Abstract
Introduction:
Blood pressure (BP) reduction is the major determinant of benefit provided by antihypertensive treatment. Although different drugs reduce peripheral BP to some extent, there may be a significant difference in their effect on central BP reduction. It has been shown that beta-blockers are efficient in reducing peripheral, but not central BP. This study was done to assess the effect of beta-1-blocker, nebivolol, in patients with essential hypertension on central aortic pressures and arterial stiffness.
Materials and Methods:
In this single arm, open-labeled study, 13 patients were given nebivolol, 5 mg orally once daily for 15 days. Primary outcome was change in central aortic pressure, and other measures of efficacy included changes in brachial BP, augmentation index (AIx%), AIx%@75 HR, augmentation pressure (AP), heart rate (HR), and carotid femoral pulse wave velocity (PWVcf).
Results:
Nebivolol 5 mg significantly reduced central aortic pressures [systolic BP, 131.5–111.6 mmHg; diastolic BP, 96.3–81.7 mmHg; Mean Arterial Pressure (MAP), 111.3–94.0 mmHg (all P<0.0001), and Pulse Pressure (PP), 35.2-29.7 mmHg (P<0.01)]. AIx%@75 HR reduced from 29 to 21.6 (P<0.001) and PWVcf reduced from 8.6 to 7.2 m/s (P<0.001). One subject was lost to followup.
Conclusion:
Nebivolol 5 mg demonstrated antihypertensive efficacy in patients with essential hypertension by reducing not only peripheral brachial pressures, but also significantly reducing central aortic pressures, augmentation index, and carotid femoral pulse wave velocity, which is the marker of arterial stiffness.
KEY WORDS: Nebivolol, central aortic pressures, arterial stiffness
Introduction
Hypertension is one of the major risk factors for cardiovascular and cerebrovascular diseases. Its prevalence is increasing due to increasing longevity, obesity, changes in diet, and a sedentary lifestyle coupled with stress.[1] It affects approximately 1 billion population worldwide. Recent data from the Framingham Heart Study suggest that individuals who are normotensive at an age of 55 years have a 90% lifetime risk for developing hypertension.[2] The relationship between blood pressure (BP) and risk of cardiovascular (CV) events is continuous, consistent, and independent of other risk factors.[2]
BP reduction is the major determinant of benefit provided by antihypertensive treatment. Although a clear reduction of CV morbidity and mortality has been observed by antihypertensive treatment, these favorable results cannot be solely attributed to the reduction of peripheral BP.[3] It appears, that improvement in vascular mechanics and central hemodynamics, which are not evident from the recording of peripheral BP, contribute to this discrepancy and one of these effects could be reduction of central (aortic) BP, which is crucially dependent on arterial stiffness. Central pressures are more modulated than peripheral pressures by changes in the functional and structural properties of large and small arteries. Hence, vascular remodeling, which is mainly induced by CV risk factors (among them hypertension is the most important), affects the central BP much more than peripheral BP. Stiffening of arterial walls results in an increase of systolic BP (SBP) and decrease of diastolic BP (DBP), and therefore a high pulse pressure (PP). Central systolic BP is further augmented by wave reflections from periphery. Vascular remodeling of large arteries results in increased stiffness, which elevates central BP more than peripheral BP.[3]
Central BP can be ideally measured invasively by the use of catheters, which is still a “gold standard” for central BP measurement. Several devices have been developed to derive central pressures from analyses of applanated radial or carotid pulses or carotid distensions. Since invasive measurements are associated with high cost and technical limitations, the noninvasive techniques are becoming widely used. They allow relatively simple estimation of central pressures (particularly indices independent from the absolute values of BP), with high reproducibility.[3]
Although different drugs reduce peripheral BP to some extent there may be significant differences in their effect on central BP reduction. It has been shown that new antihypertensive agents, such as blockers of renin–angiotensin system and calcium channel blockers, efficiently reduce both central and peripheral BP, whereas the old agents (particularly beta-blockers) are efficient in reducing peripheral, but not central BP.[3]
Newer, more selective vasodilating beta-blocking agents, such as nebivolol, carvedilol, and celiprolol, may have a greater capacity to reduce aortic systolic pressures because of their additional vasodilatory property that reduces the influence of wave reflections. The reduction in wave reflections seen with vasodilating beta-blockers appears to offset the adverse heart rate-dependent increases in augmentation index seen with traditional beta-blockers, such as atenolol. Moreover, the reduction in heart rate itself tends to be slightly less with these vasodilating agents.[4]
