Abstract
Background and Aims:
Alpha-2 agonists are being increasingly used as adjuncts in general anaesthesia, and the present study was carried out to investigate the ability of intravenous dexmedetomidine in decreasing the dose of opioids and anaesthetics for attenuation of haemodynamic responses during laryngoscopy and tracheal intubation.
Methods:
One hundred patients scheduled for elective general surgery were randomized into two groups: D and F (n=50 in each group). Group D were administered 1 μg/kg each of dexmedetomidine and fentanyl while group F received 2 μg/kg of fentanyl pre-operatively. Thiopental was given until eyelash reflex disappeared. Anaesthesia was maintained with 33:66 oxygen: nitrous oxide. Isoflurane concentration was adjusted to maintain systolic blood pressure within 20% of the pre-operative values. Haemodynamic parameters were recorded at regular intervals during induction, intubation, surgery and extubation. Statistical analysis was carried out using analysis of variance, chi-square test, Student's t test and Mann–Whitney U test.
Results:
The demographic profile was comparable. The pressor response to laryngoscopy, intubation, surgery and extubation were effectively decreased by dexmedetomidine, and were highly significant on comparison (P<0.001). The mean dose of fentanyl and isoflurane were also decreased significantly (>50%) by the administration of dexmedetomidine. The mean recovery time was also shorter in group D as compared with group F (P=0.014).
Conclusions:
Dexmedetomidine is an excellent drug as it not only decreased the magnitude of haemodynamic response to intubation, surgery and extubation but also decreased the dose of opioids and isoflurane in achieving adequate analgesia and anaesthesia, respectively.
Keywords: Dexmedetomidine, fentanyl, heart rate, isoflurane, mean arterial pressure, pressor response
INTRODUCTION
The pressor response, which is part of a huge spectrum of stress response, results from the increase in sympathetic and sympathoadrenal activity, as evidenced by increased plasma catecholamines concentrations in patients undergoing surgery under general anaesthesia.[1–5] Various drug regimens and techniques have been used from time to time for attenuating the stress response to laryngoscopy and intubation, including opioids, barbiturates, benzodiazepines, beta blockers, calcium channel blockers, vasodilators, etc.[6–10] The dose of opioids required for effective attenuation of stress response is fairly high and numerous drugs have been used as adjuncts in decreasing the dose of opioids with a varied level of success, but are not absolutely free from side-effects.[10–12]
Alpha-2 agonists like clonidine has been used extensively in the past for attenuation of sympathoadrenal stimulation caused by tracheal intubation and surgery.[4,13] Dexmedetomidine is the new alpha-2 agonist having eight-times more affinity for alpha-2 adrenoceptors as compared with clonidine, which has shown only partial agonist activity and is known to decrease the plasma catecholamines levels and suppressing the release of catecholamines also.[5,13–15]
A basic need is continuously felt among the anaesthesiologist fraternity for the desired availability of a drug that effectively suppresses all the hazardous responses to obnoxious stimuli with a maximum safety margin. With an emphasis on multidimensional features of dexmedetomidine, we designed a randomized, prospective, double-blinded study to determine whether the addition of dexmedetomidine would decrease the dose of opioids and anaesthetics for attenuation of haemodynamic response during laryngoscopy, tracheal intubation, surgery and extubation.
METHODS
After obtaining the ethics committee approval, 100 American Society of Anaesthesiologist (ASA)-I/II patients, aged 25–50 years, undergoing elective general surgical procedures were enrolled in the present study. Patients with cardiovascular disease, epilepsy, hypertension, chronic obstructive pulmonary disease, child-bearing potential, taking any antipsychotic medications or having a history of allergy to any drug and in whom the intubation attempts lasted longer than 25 s were excluded from the study. The research methodology was prospectively randomized with the help of computer-generated coded envelopes and patients were divided into two groups: Group F and group D.
