Abstract
Patients with chronic obstructive pulmonary disease (COPD) are susceptible to airway malacia, which may be unmasked following mechanical ventilation or tracheostomy decannulation. Dynamic imaging of central airways, a non-invasive test as effective as bronchoscopy to diagnose airway malacia, has increased the recognition of this disorder. We describe a 70-year-old woman admitted with adult respiratory distress syndrome. She had cardiorespiratory arrest on admission, from which she was successfully resuscitated. She had obesity, hypertension, diabetes mellitus, recurrent ventricular tachycardia, sarcoidosis with interstitial lung disease and COPD. She received short-term (18 days) mechanical ventilation with tracheostomy and developed respiratory distress following tracheostomy decannulation.
Keywords: Adult respiratory distress syndrome, chronic obstructive pulmonary disease, tracheomalacia, tracheostomy
INTRODUCTION
Tracheomalacia is an abnormal collapse of the tracheal wall characterized by flaccidity of the supporting tracheal cartilages and reduction of the anteroposterior diameter of the trachea. This pathologic narrowing can produce dynamic outflow obstruction with symptoms such as dyspnoea, orthopnoea, cough, wheezing and inability to clear secretions, predisposing the patient to recurrent infections and wheezing. There is a strong association between airway malacia and chronic obstructive pulmonary disease (COPD),[1] and it is frequently seen in patients with chronic bronchitis and emphysema.[2] The incidence may be as high as 23% among the patients with COPD undergoing bronchoscopy.[3] Dynamic imaging of the central airways, a non-invasive test as effective as bronchoscopy to diagnose airway malacia, has increased the recognition of this disorder.[1,4] We report an elderly woman admitted with adult respiratory distress syndrome (ARDS) who received short-term mechanical ventilation with tracheostomy and developed respiratory distress following tracheostomy decannulation due to severe tracheomalacia.
CASE REPORT
A 70-year-old obese woman (weight=85 kg, body mass index=33.2 kg/m2) was admitted to our institute with a history of cough, cold and breathlessness since 10 days. She was on antibiotics prescribed by the primary physician. On examination in the emergency department, she was conscious, drowsy, tachypneic (respiratory rate=45/min) and cyanosed. Her pulse rate was 136/min, blood pressure was 170/110 mmHg, arterial oxygen saturation measured by pulse oximeter was 78% and heart sounds were muffled on auscultation. The respiratory system examination showed a grossly reduced bilateral air entry with silent chest. She was a known diabetic for the past 20 years and was currently on treatment with insulin. She was on Tab. Ramipril 2.5 mg once daily for essential hypertension for the past 10 years. She was a biopsy-proven case (hilar lympadenopathy) of sarcoidosis with interstitial lung disease and COPD, for which she was on salbutamol and budesonide metered-dose inhaler. There was no history of smoking. Quinidine had been used 10 years back for treating ventricular tachycardia but, presently, she did not need treatment for the same. Coronary angiography performed at that time failed to reveal any perfusion-related problem. On further enquiry, her husband revealed that she used to sleep in a sitting position for the past 6 months. Her recent travel history included visits to places with a high risk of Swine flu (H1N1).
She was administered O2 12 L/min by a non-rebreathing mask, salbutamol nebulization, intravenous deriphylline and hydrocortisone. Her initial blood gas analysis showed pH=7.13, PaO2=111 mmHg, PaCO2=47.6 mmHg, bicarbonate=14, base deficit=–13, oxygen saturation=95.7%, Na+=131 meq/L and K+=5.89 meq/L. A chest X-ray showed diffuse infiltrates in both the lung fields. After about 45 min, she developed severe bradycardia followed by cardiac arrest and one episode of convulsion. She was successfully resuscitated and put on mechanical ventilation with pressure control mode and dopamine infusion (5 μg/kg/min). She was shifted to the surgical intensive care unit (SICU) for further management.
Possibility of Swine flu, community-acquired pneumonia and infection by atypical organisms was considered at this stage. Samples for bacterial and viral (H1N1) culture were taken from the throat, nasopahrynx, trachea and blood. Right subclavian central venous and radial artery catheters were secured. Central venous pressure and invasive blood pressure monitoring were initiated. Broad-spectrum antibiotics, bronchodilators, oseltamavir (75 mg twice daily for 5 days through a nasogastric tube), methyl prednisolone (125 mg intravenously, thrice daily), low-molecular weight heparin and sedation were started. She developed two episodes of severe bronchospasm on separate occasions following tracheal suctioning that required treatment with inhaled salbutamol, ipratropium bromide, adrenaline and infusion of intravenous lignocaine and magnesium sulphate. Results of the laboratory tests ruled out H1N1 infection.