However, unlike other third-generation β-blockers, such as labetalol, carvedilol, and bucindolol, whose vasodilatory effect is mediated by α1-adrenoceptor antagonism, nebivolol mediates endothelium-dependent vasodilatation via the L-arginine-nitric oxide (NO)-dependent pathway. These effects are thought to be due to the L-enantiomer, whereas the BP-reducing properties of nebivolol appear to be provided by the D-enantiomer. The combination of β-adrenoceptor antagonism and NO-mediated vasodilatation not only potentiates the BP-lowering activity of nebivolol but also confers a broader favorable metabolic profile, which is clinically relevant in the treatment of hypertensive patients.[5]
New evidence from recently completed clinical studies of nebivolol confirms previous findings that nebivolol differs from other beta-blockers, is a highly selective beta-1-blocker with additional vasodilating activity mediated by endothelial NO release.[5]
In view of these findings, this study was undertaken to assess the effect of beta-1-blocker, nebivolol, in patients with essential hypertension on central aortic pressures and arterial stiffness.
Materials and Methods
This was an open-labeled study designed to assess the effect of beta-1-blocker, nebivolol, in patients with essential hypertension. The study received Institutional Ethics Committee approval and was performed in accordance to the Declaration of Helsinki. The patients were recruited from Outpatient Department of General Medicine and Nephrology. All the patients provided written informed consent before the start of the study.
Patients of either sex, between 18 and 55 years of age with body mass index (BMI) of 18–30 kg/m2 with newly diagnosed essential hypertension or those who have discontinued antihypertensive medications for more than a month having msSBP 140–159 mmHg and/or msDBP 90–99 mmHg (JNC-7, Stage I Essential Hypertension) were included in the study.
Patients with secondary hypertension, uncontrolled hypertension, history of severe bradycardia, decompensated heart failure, sick sinus syndrome, angina, myocardial infarction, bronchospastic disorders, diabetes, peripheral arterial disease or severe hepatic and renal dysfunction, history of any psychiatric disorder, or history of hypersensitivity to beta-blockers were excluded from the study. Patients were also excluded if they were pregnant or lactating, if they had participated in any trials within 30 days prior to screening visit, or if they had taken other CYP2D6 inhibitors within 15 days prior to screening visit.
There were altogether 3 visits in the study. Screening visit (visit 1) was to ensure that the subjects met the eligibility criteria. The eligible patients’ baseline data for efficacy and safety were recorded at visit 2 and first dose of nebivolol 5 mg (Nebicard, Torrent Pharmaceuticals) was administered under supervision. Patients were then dispensed nebivolol 5 mg tablets for the next 15 days and they were instructed to take one tablet daily orally in the morning between 7 and 9 AM. Patients were instructed to come for visit 3, after 15 days, at which efficacy and safety data were recorded and drug accountability was reviewed. After the study, all the patients were referred back to the treating physician.
Brachial BP was measured using a validated, automated oscillometric device (Omron, SEM-1 model, Omron Healthcare, Japan). For central hemodynamics measurement, subjects were asked to lie down in a quiet room, at controlled temperature for 15 min, after which BP was measured over the brachial artery 3 times at 2 min intervals. The mean of three readings was recorded as representative of brachial BP (if the readings did not differ from each other by more than 10 mmHg, if any of the readings differed by more than 10 mmHg, another set of 3 readings were taken). After the last measurement, radial pulse of right arm was detected by a noninvasive sensor using applanation tonometry, and pressure waveforms were sampled over 20 s (Sphygmocor, Atcor Medicals, Version 8, NSW2114, Australia) using pulse wave analysis mode. Three consecutive radial pressure waveforms were recorded with operator index more than 85, and the mean of resulting central aortic pressure parameters was considered. For pulse wave velocity (Sphygmocor), the distance from suprasternal notch to site of maximum pulsation of right carotid artery and from suprasternal notch to the site of maximum pulsation of right femoral artery were measured and distance entered in millimeter. Then, carotid artery waveform was first recorded for 20 s by placing the sensor on carotid artery pulse followed by femoral arterial waveform recording for 20 s on femoral artery pulse. The mean of two consecutive readings of carotid–femoral pulse wave velocity, which did not differ by more than 0.5 m/s, was taken as representative reading.