Patients were premedicated with tab ranitidine 150 mg and tab alprazolam 0.25 mg a night before and 2 h before on the morning of surgery with a sip of water. In the pre-op room, a good intravenous access was secured and baseline parameters were observed and recorded, which included heart rate (HR), mean arterial blood pressure (MAP), electrocardiogram respiratory rate and pulse oximetry (SpO2). All patients were pre-hydrated with 500 mL of Ringer's lactate solution. Thereafter, group F received 100 mL of normal saline 20 min pre-operatively and inj. Fentanyl 2 μg/kg 3 min before induction, while group D received 1 μg/kg of dexmedetomidine in 100 mL of normal saline over 20 min and 1 μg/kg of fentanyl 3 min before the induction of anaesthesia. A subjective sedation scale, derived from the sedation agitation scale, was employed for the purpose of evaluation of sedation effect in both the groups[16] (1=fully awake and conscious, 2=awakening on verbal command, 3=awakening on gentle shaking, 4=awakening on vigorous shaking and painful stimuli and 5=unarousable).
Induction of anaesthesia was carried out with thiopentone sodium in a dose sufficient to abolish the eyelash reflex followed by 0.1 mg/kg of vecuronium bromide to provide neuromuscular blockade. The lungs were ventilated with 50% nitrous oxide in oxygen with Bains circuit for the next 3 min. Thereafter, laryngoscopy was performed with a Macintosh laryngoscope and intubation was performed with a cuffed endotracheal tube of appropriate size with a strict and vigil monitoring of haemodynamic and respiratory parameters at regular intervals of 1 min for the first 5 min and thereafter were observed continuously but recorded at 5-min intervals till completion of surgery. Response to skin incision was also observed and recorded in a similar manner. During surgery, anaesthesia was maintained with isoflurane and 66% nitrous oxide in oxygen. The adjustment in the inspiratory concentration of isoflurane was carried out in increment or decrement values of 0.2%, as judged by the increase or decrease in HR and MAP of 20% from the baseline values. An additional 0.5 μg/kg dose of fentanyl was repeated whenever the inspiratory concentration of inhalational anaesthetic reached 1%, and the average total inspiratory concentration of isoflurane was calculated by the sum of the products of inspiratory concentration and times divided by total anaesthesia time.[17] At the end of the surgical procedure, residual neuromuscular blockade was antagonized with neostigmine 2.5 mg and glycopyrrolate 0.5 mg intravenously (IV). Extubation was carried out as routine procedure and stress response to extubation was again observed and recorded in a similar manner as done during the intubation process, and patients were shifted to the recovery ward for further observation.
Demographic data was analysed using Student's t test while all the continuous variable were analysed using analysis of variance with post hoc significance to interpret changes over time. Mann–Whitney U test and chi-square test were applied whenever there was a statistically significant difference between two different sets of data for each variable. Power analysis was carried out using SPSS version 15 for windows and assuming α=0.10 and β=0.8, the effective sample size on the basis of haemodynamic differences turned out be 43 for the comparison of independent means. A value of P<0.05 was considered significant and P<0.001 was considered highly significant.
RESULTS
All the data is expressed as mean and standard deviation. The two groups were comparable in patient characteristics with respect to age, gender, ASA physical status and mean weight (P>0.05) [Table 1]. The infusion of dexmedetomidine was well tolerated and sedative effects of the drugs started appearing during infusion only in group D, but patients were arousable on verbal commands. The most strikingly significant feature after the completion of dexmedetomidine infusion in addition to lower blood pressure and HR was the fall in oxygen saturation (SpO2) up to 94–95%, which returned to normal on waking up the patients in group D [Figure 1]. There was, however, not much difference in the haemodynamics on awakening except for a 2–3% increase in HR and MAP.
Table 1.
Demographic profile of patients in both the groups
Figure 1.