On the 4th day, her blood gas improved (pH=7.37, PaO2=70 mmHg, PaCO2=44 mmHg, bicarbonate=24, base deficit=–0.4 and oxygen saturation=93% on 50% O2 with PEEP=8 cmH2O). Her chest X-ray showed bilateral pericardiac/perihilar interstitial inhomogeneous opacities with waxing/waning airspace consolidation. The oral endotracheal tube was changed to nasal; she was weaned from sedation and ventilation and put on pressure support ventilation. On the 6th day, the trachea was extubated and she was put on non-invasive ventilation, which she tolerated only for 2 h. She was reintubated and put back on mechanical ventilation. A surgical tracheostomy was performed on the next day in view of anticipated difficulty in weaning and for better pulmonary care. Six days after the tracheostomy, the tube was changed to a silicon tracheostomy tube. Aggressive chest physiotherapy was started. On the 11th day, the chest X-ray showed segmental collapse of the right lower lobe. The tracheal aspirate grew Acinetobacter, and antibiotics were changed accordingly. An echocardiography showed concentric left ventricular hypertrophy, moderate pulmonary artery hypertension (56 mmHg) and 60% left ventricle ejection fraction.
She was successfully weaned from the ventilator and put on T-piece on the 18th day and transferred to the ward 4 days later after decannulating tracheostomy. Three days later, she started developing intermittent stridor on exertion. She was evaluated for the cause of stridor. An indirect laryngoscopy showed a normal upper airway and vocal cords. A lateral neck X-ray showed soft tissue swelling in the region of thyroid gland and tracheal narrowing just below the site of the tracheostomy. The computed tomography (CT) scan [Figures 1 and 2] demonstrated a short segment narrowing of the trachea (luminal caliber 0.8 cm × 0.7 cm) just distal to the partially closed tracheostomy stoma. There appeared a definitive bulge into the tracheal lumen at the above-mentioned region, both from the anterior as well as the posterior walls. An abnormal thyroid encircling the trachea (above the stoma) was also noted without any tracheal narrowing at that level.
Figure 1.
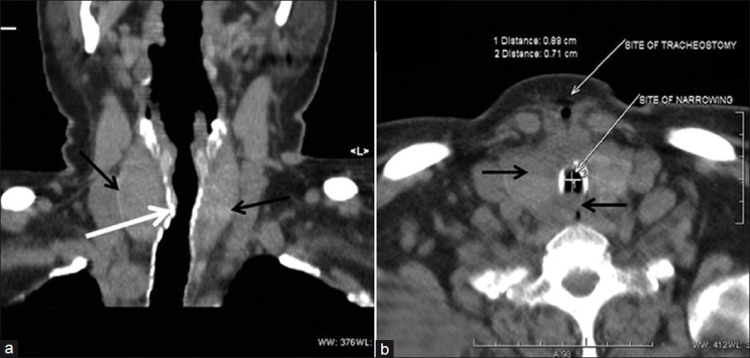
Neck computed tomography scan: Coronal (a) and transverse section (b) showing tracheal narrowing (white arrows) and thyroid gland (black arrow) abnormally encircling the trachea
Figure 2.
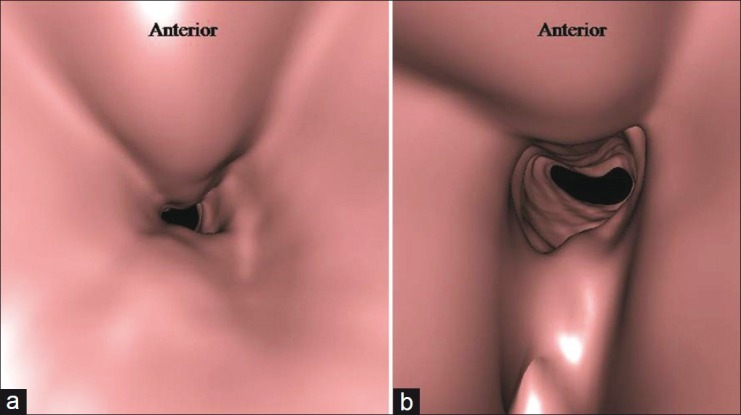
Computed tomography with virtual bronchoscopy. Severe narrowing of the trachea just distal to the tracheostomy site
She was shifted to another centre which dealt with tracheal problems. She developed severe stridor there and had to be intubated emergently. She was subjected to a bronchoscopic evaluation, which showed a long-segment tracheomalacia up to the carina with dynamic collapse of the anterior tracheal wall. The patient was offered the option of tracheal stenting or conservative management, but the relatives of the patient opted for the latter. At the time of writing this report (1 year later), the patient was on conventional T-tube and was doing well at home. The patient gave consent for publishing this report.