The prespecified primary measure of efficacy in the study was the change in central aortic pressure between the baseline and end of 15 days treatment.
Secondary measures of efficacy included (1) changes in brachial pressure, (2) changes in measures of arterial stiffness [augmentation index (AIx%), augmentation pressure (AP), carotid-femoral pulse wave velocity (PWV c-f)].
Measures of safety included frequency and severity of reported adverse events and tolerability was assessed by number of subjects who discontinued the study due to side effects.
Statistical analysis of primary and secondary efficacy variables was done by using paired t test (Graph Pad) to compare the changes between baseline and end of treatment visit. Mean percentage change from baseline was also computed for all primary and secondary efficacy variables.
Sample size calculation: A sample size of 12 patients with anticipated 20% dropout rate was estimated assuming reduction of aortic systolic pressure after treatment with nebivolol by at least 15 mmHg with anticipated standard deviation of 5 considering power of 99% at 0.001% level of significance.
Results
Of the 21 patients screened, 8 patients were found to be ineligible at screening, due to failure to meet the selection criteria (5 patients had brachial BP <140/90 mmHg, 1 patient was diabetic, and 2 patients had brachial BP > 159/99 mmHg).
Of the 13 patients, who were enrolled into open label treatment period, 12 patients completed the study and 1 patient was lost to followup. Data of all the 13 patients was included in safety analysis but data of only 12 patients was included in efficacy analysis.
The study population was predominantly male (61.5%), with a mean age of 39.4 years and mean BMI of 26.4 kg/m2 [Table 1].
Table 1.
Demographic characteristics of patients (n=13)

Nebivolol produced significant reductions in the central aortic pressures from baseline. There was a significant (P<0.0001) reduction in central SBP, DBP, MAP, and central PP also reduced significantly (P<0.01) [Table 2].
Table 2.
Hemodynamic variables before and after 15 days treatment with nebivolol (5 mg) in hypertensive patients
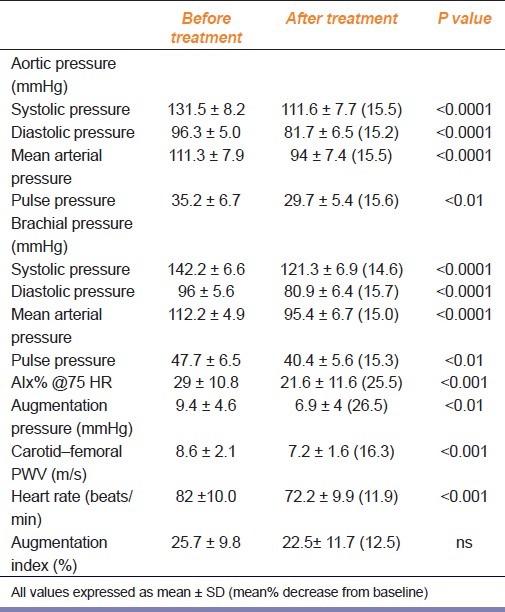
The brachial BPs were also significantly decreased compared with baseline [brachial SBP, DBP, MAP (P<0.0001), and brachial PP (P<0.01)] [Table 2].
As shown in Table 2, nebivolol significantly reduced AIx%@75 HR (P<0.001), augmentation pressure (P<0.01) and HR (P<0.001) from baseline. The mean percentage decrease from baseline for AIx% @75 HR was 25.5%, for augmentation pressure was 26.5%, for PWVcf was 16.3%, and for HR was 11.9%. The change in AIx% from baseline was not statistically significant. Significant reduction was seen in carotid-femoral PWV with respect to baseline (P<0.001) [Table 2]. The mean percentage decrease from baseline for central SBP, DBP, MAP, and PP were 15.5%, 15.2%, 15.5%, and 15.6%, respectively; and for brachial pressures SBP, DBP, MAP, and PP were 14.6%, 15.7%, 15%, and 15.3%, respectively [Figure 1].
