normal rats) [Figures 1b and c]. The increase of the blood glucose level was seen after 20 days of the treatment. At the 55th day, the diet significantly (P<0.05) increased the preprandial and postprandial blood glucose levels by 65.62 ± 4.56% and 115.58 ± 4.83%, respectively.
Figure 1.
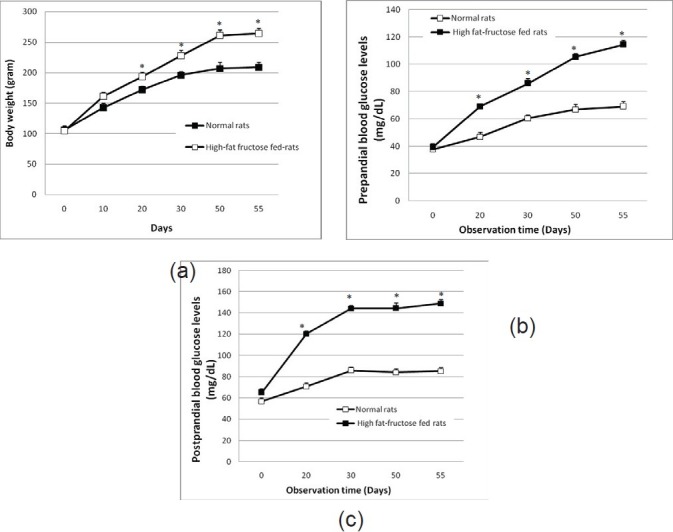
Effect of high-fructose-fat feeding on rat body weight gain (a), and on preprandial (b) and postprandial (c) blood glucose levels (mg/dL) in rats. Data represent mean±SEM, and are five to six independent experiments. *Significant difference P<0.05 compared to the control value
High-fructose-fat diet in the rats also significantly increased triglyceride, LDL, and cholesterol (P<0.05) in comparison to the control group [Figure 2]. The diet increased the blood triglyseride, LDL, and cholesterol level by 27.41 ± 1.78%, 34.41 ± 5.48%, and 21.11 ± 2.54% at the 55th day, respectively.
Figure 2.
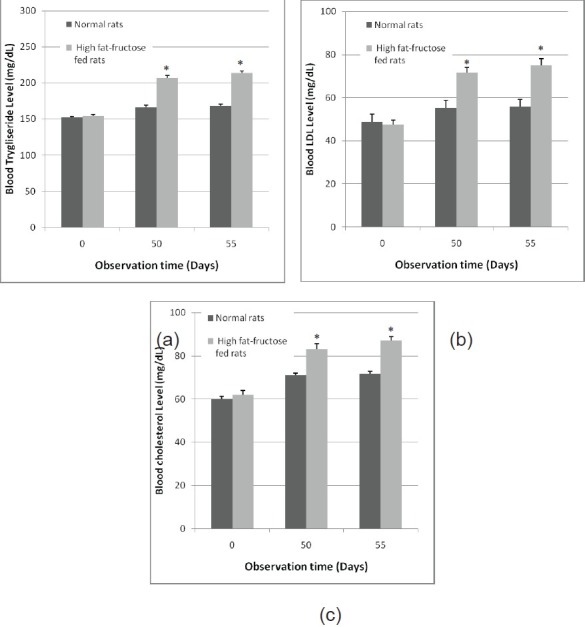
Effect of high-fructose-fat feeding triglyceride (a), LDL (b), and cholesterol (c) (mg/dL). Data represent mean±SEM, and are five to six independent experiments. *P<0.05 compared to the control value
Blood Glucose Levels
Administration of the purified extract of Andrographis paniculata (Burm. f.) Nees for 5 days significantly decreased (P<0.05) preprandial and postprandial blood glucose level in high-fat-fructose-fed rats in a dose-dependent manner [Figures 3a and b]. At doses of 434.6 and 1303.8 mg/kg b.wt twice daily the extract decreased the preprandial blood glucose level by 41.12 ± 7.50% and 45.76 ± 9.89%, respectively. The extract also decreased the postprandial blood glucose level by 53.55 ± 13.75% and 60.14 ± 12.39%, respectively. Treatment of andrographolide compound for 5 days also decreased preprandial and postprandial blood glucose level in high-fat-fructose-fed rats in a dose-dependent manner. The compound doses 1.5 and 4.5 mg/kg BW twice daily decreased preprandial blood glucose level by 33.29 ± 6.40% and 44.41 ± 7.40%, respectively. It also decreased the postprandial blood glucose level by 38.33 ± 10.90% and 62.31 ± 6.35%, respectively, whereas metformin decreased both preprandial and postprandial blood glucose level by 45.24 ± 3.23% and 58.11 ± 4.04%, respectively.
Figure 3.
