Abstract
Background:
To compare the efficacy and safety of local anaesthetics under cervical epidural anaesthesia (CEA) using lignocaine (1%), bupivacaine (0.25%) and ropivacaine (0.5%) for thyroid surgery.
Methods:
In a prospective, randomized fashion, 81 patients were selected for thyroid surgery under CEA. They were assigned to one of three groups: Group L, B and R to receive 10 mL of 1% lignocaine, 0.25% bupivacaine and 0.5% ropivacaine, respectively. We compared their efficacy in terms of pulmonary and haemodynamic parameters, blockade quality and complications.
Results:
Of the total, 74 patients completed the study successfully. Sensory block attained the median dermatomal range of C2-T4/T5 in all the groups. Motor block was more pronounced in the ropivacaine group. Cardiorespiratory parameters decreased significantly in all the groups; however, none of the patients had any major complications except for bradycardia in two patients. Among the measured variables, the decrease in heart rate and peak expiratory force was more in the lignocaine group while forced vital capacity and forced expiratory volume at 1 sec declined to a greater extent in the ropivacaine group. The lignocaine group required significantly more epidural top-ups compared with the other two groups.
Conclusion:
We conclude that cervical epidural route can be safely used for surgery on thyroid gland in patients with normal cardiorespiratory reserve, using either of local anaesthetics chosen for our study. Under the selected dose and concentrations, the decrease in cardiorespiratory parameters was lesser with bupivacaine.
Keywords: Bupivacaine, cervical epidural anaesthesia, lignocaine, ropivacaine, thyroid surgery
INTRODUCTION
Thyroid surgeries are conventionally performed under general anaesthesia (GA). With the rising concern for GA-related implications on cardiorespiratory, metabolic and immune status of the patient, a preference for regional anaesthetic techniques has increased worldwide. Epidural anaesthesia is a ubiquitous technique in regional anaesthesia. While the earlier trials have widely focused on lumbar or thoracic epidurals, the cervical approach has been an upcoming technique since the past few years and has attracted investigators to explore its viability for various surgeries. Administration of local anaesthetic into the cervical epidural space results in anaesthesia of cervical plexus, brachial plexus and superior thoracic dermatomes. Additional advantages are lower cost, reduced intraoperative blood loss, stable cardiovascular status, reduced stress response, post-operative analgesia and early ambulation of the patient.[1] Some studies have documented the efficacy and safety of cervical epidural anaesthesia (CEA) as a sole anaesthetic technique for upper extremity and thoracic wall surgeries.[2,3] However, this technique is relatively rarely used for thyroid surgeries, particularly in a randomized controlled setting; the data are limited to case reports or pilot studies only.[1,4]
Data regarding the comparison of various local anaesthetics in CEA for neck surgeries are scarce in the international literature. The present trial was undertaken to compare the efficacy of three different formulations of local anaesthetics (lignocaine, bupivacaine and ropivacaine) for the conduct of thyroid surgeries under CEA.
METHODS
After ethical committee approval and written informed consent, 81 euthyroid cases of ASA physical status I–III, aged 40–60 years, height 145–165 cm, body mass index 25±10%, posted for thyroid surgeries (subtotal thyroidectomy, lobectomy) in between August 2009 and July 2011 were included in this trial. Exclusion criteria included deranged coagulation profile, history of allergy to local anaesthetics, retrosternal goitre, cardiorespiratory disease or any contraindication to regional anaesthesia.
These patients were randomly allotted to three groups using computer-generated random numbers: Group L, R or B to receive 10 mL of lignocaine (1%), ropivacaine (0.5%) or bupivacaine (0.25%) injected via a cervical epidural catheter, respectively. Concealment (sealed opaque envelope) was done by an investigator intended to prepare the studied drug solution. Two experienced anaesthesiologists (blinded to group allocation) familiar with the CEA technique participated in the procedure; one for performing the procedure and the other for assistance. The data collection was done by an investigator blinded to group allocation.