Figure 1.
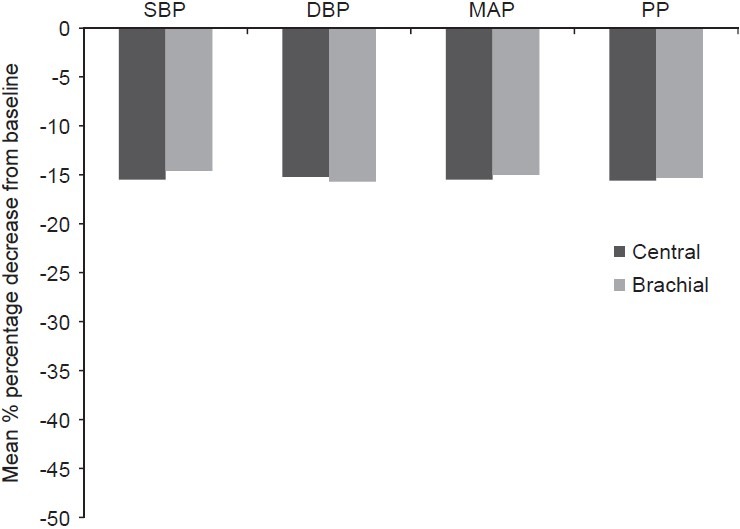
Mean percentage decrease from baseline for aortic and brachial pressure after 15 days treatment with nebivolol in hypertensive patients
Nebivolol 5 mg was well tolerated. Three patients reported side effects, of which most common was dizziness observed in 2 patients, numbness of tongue for an hour postdose in 1 patient, and myalgia in upper limb in 1 patient. None of these side effects led to discontinuation of therapy or any changes to therapy.
Discussion
This study demonstrated the antihypertensive efficacy of nebivolol (5 mg, once daily) in patients with essential hypertension, by reducing both brachial and central aortic BPs. This finding that nebivolol significantly reduced central aortic pressures is consistent with the results of the studies conducted by Dhakam et al.[6] and Azra Mahmud and Feily[7] and that it significantly reduced brachial pressures is consistent with the studies of Weiss et al.[8] and Saunders et al.[9]
This study therefore supports the recent evidence that nebivolol differs from other beta-blockers,[5,7,10,11] by its favorable hemodynamic effects, which may translate into improved cardiovascular outcomes in patients with hypertension.[11]
Nebivolol is the most beta-1-selective adrenoceptor antagonist currently available in clinical use and its enantiomers have different pharmacologic properties—D-isomer providing the beta-blocking activity and both the D – and L-isomers have an endothelial NO-dependent vasodilating effect.[5,11] NO has powerful antiatherogenic effects and thus treatment with nebivolol may have favorable effect on vascular complications of hypertension either directly by reducing BP or indirectly by increasing the bioavailability of NO.[11] It might also improve endothelial function by increasing NO synthesis through a stimulation of constitutive NO-synthase (eNOS), or by reducing oxidative inactivation of NO. An increased oxidative stress or a decreased NO production may be involved in the development of structural alterations in large arteries, including changes in the mechanical properties. Therefore, the NO-mediated effects of nebivolol may reverse endothelial dysfunction and lead to a decrease of large artery stiffness and systemic vascular resistance.[10]
In addition, the antioxidant properties and neutral or favorable effects on both carbohydrate and lipid metabolism of nebivolol are also well documented.[5] Beta-blockers have been associated with a risk of sexual dysfunction. In a recent clinical trial by Doumas et al. on hypertensive men who complained of erectile dysfunction while taking atenolol, metoprolol, or bisoprolol, the researchers found that after switching to nebivolol therapy, there was significant improvement in erectile function without significant change in BP.[12] All these properties help to differentiate nebivolol from nonvasodilating β-blockers, such as atenolol, metoprolol, or bisoprolol.[5]
Central aortic pressure is a strong predictor of cardiovascular morbidity and mortality, but classic beta-blockers as atenolol have little effect on reducing central aortic pulse pressure in hypertensive patients. The lack of efficacy of atenolol in reducing central aortic pressure may have a direct effect on cardiovascular outcomes.[11]