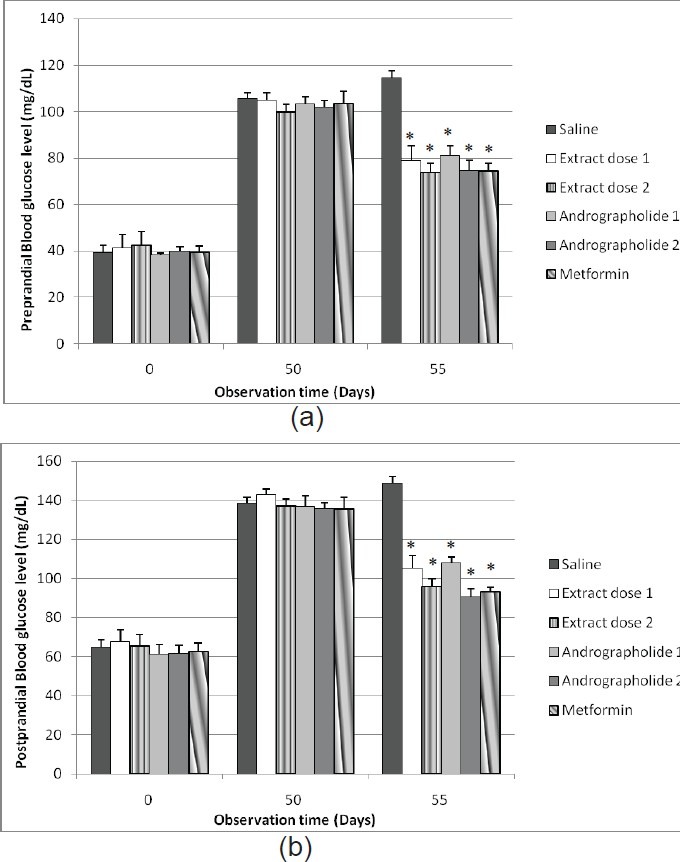
Effect of the purified extract of Andrographis paniculata (Burm. f.) Nees (434.6 and 1303.8 mg/kg BW), andrographolide (1.5 and 4.5 mg/kg BW), or metformin (45 mg/kg BW) twice daily on preprandial (a) and postprandial (b) blood glucose levels (mg/dL) in high-fructose-fat-fed rats. Data represent mean±SEM, and are five to six independent experiments. *P<0.05 compared to the negative control value
Blood LDL, Triglyceride, and Cholesterol Levels
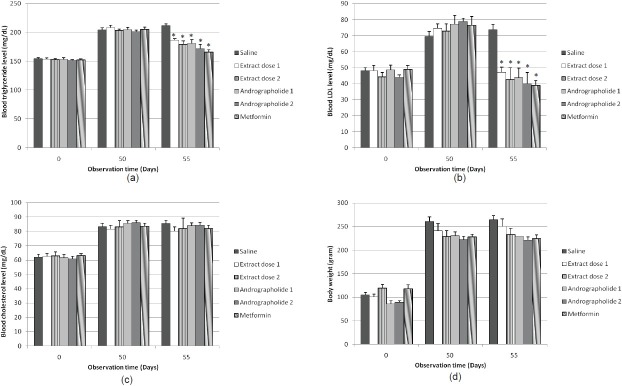
Administration of the purified extract of Andrographis paniculata (Burm. f.) Nees or andrographolide for 5 days significantly decreased (P<0.05) the LDL and triglyceride in high-fat-fructose-fed rats in a dose-dependent manner [Figure 4a and b]. However, no changes were observed in blood cholesterol levels [Figure 4c]. The purified extract of Andrographis paniculata (Burm. f.) Nees (434.6 and 1303.8 mg/ kg BW) significantly (P<0.05) decreased blood LDL by 102.53 ± 17.13% and 130.68 ± 46.25%, respectively. The extract also significantly (P<0.05) decreased triglyceride by 38.61 ± 4.26% and 49.54 ± 8.37%, respectively.
Figure 4.
Effect of the purified extract of Andrographis paniculata (Burm. f.) Nees (434.6 and 1303.8 mg/kg BW), andrographolide (1.5 and 4.5 mg/kg BW), or metformin (45 mg/kg BW) twice daily on the levels of trigliceride (a), LDL (b), cholesterol (c) (mg/dL), and on rats body weight gain (d) (gram) in high-fructose-fat-fed rats. Data represent mean±SEM, and are five to six independent experiments. *P<0.05 compared to the negative control value
Andrographolide at the doses of 4.5 and 13.5 mg/kg BW also significantly (P<0.05) suppressed the levels of blood LDL by 105.20 ± 18.06% and 109.73 ± 17.07%, respectively. The compound also significantly (P<0.05) decreased the level of blood triglyceride by 48.52 ± 10.89% and 56.95 ± 12.89%, respectively.