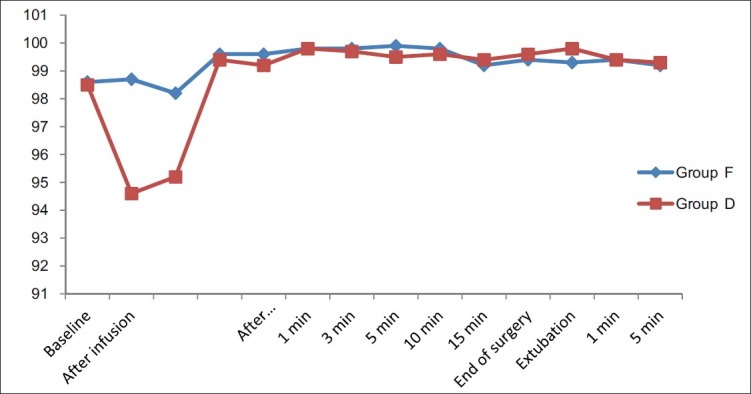
Graphical comparison of oxygen saturation in both the groups during the pre-op and the entire surgical period
The mean HR (P=0.02) and MAP (P=0.015) were significantly lower in the group D 20 min after the infusion of study drug as compared with similar parameters in group F [Figures 2 and 3]. Just after the induction of anaesthesia, mean HR and MAP decreased further in both the groups, and on analyzing the magnitude of decrease in haemodynamics, it was found to be highly significant on statistical comparison (P<0.001) The laryngoscopy and intubation was associated with a significant rise of mean HR and MAP in group F as compared with group D (P<0.001). Mean HR and MAP after 1, 3 and 5 min of intubation again returned to lower values, but on comparison of rate of decrease and stabilization of haemodynamic parameters, it was highly significant in group D (P<0.001). Thereafter, till the completion of surgery, no significant difference was noted in these respective parameters (P>0.05).
Figure 2.
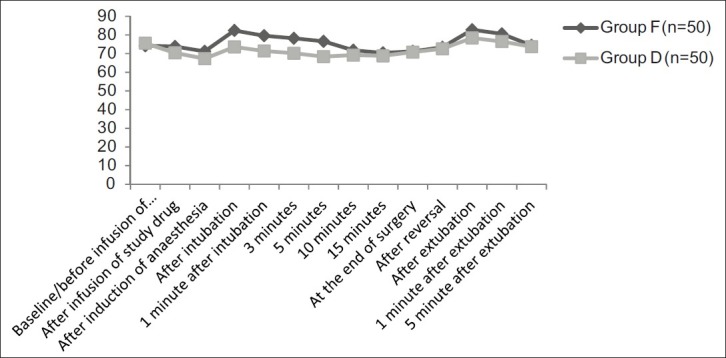
Comparison of mean heart rate in groups F and D
Figure 3.
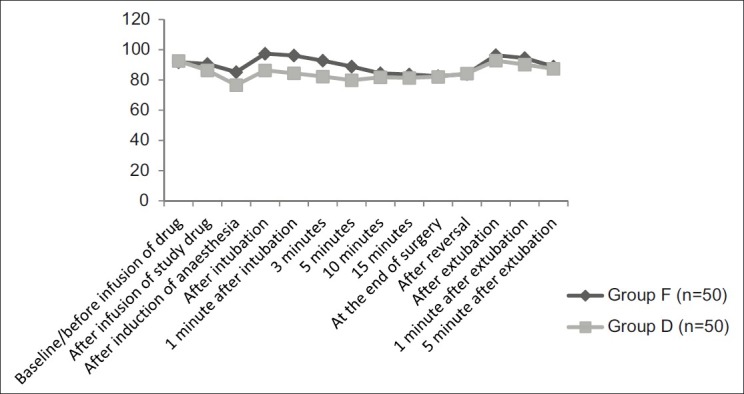
Comparison of mean arterial blood pressure in groups F and D
After the administration of injection neostigmine and glycopyrrolate for reversal of residual neuromuscular blockade, mean HR and MAP started increasing in patients of both the groups, but just before and immediately after the extubation, mean HR (P=0.009) and MAP (P=0.022) rose significantly in group F. These parameters came to the normal values in 2–3 min after the extubation.
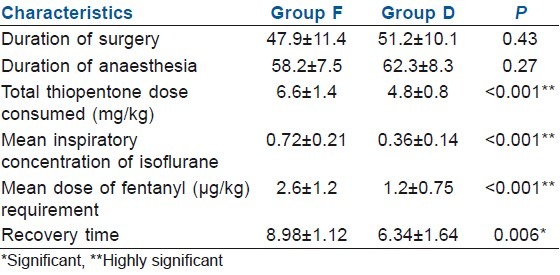
Duration of surgery and duration of anaesthesia was comparable in both the groups, but other anaesthesia characteristics exhibited a wider and significant variability between the groups [Table 2]. The requirement for total induction dose of thiopentone sodium was significantly lower in group D (P<0.001). Similarly, the requirement of fentanyl and isoflurane during the surgical period was significantly decreased in patients who received dexmedetomidine pre-operatively (P<0.001). The difference between the recovery characteristics in both the groups with regard to protrusion of tongue, sustained head lift and response to other verbal commands was significant on statistical comparison (P=0.006) [Table 2].