Studies comparing atenolol with other antihypertensive agents show that although decreases in peripheral BP are similar, treatment with atenolol results in significantly less reduction of central aortic pressure compared with either fosinopril in Chen et al.'s[13] study or eprosartan in Dhakam et al.'s[14] study.[11]
Also, in the CAFE study conducted to examine the impact of the 2 treatment regimens, that is, amlodipine ± perindopril versus atenolol ± thiazide, on derived central aortic pressures and other hemodynamic parameters measured by radial artery applanation tonometry found that despite comparable brachial systolic BPs in this subgroup of patients, central aortic pressures were significantly lower in the amlodipine/perindopril group.[11,15,16]
Taken together, the results of the CAFE study and those of other studies comparing beta-blockers with other antihypertensive agents suggest that in addition to their undesirable metabolic profile, beta-blockers have a less favorable impact on arterial stiffness and wave reflections, which may in part counterbalance the positive consequences of their BP-lowering efficacy.[16] Atenolol may be less effective at reducing central aortic pressure because of its effects on reducing heart rate, which may enhance the effect of wave reflections.[11]
Beta-blockers represent a very heterogenous class of antihypertensive drugs. Vasodilating beta-blockers, such as nebivolol and carvedilol, might not increase central BP as atenolol did in the CAFE study, because these beta-blockers exert a hemodynamic profile that is similar to those of angiotensin-converting enzyme inhibitors and calcium antagonists and contrasts markedly with that of conventional beta-blockers.[4,16]
In our present study, nebivolol has not only significantly reduced the central aortic pressures and brachial pressures, but also significantly reduced AIx%@75 HR and carotid-femoral PWV, which are independent risk markers, for cardiovascular risk.[17–20] This finding is consistent with results of a study done by Azra Mahmud et al. in which nebivolol in contrast to atenolol, has an effect on increasing PP amplification and reducing wave reflection, possibly because of increased NO levels.[7] In a study conducted by McEniery et al., the authors concluded that nebivolol decreases arterial stiffness independently of any effect on BP.[21]
In our study, nebivolol was well tolerated with minor side effects, such as dizziness and myalgia, and these findings were comparable to the results of a meta-analysis of adverse events in double-blinded placebo-controlled trials, which finds occurrence of adverse events to be no different with nebivolol as compared with placebo.[22]
Only one subject was lost to followup and was not due to side effects and all the rest had good compliance in the study. This is in line with the evidence that due to a good safety profile, it is likely that patients treated with nebivolol may have better adherence to therapy than to other beta-blockers.[5]
In conclusion, nebivolol 5 mg administered orally once daily, demonstrated antihypertensive efficacy in subjects with essential hypertension by reducing not only peripheral brachial pressures, but by also significantly reducing central aortic pressures. It has also reduced AIx%@75 HR and PWVcf, which is a marker of arterial stiffness significantly, which adds to its positive hemodynamic profile.
