Abstract
Background:
Paediatric patients often present with different painful conditions that require immediate surgical interventions. Despite a plethora of articles on the ketamine–propofol combination, comprehensive evidence regarding the suitable sedoanalgesia regime is lacking due to heterogeneity in study designs.
Methods:
This prospective, randomized, double-blind, active–controlled trial was conducted in 100 children, of age 3–14 years, American Society of Anesthesiologist physical status IE-IIE, posted for emergency short surgical procedures. Patients were randomly allocated to receive either 2 mL of normal saline (pre-induction) plus calculated volume of drug from the 11 mL of ketamine–propofol solution for induction (group PK, n=50) or fentanyl 1.5 μg/kg diluted to 2 mL with normal saline (pre-induction) plus calculated volume of drug from the 11 mL of propofol solution for induction (group PF, n=50). In both the groups, the initial bolus propofol 1 mg/kg i.v. (assuming the syringes contained only propofol, for simplicity) was followed by adjusted infusion to achieve a Ramsay Sedation Scale score of six. Mean arterial pressure (MAP) was the primary outcome measurement.
Results:
Data from 48 patients in group PK and 44 patients in group PF were available for analysis. Hypotension was found in seven patients (14.6%) in group PK compared with 17 (38.6%) patients in group PF (P=0.009). Intraoperative MAP was significantly lower in group PF than group PK when compared with baseline.
Conclusion:
The combination of low-dose ketamine and propofol is more effective and a safer sedoanalgesia regimen than the propofol–fentanyl combination in paediatric emergency short surgical procedures in terms of haemodynamic stability and lesser incidence of apnoea.
Keywords: Drug combinations, fentanyl, ketamine, paediatric emergency, procedural sedation, propofol
INTRODUCTION
Paediatric patients often present to the emergency department with different painful conditions that require immediate surgical interventions. The choice of anaesthetic agent to provide deep sedation and analgesia in these patients varies highly. Ketamine has already played a safe and effective role as a sole anaesthetic agent with a few limitations like delayed recovery, emergence phenomenon and nausea and vomiting.[1] Subsequently, there is an increase in the use of propofol due to its favourable pharmacokinetics.[2] However, propofol is associated with dose-dependent respiratory depression, hypotension and no intrinsic analgesic property. Addition of fentanyl to propofol compliments the analgesic property. Concomitant opioid use reduces the dose requirements of each other, but the respiratory depression is supposed to be heightened. Theoretically, the combination of ketamine and propofol would allow a reduction in dose requirement of each agent, like the combination of fentanyl and propofol.
Despite a plethora of articles on the ketamine–propofol combination, a comprehensive evidence is still lacking probably due to heterogeneity of clinical studies and various study designs.[3–10] Among these, a few authors have studied the mixture of ketamine–propofol[3–5,10] in the form of infusion. To the horizon of our knowledge, only one case series (of four patients) has compared continuous infusion of the ketamine–propofol mixture with propofol alone[10] in paediatric patients. Another study[7] has compared ketamine–propofol versus fentanyl–propofol, where the investigators used propofol infusion alone throughout the procedure in both the groups after the bolus administration of the respective mixtures in the designated groups.
This study was carried out to compare the effectiveness and safety of intravenous infusion of the ketamine–propofol combination with the conventional fentanyl–propofol combination for emergency short surgical procedures in paediatric patients. We hypothesised that infusion of the ketamine–propofol combination would have more favourable haemodynamics and a better recovery profile than the infusion of the fentanyl–propofol combination in case of emergency short surgical procedures in the healthy paediatric patient population.