Table 2.
Comparison of anaesthesia characteristics between the groups
DISCUSSION
Attenuation and obtundation of pressor response during laryngoscopy and surgery has been one of the most researched topics in anaesthesia, but with only a few positive established outcomes.[1–5] Numerous drugs and their combinations have been tried in the past and studies have highlighted the use of these drugs in varying doses for suppression of stress response but not without the significant incidence of quite a few side-effects, especially with higher doses of opioids.[5–10]
The analgesic, sedation, anxiolytic, sympatholytic and blunting of exaggerated haemodynamic responses by administration of dexmedetomidine are being extensively studied and are mainly mediated by the activation of alpha-2 receptors located in the post-synaptic terminals in the central nervous system (CNS), which causes decreased neuronal activity and augmentation of the vagal activity.[4,18–20] The role of alpha-2 agonists in regulating the autonomic and cardiovascular responses is well understood, whereby they inhibit release of catecholamines (nor-epinephrine) from the sympathetic nerve terminals by augmentation of a vasoconstrictive effect.[1,21,22]
In the present study, the dose of 1 μg/kg of dexmedetomidine attenuated but did not completely obtund the haemodynamic responses to laryngoscopy and tracheal intubation. Although a 2 μg/kg dose of fentanyl in the control group was used, it was not sufficient to attenuate the stress responses to laryngoscopy and tracheal intubation as 15–25% increase in HR and blood pressure was recorded.
The dosage of general anaesthetic for the induction of anaesthesia decreases significantly, as was also evident from the decreased requirement of thiopentone in group D. Even the requirement of inhalational anaesthetic for maintenance of anaesthesia during the entire surgical procedure was markedly reduced as we used a lower inspiratory concentration of isoflurane in group D as compared with 0.6–1.6% of the concentration of isoflurane used in the control group. The effects of alpha-2 agonists in decreasing the minimal alveolar concentration for volatile anaesthetics and opioid have been well studied previously also and possibly mediated through both pre- and post-synaptic alpha-receptor activation in the CNS.[5,7,13,14,22]
Various studies have used dexmedetomidine in doses ranging from 0.1 to 10 μg/kg/h with not so much conclusive data but definitely associated with a significant incidence of bradycardia and hypotension in higher doses.[12,23] We used dexmedetomidine in a pre-operative infusion dose of 1 μg/kg over 20 min and observed a moderate reduction in the MAP and HR to the extent of 10–15% from the baseline values, and the findings are very much similar to the observations of other studies and can be explained on the basis of markedly decreased CNS sympathetic activity.[15] Earlier studies have demonstrated a transient increase in HR and MAP initially during the administration of dexmedetomidine infusion, which is followed by a decrease.[13,14,24,25] A similar kind of phenomenon was encountered during the present study as a transient increase in HR and MAP for 3–5 min was observed after the start of dexmedetomidine infusion and is probably due to the vasoconstriction effect of dexmedetomidine appearing earlier than the central sympathetic action. Studies using dexmedetomidine have commonly reported cardiac side-effects like bradycardia and sinus pause, which is mainly due to sympatholytic effect as well as preservation of baroreflex mechanisms. But, none of the patients in the present study had such an incidence, which could have warranted the use of atropine possibly due to the usage of a lower dose of dexmedetomidine.[17,24,26,27] However, to substantiate the cardiovascular safety of such drugs, such a small study of ours is not sufficient and larger meta-analytical studies are required. The rapid speed of infusion also determines, to a large extent, the higher incidence of side-effects such as apnoea and irregular ventilation, and occurs due to increased central sedation rather than direct respiratory depressant effect.[25,28] On the contrary, it is demonstrated that a lower dose of dexmedetomidine decreases the risk of apnoea and is considered a better alternative in critical patients in whom narcotics can cause severe respiratory depression.[29,30]
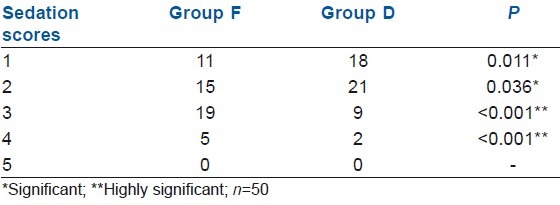
Another strikingly significant observation was the clear consciousness and alert state of mind in the patients who received dexmedetomidine and a lower fentanyl requirement as compared with the patients in the control group who were slightly sedated even 10–15 min after extubation, and the findings are almost similar with other such studies as sedation scores were significantly better in group D patients[2,5,11,12,14,18] [Table 3].