All patients were premedicated with lorazepam (2 mg) 2 h prior to the procedure. On arrival to the operative room, standard monitors were attached and all patients were positioned in the right lateral decubitus position with the neck flexed and chin on chest. The cervical epidural space was identified with an 18-gauge Tuohy epidural needle, at the C7–T1 interspace using the loss of resistance technique via a midline cephalad approach. A 19-gauge end-holed catheter was then introduced 4 cm into the epidural space. After negative aspiration, the catheter was tunnelled subcutaneously and patients were laid supine. The cephalad position of the catheter's tip was confirmed radiographically using Iohexol dye (iodine concentration 350 mg/mL, 0.5–1 mL). The test dose of prepared drug solution (3 mL) was injected via an epidural catheter as per group allocation; vitals [breathing, SpO2, consciousness, heart rate (HR), non-invasive blood pressure and electrocardiogram] were monitored for 5 min for any sign of deterioration. In the absence of such signs, the remainder of the mixture was administered through the catheter. Any cases of failed CEA were managed by giving GA and excluded from the study. After measuring the pulmonary variables at 30 min post-CEA, drapes were applied and surgery was started. Monitoring was done throughout the operation and vitals were recorded on monitors every 5 min. The patients were kept in a state of conscious sedation with midazolam (mean dose, 0.04 mg/kg IV) throughout the surgery. Vocal cord functions were monitored intermittently by verbal contact with the patient. Any intraoperative discomfort in the neck or request for rescue analgesic was managed by administering epidural top-ups (4 mL) of studied drug solution as per group allocation. Post-operatively, epidural top-ups were given on complain of the patient (score of ≥5 on a 10-point numerical score).
The block profile was evaluated at 5-min intervals for first 30 min after the induction of CEA and at 1-h intervals thereafter. The level of sensory block was tested bilaterally (defined as loss of sensation to pinprick) in an ascending fashion starting from the T12 dermatome. The onset of sensory block was defined as the time to loss of sensation to pinprick in the C3 dermatome. The degree of upper limb motor block was assessed according to the following scale: 1 – absence of motor block, 2 – partial motor block (weakness appreciable but movement possible against resistance), 3 – motor block almost complete (possible movement but not against resistance) and 4 – complete motor block (absence of movement).[5] The haemodynamic variables [HR, mean arterial pressure (MAP)] were recorded at baseline, 30 min post-CEA and end of surgery. The pulmonary functions were measured by a bedside spirometer. The recorded variables included vital capacity (VC), peak expiratory force (PEF) and fraction of the vital capacity expired during the first second of a forced expiratory volume (FEV1) measured at baseline, 30 min post-CEA and end of the surgery.
The demographics, baseline parameters and sensory and motor characteristics were compared by the Mann-Whitney U test. The paired data including the haemodynamic and respiratory parameters were compared by the Wilcoxon Signed Rank test. The need of epidural top-ups and any perioperative complications were compared by Fisher's exact test/Chi square test, whichever was appropriate (SPSS 16.0 statistic software). Difference of P value <0.05 was considered significant.
To detect a 20% difference in the measured variables with an expected standard deviation of 20% estimated from initial pilot observations, with 90% power and 5% alpha error, a sample size of 22 subjects per group was required. The admission rate for thyroid surgeries in our hospital is about four to five per month. All the admitted patients during the study period were screened for our study, with the target to include at least 22 patients per group. Sample size was calculated using power and sample size calculator by the Department of Biostatics, Vanderbilt University, USA.
RESULTS
In total, 81 patients were initially randomized into three study groups. Seven patients were later excluded: Two due of protocol violation, two due to haemorrhagic tap during localization of epidural space and three due to patchy anaesthesia. These patients were managed under conventional GA. Thus, 74 patients completed the study successfully.
The demographics, baseline haemodynamics and pulmonary functions were comparable between the study groups. There was no significant difference in the duration of surgery, IV fluid infused and estimated blood loss between the groups [Table 1]. The upper level of sensory block was situated at the C2 dermatome in all the patients. The caudad dermatomal levels were noted at T5 in groups L and B, and T4 in group R. Median time to onset of sensory block was significantly shorter in group L [(10 min (5-10))] as compared with the group R [(15 min (10-20))] and B [(10 min (10-15))]. Greater motor blockade was observed in group R as compared with the other two groups. The group L required significantly more epidural top-ups to maintain the intraoperative anaesthesia [Table 1].
Table 1.