METHODS
This prospective, randomized, double-blind, active-controlled trial was conducted after getting the permission from the Hospital Ethics Committee. Children aged 3–14 years, of American Society of Anesthesiologist (ASA) physical status IE-IIE, posted for emergency short surgical procedures like reduction of fracture dislocation, incision and drainage of abscess and dressing-debridement of wounds, were included in this study. The exclusion criteria were known allergy or contraindication to either study drug, patient's/parent's refusal, head injury, seizure disorder, psychological disorders, ingestion of psychotropic or sedative medication, congenital heart disease, severe obesity (body mass index >35 kg/m2) and full stomach patients. Written informed consent was obtained from the guardian of the patients after proper discussion of the study procedure and risk benefits in the language they understand. We performed a pilot study to calculate the sample size. We found hypotension in 50% of the patients in the propofol–fentanyl group compared to with 20% in the ketamine–propofol group. Considering the level of significance (α) as 0.05, power of the study (1-β) as 80% and expecting an improvement in the incidence of hypotension of about 30% between the two groups, the sample size was estimated to be 40 in each group. Considering a dropout ratio of 25%, a total of 100 patients were included in the study. Patients were randomly allocated by the “sealed envelope technique” to one of two groups pre-operatively, and received either of the following two regimens:
In group PK (n=50): 2 mL of normal saline (pre-induction) and calculated volume of drug from 11 mL of the ketamine-propofol solution for induction
In group PF (n=50): fentanyl 1.5 μg/kg diluted to 2 mL with normal saline (pre-induction) and calculated volume of drug from 11 mL of the propofol solution for induction.
In case of group PK, a ketamine–propofol solution (1:2) was prepared by mixing 1 mL ketamine (50 mg/ mL) with 10 mL propofol 1% (10 mg/mL) so that each milliliter of the ketamine–propofol solution was contained of 9.0909 mg propofol and 4.5454 mg ketamine (approximately rounded off to 9 mg of propofol and 4.5 mg of ketamine, respectively). In case of group PF, 10 mL propofol 1% was mixed with 1 mL of normal saline so that each milliliter was contained of 9.0909 mg of propofol (rounded off to 9 mg/mL).
The anaesthesiologist who gave the drugs and assessed the parameters was blinded to the study drugs because the drugs were prepared by a separate anaesthesiologist who was not involved in the study procedures, and the colours of the prepared drugs were the same.
After taking the weight, the patient was transferred to the operating room. Monitors [electrocardiography non-invasive blood pressure (NIBP) and pulse oximeter] were attached and the baseline values were noted. An intravenous (iv) line was made and infusion with lactated Ringer's solution was started. Inj. ranitidine (1 mg/kg) and inj. metoclopramide (0.15 mg/kg) iv were slowly given as premedication, 30 min before starting the procedure.
Group PK received 2 mL of normal saline iv and group PF was given inj. fentanyl 1.5 μg/kg iv, the volume of which was made to 2 mL. Both the interventional drugs were given as an initial bolus dose of 1 mg/kg propofol iv (considering the syringes contained only propofol for simplicity). Then, infusion was started at the rate of 50 μg/kg/min. The level of sedation was assessed at 1-min intervals and the infusion rate was adjusted accordingly with a backup plan provided to the anaesthesiologist to achieve a Ramsay Sedation Scale (RSS) score of 6. As soon as the desired level of sedation was achieved, an appropriate size of laryngeal mask airway (LMA) was inserted and patients were maintained on spontaneous respiration. An end tidal carbon dioxide concentration (EtCO2) probe was attached between the LMA and the breathing system. Increase in heart rate, blood pressure, respiratory rate or body movement on initiation of the surgical procedure was considered as inadequate analgesia and were managed accordingly by increasing the study drug infusion rate, and noted.
NIBP and heart rate were measured before induction (baseline), after induction and then at every 5-min interval for 15 min and then at every 15-min interval till the completion of the procedures. EtCO2 and oxygen saturation were monitored continuously throughout the perioperative period and intermittently recorded. The patients were also assessed for desaturation or apnoea, which can be defined as a 10% decrease in SpO2 when compared with baseline or cessation of respiration for 15 s or more, respectively, and were managed accordingly by assisting ventilation. Hypotension was considered when the mean arterial pressure (MAP) dropped by >20% of the baseline MAP and managed accordingly by fluid bolus or vasopressors. Bradycardia was defined as heart rate less than 60 beats/min and managed with atropine 20 mcg/kg iv. Any movement of the patient suggestive of pain was treated with increase in the study drug infusion rate, and was noted.
The study drug infusion was discontinued at the end of the surgical procedure, and total drug consumption was noted. After reaching the RSS score of 3, LMA was removed after proper suctioning of the oropharynx if required. The recovery time (i.e., the time from discontinuation of infusion of the study drug and achievement of RSS score of 3) was noted and patients were transferred to the recovery room. The recovery room anaesthesiologist was blinded to the study medication received by the patients. The incidence of any episode of post-operative nausea and vomiting (PONV) or any other adverse events (e.g., hallucinations, agitation or pain) were noted by the recovery room anaesthesiologist and were managed accordingly. The patients’ vital signs were assessed at 5-min intervals for the first 15 min and then at every 15-min intervals. Any incidence of desaturation or hypotension was managed by giving oxygen through nasal prong or fluids and vasopressors, respectively. Patients were discharged from the recovery room after attaining an Aldrete Recovery Scale Score of 9. Time taken to achieve this score was noted.