Table 3.
Comparative sedation scores in both the groups post-operatively
Emergence from anaesthetic effects and extubation are equally crucial as is the laryngoscopy, intubation and surgical period, as the depth of anaesthesia decreases abruptly and the rising levels of catecholamines can be deleterious for high-risk patients.[31] Dexmedetomidine enables a smooth transition from the time of administration of reversal to the post-extubation phase by suppressing the CNS sympathetic activity, leading to a high quality of extubation, as was observed in the majority of our patients in group D.[31]
There were certain limitations as the present study was carried out in surgical procedures of a short duration (<1 h) and on a smaller number of patients. More studies are required to establish the effects of a single dose of dexmedetomidine in surgeries of longer duration. Moreover, the effect was seen in ASA I/II patients, but the usefulness will be of immense help in high-risk cardiac patients who we could not study because we did not have an advanced cardiac set-up at our institute. The use of a Bispectral Index system would have been ideal in drawing the conclusions.
CONCLUSIONS
Dexmedetomidine is an excellent drug when used as an adjunct to general anaesthesia for attenuation of pressor response. It not only decreased the magnitude of stress response to intubation, surgery and extubation but also decreased the dose of opioids and isoflurane in achieving an adequate analgesia and anaesthesia, respectively.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sturaitis M, Kroin J, Swamidoss C, Moric M. Effects of intraoperative dexmedetomidine infusion on hemodynamic stability during brain tumor resection. Anesthesiology. 2002;98:A-310. [Google Scholar]
- 2.Bekker A, Basile J, Gold M, Riles T, Adelman M, Cuff G, et al. Dexmedetomidine for awake carotid endarterectomy: Efficacy, hemodynamic profile, and side effects. J Neurosurg Anesth. 2004;16:126–35. doi: 10.1097/00008506-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Reich DL, Hossain S, Krol M, Baez B, Patel P, Bernstein A, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101:622–8. doi: 10.1213/01.ANE.0000175214.38450.91. [DOI] [PubMed] [Google Scholar]
- 4.Wijeysundera DN, Naik JS, Beattie WS. a-2 adrenergic agonists to prevent perioperative cardiovascular complications: A meta analysis. Am J Med. 2003;114:742–52. doi: 10.1016/s0002-9343(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 5.Yildiz M, Tavlan A, Tuncer S, Reisli R, Yosunkaya A, Otelcioglu S. Effect of dexmedetomidine on haemodynamic responses to laryngoscopy and intubation: Perioperative haemodynamics and anaesthetic requirements. Drugs R D. 2006;7:43–52. doi: 10.2165/00126839-200607010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Charuluxananan S, Kyokong O, Somboonviboon W, Balmongkon B, Chaisomboonpan S. Nicardipine versus lidocaine for attenuating the cardiovascular response to endotracheal intubation. J Anesth. 2000;14:77–81. doi: 10.1007/s005400050071. [DOI] [PubMed] [Google Scholar]
- 7.Menda F, Koner O, Sayin M, Ture H, Imer P, Aykac B. Dexmedetomidine as an adjunct to anesthetic induction to attenuate hemodynamic response to endotracheal intubation in patients undergoing fast-track CABG. Ann Card Anaesth. 2010;13:16–21. doi: 10.4103/0971-9784.58829. [DOI] [PubMed] [Google Scholar]
- 8.Gunes Y, Gunduz M, Ozcengiz D, Ozbek H, Isik G. Dexmedetomidine-remifentanil or propofol-remifentanil anesthesia in patients undergoing intracranial surgery. Neurosurg Q. 2005;15:122–6. [Google Scholar]
- 9.Powroznyk A, Vuylsteke A, Naughton C, Misso S, Holloway J, Jolin-Mellgard A, et al. Comparison of clevidipine with sodium nitroprusside in the control of blood pressure after coronary artery surgery. Eur J Anaesth. 2003;20:697–703. doi: 10.1017/s0265021503001133. [DOI] [PubMed] [Google Scholar]
- 10.Abou-Arab MH, Heier T, Caldwell JE. Dose of alfentanil needed to obtain optimal intubation conditions during rapid-sequence induction of anaesthesia with thiopentone and rocuronium. Br J Anaesth. 2007;98:604–10. doi: 10.1093/bja/aem064. [DOI] [PubMed] [Google Scholar]