Similarly, treatment with metformin (90 mg/kg BW) for 5 days in high-fat-fructose-fed rats also significantly (P<0.05) decreased the blood LDL and triglyceride levels by 135.09 ± 25.86% and 74.09 ± 10.34%, respectively
Effect of purified extract of Andrographis paniculata (Burm. f.) Nees and andrographolide on body weight
No change in the rat body weight after treatment of the purified extract of Andrographis paniculata (Burm. f.) Nees, andrographolide or metformin for 5 days was observed [Figure 4d].
Discussion
Andrographis paniculata (Burm. f.) Nees is a medicinal plant growing widely in many areas in Indonesia.[12] Traditionally this plant is used as an antidiabetes, antiinflammatory, hepatoprotective, antispasmodic, and antioxidant agents.[1] Andrographis paniculata (Burm. f.) Nees contains major constituents such as diterpenoids, flavonoids, and polyphenol.[13] The diterpenoids are andrographolide, deoxyandrographolide, 19-O-acetylanhydro-andrographolide, neoandrographolide, 14-deoxy-didehydroandrographolide, and homoandrographolide. Among them, andrographolide is a major compound and found in abundance in the plant. Therefore, andrographolide has an important role in the biological activities of Andrographis paniculata (Burm. f.) Nees. The flavonoids found in the plant are 7-O-methylwogonin; apigenin, onysilin; 3,4-dicaffeoylquinic; 5,7,2¢,3¢-tetramethoxy-flavanone, and 5-hydroxy-7,2¢,3¢-trimethoxyflavone.[5,13]
The purified extracts of Andrographis paniculata (Burm. f.) Nees and andrographolide were evaluated for antidiabetic and antihyperlipidemic activities in high-fructose-fat-fed rats in the present study. Long-term high-fructose feeding in rats was often used to induce an insulin resistance.[9,11] Previously, fructose feeding consisting of 66% fructose, 22% casein, and 12% lard for 8 weeks induced mild insulin resistance in rats characterized by increased levels of insulin, glucose, and triglyceride significantly.[14] The prolonged increase of insulin (hyperinsulinemia) can decrease insulin sensitivity. Hyperinsulinemia influences the pattern of adipocyte secretion that causes insulin resistance in both adipocytes and myocytes.[15] Long-term high-fructose feeding was reported to induce insulin resistance, hyperinsulinemia, and dyslipidemia. In addition, high quantities of fructose in liver can rapidly stimulate lipogenesis and triglyceride accumulation that can reduce the insulin sensitivity, and increase the formation of VLDL.[16]
The present study showed that the treatment of the high-fructose fat consisting of 36% fructose, 15% lard, and 5% egg yolks in 0.36 g/200 g BW for 55 days succeeded to stimulate blood glucose, triglyceride, and LDL levels in comparison to the control. The diet also moderately increased the blood cholesterol and rat body weight. In the present study, the purified extract of Andrographis paniculata (Burm. f.) Nees or its most active compound andrographolide showed hypoglycemic and hypolipidemic effects in high-fat-fructose-fed rats. Administering the purified extract of Andrographis paniculata (Burm. f.) Nees or its most active compound andrographolide for 5 days succeeded to decrease the levels of blood glucose, triglyceride, and LDL. However, they did not influence the cholesterol level. Metformin, a type 2 antidiabetic drug acting to decrease hepatic glucose production and to increase insulin action in muscle and fat,[16] also showed similar effects on these parameters. Based on these results, it is found that andrographolide has an important role in hypoglycemic and hypolipidemic effects of Andrographis paniculata (Burm. f.) Nees in high-fat-fructose-fed rats.
Andrographolide was reported to show hypoglycemic effects in streptozotocin-diabetics rats,[6] a model of type 1 diabetes mellitus (DM) or insulin-dependent DM rats.[17] The compound succeeded to improve the uptake of glucose in isolated soleus muscle of STZ-diabetic rats by increasing the mRNA and protein levels of GLUT4.[6,18] A similar effect was also shown by andrographolide analog namely andrographolide-lipoic acid conjugate that causes lowered blood glucose level, increased insulin, and stimulated GLUT4 translocation into the soleus muscle membrane in alloxan-diabetics rats, another model of type 1 DM rats.[17]
In conclusion, Andrographis paniculata (Burm. f.) Nees or its most active compound andrographolide showed hypoglycemic and hypolipidemic effects in high-fat-fructose-fed rat. However, further studies are needed to establish their action mechanisms.