RESULTS
Both the groups were comparable demographically [Table 1]. Six patients in group PF and two patients in group PK were excluded due to violation of the study protocol.
Table 1.
Demographic parameters
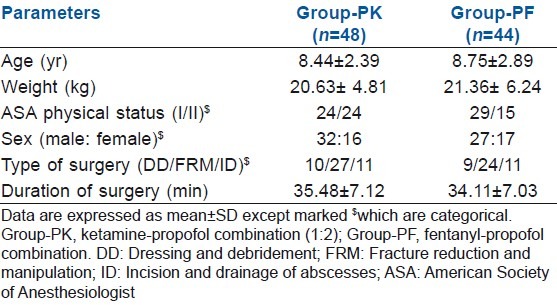
In our study, hypotension was found in seven patients (14.6%) in group PK compared with 17 (38.6%) in group PF (P=0.009). Bradycardia was found in 10 patients (22.7%) in group PF compared with none in group PK (P<0.000). Emergence reaction occurred in five of 48 patients (10.42%) in group PK, who were managed with i.v. midazolam 0.05 mg/kg. PONV occurred in five of 48 patients (10.42%) in group PK and in three of 44 (6%) patients in group PF. This was controlled with iv ondansetron 0.1 mg/kg. Apnoea occurred in 4.16% patients (two of 48) in group PK compared with 18.18% patients (eight of 44) in group PF (P=0.031) [Figure 1]. Lower EtCO2 value was observed in group PK than in group PF [Figure 2].
Figure 1.
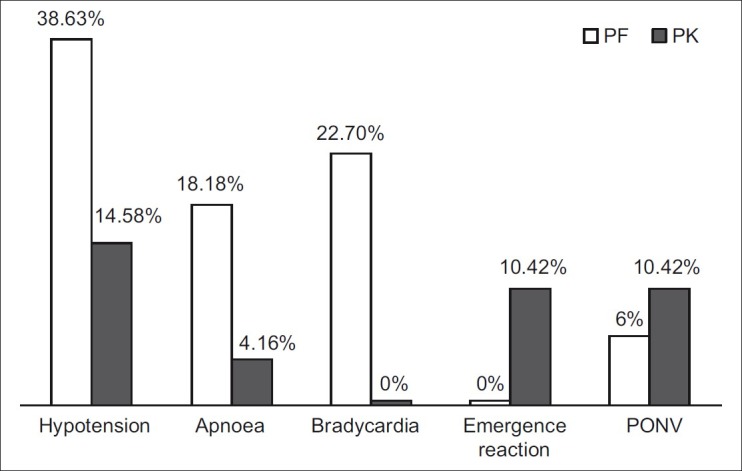
Incidences of adverse events
Figure 2.
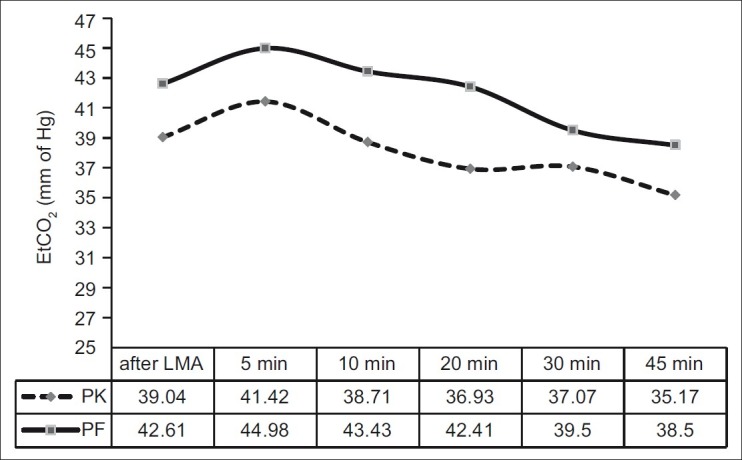
Trend of end tidal carbon dioxide concentration values throughout the intraoperative period
Intraoperative MAP was significantly higher in group PK than in group PF when compared with baseline. Intraoperative MAP was significantly lower in group PF than in group PK when compared with baseline. Although MAP decreased in both the groups after induction as well as during the intraoperative period, the decrease was much more significant in group PF [Table 2, Figure 3].