- 11.Engoren M, Luther G, Fenn-Buderer N. A comparison of fentanyl, sufentanil, and remifentanil for fast-track cardiac anesthesia. Anesth Analg. 2001;93:859–64. doi: 10.1097/00000539-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Feld JM, Hoffman WE, Stechert MM, Hoffman IW, Ananda RC. Fentanyl or dexmedetomidine combined with desflurane for bariatric surgery. J Clin Anesth. 2006;18:24–8. doi: 10.1016/j.jclinane.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Hall JE, Uhrich TD, Ebert TJ. Sedative, analgesic and cognitive effects of clonidine infusions in humans. Br J Anaesth. 2001;86:5–11. doi: 10.1093/bja/86.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guler G, Akin Z, Tosun E, Eskitascoglu , Mizrak A, Boyaci A. Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anaesthesiol Scand. 2005;49:1088–91. doi: 10.1111/j.1399-6576.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 16.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation- Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325–9. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Lerou JG, Booij LH. Model-based administration of inhalation anaesthesia.Developing a system model. Br J Anaesth. 2001;86:12–28. doi: 10.1093/bja/86.1.12. [DOI] [PubMed] [Google Scholar]
- 18.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic and analgesic properties of smalldose Dex infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 19.Ebert T, Maze M. Dexmedetomidine: Another arrow for the clinician's quiver. Anesthesiology. 2004;101:568–70. doi: 10.1097/00000542-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Gerlach AT, Dasta JF. Dexmedetomidine: An updated review. Ann Pharmacother. 2007;41:245–52. doi: 10.1345/aph.1H314. [DOI] [PubMed] [Google Scholar]
- 21.BekkerA , Sturaitis M. Dexmedetomidine for neurosurgical surgery. Operative Neurosurg. 2005;57:1–10. doi: 10.1227/01.neu.0000163476.42034.a1. [DOI] [PubMed] [Google Scholar]
- 22.Tanskanen P, Kytta J, Randell T, Aantaa R. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumor surgery: A double-blind, randomized and placebo-controlled study. Br J Anaesth. 2006;97:658–65. doi: 10.1093/bja/ael220. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey MA, Saha D, Hebeler RF. Tracheal resection in the morbidly obese patient: The role of dexmedetomidine. J Clin Anesth. 2006;18:452–4. doi: 10.1016/j.jclinane.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–70. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 25.Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004;101:787–90. doi: 10.1097/00000542-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 26.Kunisawa T, Nagata O, Iwasaki H. Pharmacokinetic simulation of high-dose administration of dexmedetomidine for decubitus treatment. Masui. 2006;55:995–8. [PubMed] [Google Scholar]
- 27.Coursin DB, Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care. 2001;7:221–6. doi: 10.1097/00075198-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Bajwa SJ, Gupta S, Kaur J, Singh A, Parmar SS. Reduction in the incidence of shivering with perioperative dexmedetomidine: A randomized prospective study. J Anaesthesiol Clin Pharmacol. 2012;28:86–91. doi: 10.4103/0970-9185.92452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortinez LI, Hsu YW, Sum-Ping ST. Dexmedetomidine pharmacodynamics: Part I: Crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1066–76. doi: 10.1097/00000542-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Hofer RE, Sprung J, Sarr MG, Wedel DJ. Anesthesia for a patient with morbid obesity using dexmedetomidine without narcotics. Can J Anaesth. 2005;52:176–80. doi: 10.1007/BF03027725. [DOI] [PubMed] [Google Scholar]
- 31.Güler G, Akın A, Tosun Z, Eskitaşoğlu E. During the extubation the effects of dexmedetomidine on cardiovascular changes and quality of extubation in the old patients undergoing cataract surgery. Turkish J Anaesthesia. 2005;33:18–23. [Google Scholar]