Comparison of demographic characteristics, baseline cardiorespiratory parameters and block profile between the studied groups
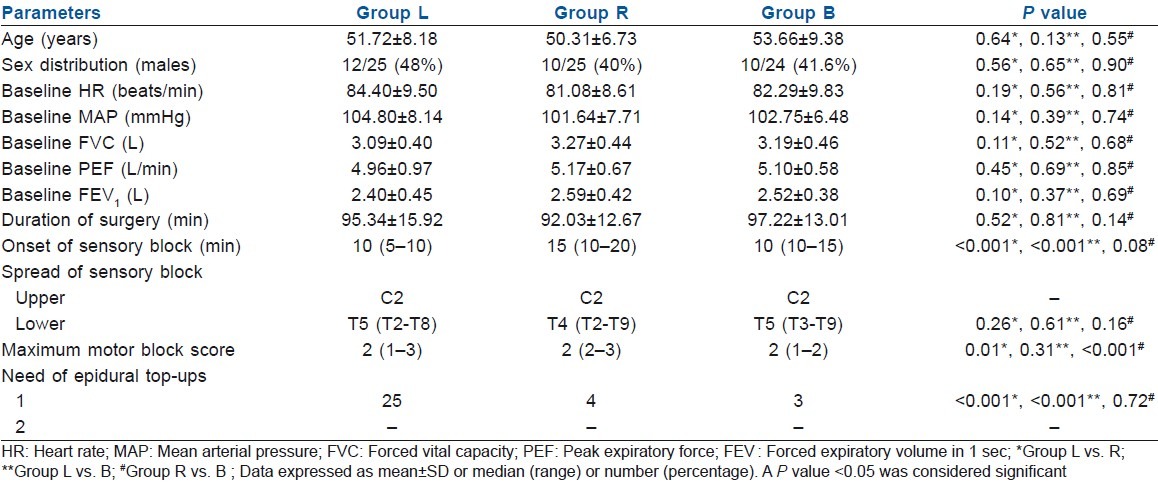
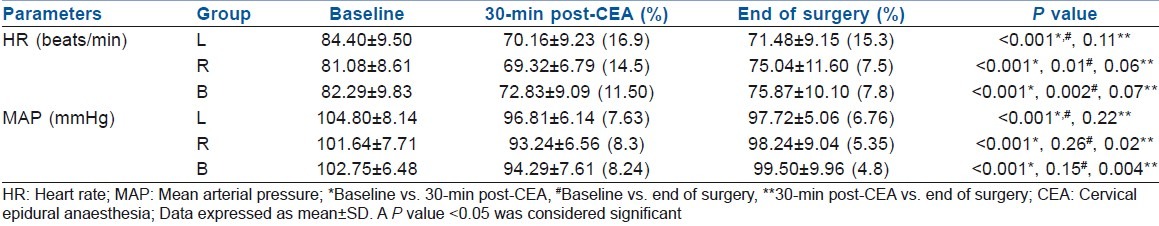
HR decreased significantly in all the three groups (30 min post-CEA), although the decline was higher in L group (16.9%, P>0.001) than in the B (11.5%, P>0.001) or R (14.5%, P>0.001) groups as compared with their respective baseline values. A modest fall in MAP was also observed (all three groups), but it improved significantly and was comparable to baseline values at end of the surgery in groups R and B [Table 2]. No vasopressor agent was required in any of the cases; however, two patients developed one episode of bradycardia, which was managed promptly by a bolus dose of Atropine 0.6 mg IV.
Table 2.
Comparison of intraoperative haemodynamic parameters between the studied groups
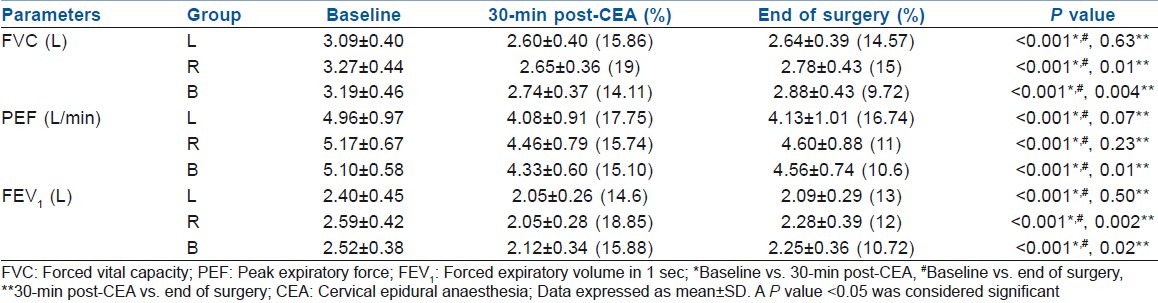
Pulmonary variables demonstrated a significant decline in forced vital capacity (FVC) and FEV1 in all the patients, although to a greater extent in the R group (19%, 18.8%). A similar decline in PEF was also observed, although the percentage fall was higher in the L group (17.75%). A significant improvement was observed in FVC and FEV1 towards the end of surgery in groups R and B; however, PEF improved significantly in the B group only [Table 3]. None of the patients developed dyspnoea or hoarseness during the perioperative period.