Table 2.
Intraoperative MAP between the two groups
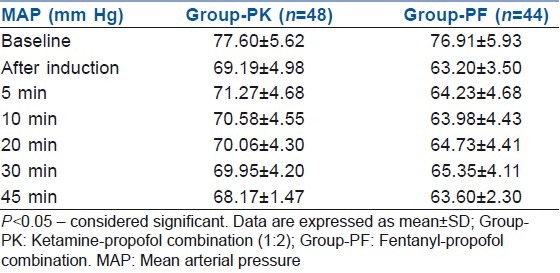
Figure 3.
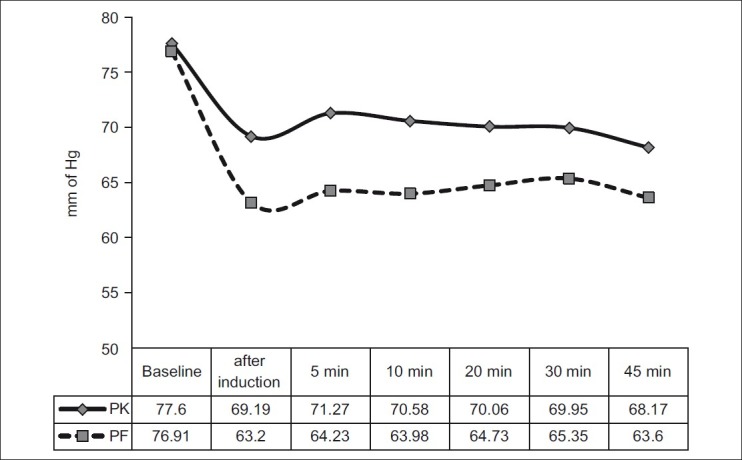
Intraoperative mean arterial pressure between the two groups
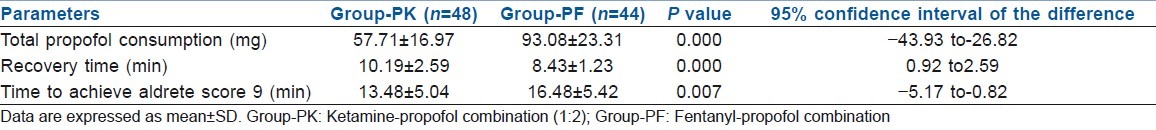
Total drug consumed in terms of calculated propofol was higher in group PF compared with group PK (93.08±23.31 mg and 57.71±16.97 mg, respectively, P=0.000). Recovery times were significantly longer in group PK compared with group PF (10.19±2.59 min and 8.43±1.23 min, respectively, P=0.000). But, the time to achieve Aldrete Recovery Scale Score of 9 is significantly higher in group PF than in group PK (16.48±5.42 min and 13.48±5.04 min, P=0.007) [Table 3].
Table 3.
Propofol consumption and recovery profile
DISCUSSION
In the present study, hypotension occurred in 17 of 44 patients (38.63%) in group PF compared with seven of 48 patients (14.58%) in group PK. The ketamine–propofol combination is thought to act by counteracting the side-effects of each other, preserving the sedative efficacy. The present study also showed that the amount of propofol needed to achieve a deep sedation level was much lower in case of the ketamine–propofol group than in case of the fentanyl–propofol group, which contributed to the lower incidence of hypotension and apnoea. Akin and colleagues found better maintenance of MAP without prolonging recovery in the ketamine–propofol (3:1) combination group than in the propofol monotherapy group.[11]
In the current study, continuous infusion was used to maintain a steady state sedation level. To quantify the level of sedation, the RSS score has been used. This score is simple, easy to use and especially useful in any set-up where monitors measuring the consciousness level like electroencephalography (EEG) and bispectral index (BIS) are absent. It is difficult to maintain moderate sedation due to inter-individual variation in central nervous system sensitivity and drug-redistribution. Antisialogogue was avoided to prevent drying up of bronchial gland secretion and undue increase in dead space. LMA, like tracheal tube, bypasses the physiological humidification. Maintenance of normal salivary secretion was used as a natural lubricant for LMA insertion. Regular checks for any abnormal accumulation of secretions were performed. No immediate post-operative adverse events like breath holding, laryngospasm, bronchospasm or peripheral arterial haemoglobin oxygen desaturation have been observed in the present study in spite of not using any antisialogogue.