Acknowledgments
We gratefully thank to Indonesia Managing Higher Education For Relevance and Efficiency (I-MHERE) Universitas Gadjah Mada Yogyakarta Indonesia through Research Grant 2011.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Niranjan A, Tewari S, Lehri A. Biological activities of Kalmegh (Andrograpis paniculata Nees) Indian J Nat Proc Resour. 2010;1:125–35. [Google Scholar]
- 2.Zhang XF, Tan BK. Anti-diabetic property of ethanolic extract of Andrographis paniculata in streptozotocin-diabetic rats. Acta Pharmacol Sin. 2000;21:1157–64. [PubMed] [Google Scholar]
- 3.Dandu AM, Inamdar NM. Evaluation of beneficial effects of antioxidant properties of aqueous leaf extract of Andrographis paniculata in STZ-induced diabetes. Pak J Pharm Sci. 2009;22:49–52. [PubMed] [Google Scholar]
- 4.Wibudi A, Kiranadi B, Manalu W, Winarto A, Suyono S. The traditional plant, Andrographis paniculata (Sambiloto), exhibits insulin-releasing actions in vitro. Acta Med Indones. 2008;40:63–8. [PubMed] [Google Scholar]
- 5.Chao WW, Lin BF. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian) Chin Med. 2010;5:17. doi: 10.1186/1749-8546-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu BC, Hung CR, Chen WC, Cheng JT. Antihyperglycemic effect of andrographolide in streptozotocin-induced diabetic rats. Planta Med. 2003;69:1075–9. doi: 10.1055/s-2003-45185. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Jiang J, Yu P, Zeng X, Larrick JW, Wang Y. Hypoglycemic and beta cell protective effects of andrographolide analogue for diabetes treatment. J Transl Med. 2009;7:62. doi: 10.1186/1479-5876-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subramanian R, Asmawi MZ, Sadikun A. In vitro alpha-glucosidase and alpha-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim Pol. 2008;55:391–8. [PubMed] [Google Scholar]
- 9.Suga A, Hirano T, Kageyama H, Osaka T, Namba Y, Tsuji M, et al. Effects of fructose and glucose on plasma leptin, insulin, and insulin resistance in lean and VMH-lesioned obese rats. Am J Physiol Endocrinol Metab. 2000;278:E677–83. doi: 10.1152/ajpendo.2000.278.4.E677. [DOI] [PubMed] [Google Scholar]
- 10.Thorburn AW, Storlien LH, Jenkins AB, Khouri S, Kraegen EW. Fructose-induced in vivo insulin resistance and elevated plasma trigyceride levels in rats. Am J Clin Nutr. 1989;49:1155–63. doi: 10.1093/ajcn/49.6.1155. [DOI] [PubMed] [Google Scholar]
- 11.Pooranaperundevi M, Sumiyabanu MS, Viswanathan P, Sundarapandiyan R, Anuradha CV. Insulin resistance induced by a high – fructose diet potentiates thioacetamide hepatotoxicity. Singapore Med J. 2010;51:389–98. [PubMed] [Google Scholar]
- 12.Widyawati T. Aspek Farmakologi Sambiloto (Andrographis paniculata Nees) Majalah Kedokt Nusantara. 2007;40:216–22. [Google Scholar]
- 13.Rao YK, Vimalamma G, Rao CV, Tzeng YM. Flavonoids and andrographolides from Andrographis paniculata. Phytochemistry. 2004;65:2317–21. doi: 10.1016/j.phytochem.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 14.D’Angelo G, Elmarakby AA, Pollock DM, Stepp DW. Fructose feeding increases insulin resistance but not blood pressure in sprague-dawley rats. Hypertension. 2005;46:806–11. doi: 10.1161/01.HYP.0000182697.39687.34. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Veledo S, Nieto-Vazquez I, de Castro J, Ramos MP, Bruderlein S, Moller P, et al. Hyperinsulinemia induces insulin resistance on glucose and lipid metabolism in a human adipocytic cell line: Paracrine interaction with myocytes. J Clin Endocrinol Metab. 2008;93:2866–76. doi: 10.1210/jc.2007-2472. [DOI] [PubMed] [Google Scholar]
- 16.Basciano H, Miller AE, Naples M, Baker C, Kohen R, Xu E, et al. Metabolic effects of dietary cholesterol in an animal model of insulin resistance and hepatic steatosis. Am J Physiol Endocrinol Metab. 2008;297:E462–73. doi: 10.1152/ajpendo.90764.2008. [DOI] [PubMed] [Google Scholar]
- 17.Szkudelski T. The Mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:536–46. [PubMed] [Google Scholar]
- 18.Yu BC, Chang CK, Su CF, Cheng JT. Mediation of beta-endorphin in andrographolide-induced plasma glucose-lowering action in type I diabetes-like animals. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:529–40. doi: 10.1007/s00210-007-0240-0. [DOI] [PubMed] [Google Scholar]