Table 3.
Comparison of intraoperative respiratory parameters between the studied groups
DISCUSSION
Our investigation indicates that thyroid surgery can be safely performed under CEA using either of local anaesthetics chosen for this study. Blockade of the sympathetic fibres originating in the cervical and thoracic region is deemed to occur during CEA.[6] The resultant decline in HR and MAP is beneficial for patients, especially with limited myocardial reserve, due to prolongation of coronary perfusion time and reduced left ventricular afterload.[3,7] We observed a similar decline in post-induction haemodynamic parameters in all the study groups. This is desirable during thyroid surgeries owing to a lower risk of perioperative blood loss and a lesser need for blood transfusions. In the lignocaine group, the MAP remained depressed till the end of surgery, which correlates with the significantly higher need of epidural top-ups in this group.
During CEA, a moderate decrease in pulmonary functions is known to occur due to partial phrenic nerve block and paralysis of intercostal muscles.[8–10] Similar results have followed after physical or pharmacological sectioning of the phrenic nerves in animal models.[11] These changes are usually clinically irrelevant in patients without any pre-existing lung disease.[1,8] We observed a significant decline in spirometric values in all the study groups, although none of the cases developed any respiratory complaints or variations in SpO2 during the perioperative period. As patients with pre-existing respiratory disease were excluded in this trial, the safety of CEA in such patients cannot be warranted by this study. Michalek et al. documented a decrease of 17–23% in the above parameters using 0.75% ropivacaine, although it was significant for forced vital capacity only.[5] Capdevila et al. showed a decrement of 18–26% in the above respiratory parameters using 0.25% bupivacaine solution.[8] Various studies performed using 2% lignocaine in CEA have documented a decrease of 15–20% in the above respiratory parameters.[9–10] On the contrary, Dohi et al. and Stevens et al. reported a non-significant decrease in pulmonary parameters on using 1.5–2% lignocaine solution in CEA.[12,13] A strong emphasis cannot be laid upon all these studies as these were not suitably powered trials and performed on pilot basis. In our study, a significant improvement in FVC and FEV1 was observed in the ropivacaine and bupivacaine groups at the end of surgery, indicating recovery phase of anaesthetic block. The PEF values on the contrary improved significantly in the bupivacaine group only; the reason for this sole observation is unexplainable. As bupivacaine and ropivacaine share a similar pharmacological profile, one possible explanation could be differences in the dose–response relationship of the above drugs at this particular concentration, volume and site.
Motor block is an undesirable side-effect of CEA, which may increase the need for assisted ventilation by causing the paresis of respiratory muscles. To minimize this effect, diluted concentration of local anaesthetics (i.e., lignocaine 1% and bupivacaine 0.25%) has been successfully tested in previous studies to conduct surgeries under CEA.[1,3,8] Christelis et al. showed a denser and more prolonged motor block with epidural ropivacaine (0.75%) as compared with bupivacaine (0.5%) in elective caesarean section.[14] In our study, greater motor blockade with ropivacaine could be attributed to its relatively lesser dilution (i.e., 2/3 or 0.5% concentration) as compared with the other two local anaesthetics (i.e., ½ dilutions) chosen for comparison. Because the concentration of ropivacaine <0.5% has not been previously studied in CEA, we selected the minimum effective dose/concentration of ropivacaine (0.5%) chosen in earlier trials.[15] Most studies have successfully conducted surgeries under cervical epidural anaesthesia using 10–15 mL of local anaesthetic volumes. The rationale behind using these volumes is that the requirement of local anaesthetic is approximately 1.2 and 1.5 mL per segment in cervical and thoracic epidural space, respectively (i.e., nearly 10–15 mL volume for spread to eight to 10 segments).[4] Therefore, we chose minimum effective concentrations of studied drugs in optimal and equal volumes (to ensure blinding) for our study.