In the present study, improved ventilation (evident by lower EtCO2 value) was seen in case of the ketamine–propofol group than in case of the fentanyl–propofol group. This may be due to ketamine-induced sympathoadrenal activation. Simultaneous use of EtCO2 and pulse oximeter helped us to quickly detect the incidences of apnoea and thus prevent any significant desaturation. Capnography has been recommended as a mandatory monitoring during sedation.[12]
Ketamine and propofol have been shown to be pharmaceutically compatible when mixed together in the same syringe.[13] The mixture of ketamine and propofol into a single syringe in a 1:2 ratio offers a simple and practical approach to medication preparation and use. Several authors[3,14,15] have used ketamine–propofol combinations in various ratios (1:1 to 1:5). All such combinations maintained haemodynamic stability. Increased discharged time was found where a higher proportion of ketamine was used. In the present study, the recovery time in group PK was within the acceptable range apparently but was significantly longer than that of group PF. Slower clearance of ketamine in comparison with fentanyl is probably responsible for this. Higher incidence of apnoea has been observed intraoperatively in group PF. This higher incidence of respiratory depression and hypotension in group PF might have contributed to the late achievement of the Aldrete score of 9, because this score consists of parameters like respiration and circulation besides other parameters (activity, consciousness and colour).
Emergence reactions and vomiting are considered to be significant adverse effects of ketamine usage, occurring more often in adults than in children.[16] Although there is a higher incidence of emergence reaction and PONV in group PK compared with group PF, this incidence rate is lower than the usual incidence rate of ketamine alone. Emergence phenomena as high as 50% in adults has been reported by others.[17] This can probably be explained by the counteraction of propofol, with its sedative and antiemetic properties, which reduced the overall incidence rates of both these adverse events of ketamine.[15,16] Nil per oral (NPO) is usually a part of sedation policies. But, the emergency department did not have the luxury of prolonged NPO status. Recent studies also found no association between the period of fasting and the incidence of adverse events.[1,18]
Positive outcome of this study can be expressed in the form of number-needed-to-treat (NNT). While calculating NNT, it was approximately four. Therefore, four extra patients in group PF would need to be treated with newer regimen ketamine–propofol (1:2) to achieve prevention of hypotension in one patient.
CONCLUSION
The current study concludes that the ketamine–propofol combination provides better sedoanalgesia and decreases the incidence of hypotension than the fentanyl-propofol combination. Low-dose ketamine–propofol infusion is a more effective and a safer sedoanalgesia regimen than propofol–fentanyl infusion in paediatric emergency short surgical procedures in terms of haemodynamic stability and lesser incidence of apnoea. Although the compatibility of the ketamine–propofol mixture in the same syringe has been studied by other authors previously, the physical stability of ketamine-propofol mixtures of different ratios and maintenance of that stability over time are yet to be elucidated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Krauss B, Green SM. Procedural sedation and analgesia in children. Lancet. 2006;367:766–80. doi: 10.1016/S0140-6736(06)68230-5. [DOI] [PubMed] [Google Scholar]
- 2.Cravero JP, Beach ML, Blike GT, Gallagher SM, Hertzog JH. The incidence and nature of adverse events during pediatric Sedation/Anesthesia with propofol for procedures outside the operating room: A Report From the Pediatric Sedation Research Consortium. Anesth Analg. 2009;108:795–804. doi: 10.1213/ane.0b013e31818fc334. [DOI] [PubMed] [Google Scholar]