Acknowledgments
Our sincere thanks to Indian Council of Medical Research (ICMR) for sponsoring the instrument sphygmocor, which was used in this study.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.George T, Ajit SM. Atenolol in Hypertension. J Assoc Physicians India. 2009;57(Suppl):S22–5. [Google Scholar]
- 2.JNC 7 Express: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure U.S. Department Health and Human Services, NIH, NHLBI [Google Scholar]
- 3.Sabovic M, Safar ME, Blacher J. Is there any additional prognostic valueof central blood pressure wave forms beyond peripheral blood pressure. Curr Pharm Des. 2009;15:254–66. doi: 10.2174/138161209787354249. [DOI] [PubMed] [Google Scholar]
- 4.McEniery CM. Antihypertensive drugs and central blood pressure. Curr Hypertens Rep. 2009;11:253–9. doi: 10.1007/s11906-009-0043-4. [DOI] [PubMed] [Google Scholar]
- 5.Agabiti Rosei E, Rizzoni D. Metabolic profile of nebivolol, a beta adrenoceptor antagonist with unique characteristics. Drugs. 2007;67:1097–107. doi: 10.2165/00003495-200767080-00001. [DOI] [PubMed] [Google Scholar]
- 6.Dhakam Z, Yasmin, McEniery CM, Burton T, Brown MJ, Wilkinson IB. A comparison of atenolol and nebivolol in isolated systolic hypertension. J Hypertens. 2008;26:351–6. doi: 10.1097/HJH.0b013e3282f283c9. [DOI] [PubMed] [Google Scholar]
- 7.Mahmud A, Feely J. Beta-blockers reduce aortic stiffness in hypertension but nebivolol, not atenolol, reduces wave reflection. Am J Hypertens. 2008;21:663–7. doi: 10.1038/ajh.2008.156. [DOI] [PubMed] [Google Scholar]
- 8.Weiss RJ, Weber MA, Carr AA, Sullivan WA. A randomized, double-blind placebo controlled parallel-group study to assess the efficacy and safety of nebivolol, a novel β-blocker, in patients with mild to moderate hypertension. J Clin Hypertens. 2007;9:667–76. doi: 10.1111/j.1524-6175.2007.06679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders E, Smith WB, DeSalvo KB, Sullivan WA. The efficacy and tolerability of nebivolol in hypertensive African American patients. J Clin Hypertens. 2007;9:866–75. doi: 10.1111/j.1524-6175.2007.07548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agabiti-Rosei E, Porteri E, Rizzoni D. Arterial stiffness, hypertension, and rational use of nebivolol. Vasc Health Risk Manag. 2009;5:353–60. doi: 10.2147/vhrm.s3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cockcroft J. A review of the safety and efficacy of nebivolol in the mild hypertensive patient. Vasc Health Risk Manag. 2007;3:909–17. [PMC free article] [PubMed] [Google Scholar]
- 12.Doumas M, Tsakiris A, Douma S, Grigorakis A, Papadopoulos A, Hounta A, et al. Beneficial effects of switching from β-blockers to nebivolol on the erectile function of hypertensive patients. Asian J A ndrol. 2006;8:177–82. doi: 10.1111/j.1745-7262.2006.00076.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen CH, Ting CT, Lin SJ, Hsu TL, Yin FC, Siu CO, et al. Different effects of fosinopril and atenolol on wave reflection in hypertensive patients. Hypertension. 1995;25:1034–41. doi: 10.1161/01.hyp.25.5.1034. [DOI] [PubMed] [Google Scholar]
- 14.Dhakam Z, McEniery CM, Yasmin, Cockcroft JR, Brown MJ, Wilkinson IB. Atenolol and eprosartan: Differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens. 2006;19:214–9. doi: 10.1016/j.amjhyper.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, et al. Differential impact of blood pressure lowering drugs on central aortic pressure and clinical outcomes.Principal Results of the Conduit Artery Function Evaluation (CAFE) Study. Circulation. 2006;113:1213–25. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 16.Burnier M, Bullani R, Vogt B. Beta-blockers for the treatment of essential hypertension: What are the arguments against their use as first line therapy? Curr Hypertens Rev. 2007;3:15–20. [Google Scholar]
- 17.Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–9. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 18.Safar H, Mourad JJ, Safar M, Blacher J. Aortic pulse wave velocity, an independent marker of cardiovascular risk. Arch Mal Coeur Vaiss. 2002;95:1215–8. [PubMed] [Google Scholar]
- 19.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic valueof aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–70. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 20.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 21.McEniery CM, Schmitt M, Qasem A, Webb DJ, Avolio AP, Wilkinson IB, et al. Nebivolol increases arterial distensibility in vivo. Hypertension. 2004;44:305–10. doi: 10.1161/01.HYP.0000137983.45556.6e. [DOI] [PubMed] [Google Scholar]
- 22.Lacourcière Y, Arnott W. Placebo-controlled comparison of the effects of nebivolol and low-dose hydrochlorthiazide as monotherapies and in combination on blood pressure and lipid profile in hypertensive patients. J Hum Hypertens. 1994;8:283–8. [PubMed] [Google Scholar]