We used midazolam (mean dose 0.04 mg/kg IV) for conscious sedation in all the cases (40–60 years age group) intraoperatively. Previous investigations conducted on midazolam by Fujisawa et al. showed that doses used for conscious sedation have no effect on pulmonary function tests (PFTs) in supine patients under the 60 years age group (midazolam dose 0.074±0.026 mg/kg). In patients above 65 years of age (midazolam dose 0.045±0.012 mg/kg), vital capacity declines while there is no effect on FEV1.[16] Thus, conscious sedation may have no influence on PFT under the methodology used in our study.
Enlarged thyroid gland can cause tracheal deviation or compression, or both, and is a known risk factor for difficult intubation.[17] Cervical epidural provides a safe and effective anaesthesia for thyroid surgery thus avoiding the potential complications associated with both difficult airway and GA.[1] It allows early recognition of hoarseness associated with recurrent laryngeal nerve injury through verbal communication with the patient during thyroid surgeries. CEA is not used in routine anaesthesia practice, commonly due to operator inexperience and risk of potential complications like dural puncture, neurovascular injury, epidural bleed/haematoma or abscess formation. Bonnet et al. noted, in a retrospective analysis of 394 patients, dural puncture in two (0.5%) and epidural venipuncture in six (1.5%) patients.[18] Hakl et al. reported migration of local anaesthetic solution into subarachnoid space in six (2.8%), failed epidural puncture in three (1.4%) and bloody epidural tap in four patients (1.8%).[19] We observed a haemorrhagic tap in two patients during epidural space localization thus mandating conversion to GA. Previous studies have documented a proportionately higher risk of hypotension and arrhythmias during thyroid surgeries under GA.[1,20] In contrast, Khanna et al. mentioned no cardiovascular complications during thyroid surgeries under CEA.[1] We observed a single episode of bradycardia in two patients, managed promptly using a vagolytic. Bilateral phrenic nerve block is also a reported complication of CEA.[18] We were unable to investigate this parameter owing to inexperience in monitoring diaphragmatic movements and unavailability of advanced equipments that can measure electrical potentials along the phrenic nerve. The implications of this aspect can be tested on normal and respiratory-compromised patients in future trials on various neck surgeries.
CONCLUSION
We conclude that the surgeries on thyroid gland can safely be performed under CEA using any of the three formulations of local anaesthetics chosen for our study. Although there is a considerable fall in post-induction cardiorespiratory parameters, these effects are clinically insignificant and well tolerated in individuals with no pre-existing cardiorespiratory disease. CEA appears to be a promising alternative in the anaesthetic management of thyroid surgery. Further research is warranted in order to fully assess its safety and efficacy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Khanna R, Singh DK. Cervical epidural anaesthesia for thyroid surgery. Kathmandu Univ Med J. 2009;7:242–5. doi: 10.3126/kumj.v7i3.2731. [DOI] [PubMed] [Google Scholar]
- 2.Guevara-López U, Bárcenas-Olivares J, Gutiérrez-Sougarret B, Aldrete JA, Olascoaga-Ortega G. Cervical epidural anesthesia for upper extremity surgery using three different formulations of local anesthetics. Cir Cir. 2005;73:273–81. [PubMed] [Google Scholar]
- 3.Biboulet P, Deschodt J, Capdevilla X, Landreau L, Aubas P, Du Cailar J, et al. Hemodynamic effects of 0.375% bupivacaine versus 0.25% bupivacaine during cervical epidural anesthesia for hand surgery. Reg Anesth. 1995;20:33–40. [PubMed] [Google Scholar]