- 3.Badrinath S, Avramov MN, Shadrick M, Witt TR, Ivankovich AD. The use of a ketamine-propofol combination during monitored anesthesia care. Anesth Analg. 2000;90:858–62. doi: 10.1097/00000539-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Frey K, Sukhani R, Pawlowski J, Pappas AL, Mikat-Stevens M, Slogoff S. Propofol versus propofol-ketamine sedation for retrobulbar nerve block: Comparison of sedation quality, intraocular pressure changes, and recovery profiles. Anesth Analg. 1999;89:317–21. doi: 10.1097/00000539-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Mortero RF, Clark LD, Tolan MM, Metz RJ, Tsueda K, Sheppard RA. The effects of small-dose ketamine on propofol sedation: Respiration, postoperative mood, perception, cognition, and pain. Anesth Analg. 2001;92:1465–9. doi: 10.1097/00000539-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Sharieff GQ, Trocinski DR, Kanegaye JT, Fisher B, Harley JR. Ketamine-propofol combination sedation for fracture reduction in the pediatric emergency department. Pediatr Emerg Care. 2007;23:881–4. doi: 10.1097/pec.0b013e31815c9df6. [DOI] [PubMed] [Google Scholar]
- 7.Tosun Z, Esmaoglu A, Coruh A. Propofol-ketamine vs propofol-fentanyl combinations for deep sedation and analgesia in pediatric patients undergoing burn dressing changes. Pediatr Anesth. 2008;18:43–7. doi: 10.1111/j.1460-9592.2007.02380.x. [DOI] [PubMed] [Google Scholar]
- 8.Messenger DW, Murray HE, Dungey PE, Vlymen JV, Sivilotti Marco LA. Subdissociative-dose ketamine versus fentanyl for analgesia during propofol procedural sedation: A randomized clinical trial. Acad Emerg Med. 2008;15:877–86. doi: 10.1111/j.1553-2712.2008.00219.x. [DOI] [PubMed] [Google Scholar]
- 9.Goh PK, Chiu CL, Wang CY, Chan YK, Loo PL. Randomised double-blind comparison of ketamine-propofol, fentanyl-propofol and propofol-saline on haemodynamic and laryngeal mask airway insertion conditions. Anaesth Intensive Care. 2005;33:223–8. doi: 10.1177/0310057X0503300211. [DOI] [PubMed] [Google Scholar]
- 10.Junzheng WU. Deep sedation with intravenous infusion of combined propofol and ketamine during dressing changes and whirlpool bath in patients with severe epidermolysis bullosa. Pediatr Anesth. 2007;17:592–6. doi: 10.1111/j.1460-9592.2006.02177.x. [DOI] [PubMed] [Google Scholar]
- 11.Akin A, Esmaoglu A, Guler G, Demircioglu R, Narin N, Boyaci A. Propofol and propofol ketamine in pediatric patients undergoing cardiac catheterization. Pediatr Cardiol. 2005;26:553–7. doi: 10.1007/s00246-004-0707-4. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Pediatrics Committee on Drugs, “Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures”. Pediatrics. 1992;89:1110–5. [PubMed] [Google Scholar]
- 13.Trissel LA, Gilbert DL, Martinez JF. Compatibility of propofol injectable emulsion with selected drugs during simulated Y-site administration. Am J Health Syst Pharm. 1997;54:1287–92. doi: 10.1093/ajhp/54.11.1287. [DOI] [PubMed] [Google Scholar]
- 14.Akin A, Esmaoglu A, Tosun Z, Gulcu N, Aydogan H, Boyaci A. Comparison of propofol with propofol-ketamine combination in paediatric patients undergoing auditory brainstem response testing. Int J Pediatr Otorhinolaryngol. 2005;69:1541–5. doi: 10.1016/j.ijporl.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Willman EV, Andolfatto G. A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;49:23–30. doi: 10.1016/j.annemergmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Green SM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation in children. Ann Emerg Med. 2004;44:460–71. doi: 10.1016/S0196064404006365. [DOI] [PubMed] [Google Scholar]
- 17.Chudnofsky CR, Weber JE, Stoyanoff PJ, Colone PD, Wilkerson MD, Hallinen DL, et al. A combination of midazolam and ketamine for procedural sedation and analgesia in adult emergency department patients. Acad Emerg Med. 2000;7:228–35. doi: 10.1111/j.1553-2712.2000.tb01064.x. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal D, Manzi SF, Gupta R, Krauss B. Preprocedural fasting state and adverse events in children undergoing procedural sedation and analgesia in a pediatric emergency department. Ann Emerg Med. 2003;42:636–46. doi: 10.1016/s0196-0644(03)00516-x. [DOI] [PubMed] [Google Scholar]