- 4.Patel MG, Swadia VN. Cervical epidural anaesthesia for various neck surgeries. J Anaesth Clin Pharmacol. 2009;25:297–300. [Google Scholar]
- 5.Michalek P, Ivan David, Adamec M, Janousek L. Cervical epidural anesthesia for combined neck and upper extremity procedure: A Pilot Study. Anesth Analg. 2004;99:1833–6. doi: 10.1213/01.ANE.0000137397.68815.7B. [DOI] [PubMed] [Google Scholar]
- 6.Waldman S. Cervical epidural nerve block. In: Waldman SD, editor. Interventional pain management. 2nd ed. Philadelphia: WB Saunders; 2001. pp. 373–81. [Google Scholar]
- 7.Michalek P, Adamec M, Janousek L, Tosenovsky P. The effect of regional anaesthesia on phrenic nerve in carotid artery surgery: A comparison of cervical epidural anaesthesia and cervical plexus block. Int Monitor Reg Anaesth. 1999;11:27. [Google Scholar]
- 8.Capdevilla X, Biboulet P, Rubenovitch J, Serre-Cousine O, Peray P, Deschodt J, et al. The effects of cervical epidural anesthesia with bupivacaine on pulmonary function in conscious patients. Anesth Analg. 1998;86:1033–8. doi: 10.1097/00000539-199805000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Huang CH, Hsu JL, Chen WS. Effects of lidocaine cervical epidural blockade on respiratory function. Ma Zui Xue Za Zhi. 1990;28:311–6. [PubMed] [Google Scholar]
- 10.Huang CH. Effect of cervical epidural blockade with 2% lidocaine plus epinephrine on respiratory function. Acta Anaesthesiol Taiwan. 2007;45:217–22. [PubMed] [Google Scholar]
- 11.Stradling JR, Kozar LF, Dark J, Kirby T, Andrey SM, Phillipson EA, et al. Effect of acute diaphragm paralysis on ventilation in awake and sleeping dogs. Am Rev Respir Dis. 1987;136:633–7. doi: 10.1164/ajrccm/136.3.633. [DOI] [PubMed] [Google Scholar]
- 12.Dohi S, Takeshima R, Naito H. Ventilatory and circulatory responses to carbon dioxide and high level sympathectomy induced by epidural blockade in awake humans. Anesth Analg. 1986;65:9–14. [PubMed] [Google Scholar]
- 13.Stevens RA, Frey K, Sheikh T, Kao TC, Mikat-Stevens M, Morales M. Time course of the effects of cervical epidural anesthesia on pulmonary function. Reg Anesth Pain Med. 1998;23:20–4. doi: 10.1016/s1098-7339(98)90106-7. [DOI] [PubMed] [Google Scholar]
- 14.Christelis N, Harrad J, Howell PR. A comparison of epidural ropivacaine 0.75% and bupivacaine 0.5% with fentanyl for elective caesarean section. Int J Obstet Anesth. 2005;14:121–8. doi: 10.1016/j.ijoa.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Seraglio P, Campostella FA. Cervical plexus block and continuous cervical peridural block with ropivacaine for carotid surgery: A comparison between the 2 methods. Minerva Anestesiol. 2001;67(9 Suppl 1):65–70. [PubMed] [Google Scholar]
- 16.Fujisawa T, Suzuki S, Kurozumi A, kimura Y, Iida A, fukushima K. Influence of intravenous sedation with midazolam on respiratory function and muscle activity in elderly and young patients. Asian J Oral Maxillfac Surg. 2002;14:209–14. [Google Scholar]
- 17.Bacuzzi A, Dionigi G, Bosco AD, Cantone G, Sansone T, Losa ED, et al. Anaesthesia for thyroid surgery: Perioperative management. Int J Surg. 2008;6:82–5. doi: 10.1016/j.ijsu.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Bonnet F, Derosier JP, Pluskwa F, Abhay K, Gaillard A. Cervical epidural anaesthesia for carotid artery surgery. Can J Anaesth. 1990;37:353–8. doi: 10.1007/BF03005590. [DOI] [PubMed] [Google Scholar]
- 19.Hakl M, Sevcik P, Pavlikova J, Kraus R. Cervical epidural anaesthesia for carotid artery surgery: New experience. Int Monitor Reg Anaesth. 1998;10:79. [Google Scholar]
- 20.Bird CG, Hayward I, Howells TH, Jones GD. Cardiac arrhythmias during thyroid surgery.Incidence with various anaesthetic techniques. Anaesthesia. 1969;24:180–9. doi: 10.1111/j.1365-2044.1969.tb02836.x. [DOI] [PubMed] [Google Scholar]