DISCUSSION
A number of late complications of tracheostomy have been recognised, including the formation of granulation tissue, tracheal stenosis, tracheomalacia, tracheoinnominate artery fistula, tracheoesophageal fistula, ventilator-associated pneumonia and aspiration.[5] Tracheobronchomalacia is a dynamic form of central airway obstruction characterized by an expiratory decrease of 50% or more in the cross-sectional area of the tracheobronchial lumen.[1] Tracheomalacia may be primary or secondary.[6] COPD is the most common cause of adult tracheomalacia.[7] The prevalence of airway malacia in patients with COPD has been reported to be up to 53%.[1] Current treatments include techniques that splint the central airways, such as continuous positive airway pressure,[8] airway stents[9] and surgical tracheobronchoplasty[7] or tracheal resection. Conservative management may be the best approach in mild cases.[5]
In patients who have undergone prolonged mechanical ventilation, tracheomalacia results from an ischaemic injury to the tracheal cartilages with subsequent destruction and necrosis. These patients usually present with dyspnoea weeks to months after tracheostomy decannulation. They may also present as failure to wean from mechanical ventilation in the acute setting.[5] Also, tracheomalacia may be underdiagnosed because of the presentation with a variety of non-specific symptoms similar to patients with asthma and COPD.[10] However, early tracheomalacia as a result of tracheal ring fracture during percutaneous dilatational tracheostomy, which presented as difficult decannulation about a month after tracheostomy, has been reported.[11] A case of concealed tracheomalacia leading to airway obstruction immediately after tracheal intubation for elective operation has been reported and, here too, the patient had COPD.[12] In a study examining the pathologic changes after percutaneous tracheostomy, autopsies of patients showed mucosal ulceration and necrosis of the tracheal cartilage beyond the tracheostomy site as early as 10 days after the procedure.[13]
Timely diagnosis of tracheomalacia in a mechanically ventilated patient requires a high index of suspicion. Bronchoscopy has been considered the “gold standard” in diagnosing tracheomalacia.[6,14,15] Fluoroscopy may demonstrate dynamic collapse.[6] Dynamic expiratory CT scan has gained acceptance as a reliable non-invasive diagnostic tool.[14,15] CT may yield false-negative evidence in patients who have an endotracheal/tracheostomy tube in situ during imaging.[14] Variable intrathoracic obstruction may be evident on flow-volume loop in a spontaneously breathing patient with tracheobronchomalacia.[5]
In our patient, initially, we had attributed her history of orthopnoea to sarcoidosis with interstitial lung disease and COPD. Clinical features of this patient following her discharge from the SICU suggested a tracheal narrowing. Our initial concerns were about the tracheal stenosis or intermittent obstruction by the tracheal ring flap. We did not expect tracheomalacia to develop so early because of the short duration (18 days) of mechanical ventilation.
We decided not to subject her for bronchoscopy in our institute and shifted her to another centre in which tracheal problems are managed regularly. This decision was based on the following reasons: (1) our centre was not specialized in managing such patients, (2) bronchoscopy would have been essentially a diagnostic procedure in our institute and (3) the patient's susceptibility to reactive airway disease, which could have precipitated another episode of bronchospasm during bronchoscopy.
In summary, it was a challenging case to manage in view of the ARDS and her past history of diabetes mellitus, hypertension, sarcoidosis with interstitial lung disease, COPD, recurrent ventricular tachycardia and obesity. Management of this patient required a multidisciplinary coordination involving the anaesthesiologist, physician, cardiologist, pulmonologist, otorhinolaryngologist, radiologist and physiotherapist.
CONCLUSION
Patients with COPD are susceptible to airway malacia, which may be unmasked following mechanical ventilation or tracheostomy decannulation. Appropriate imaging studies or a diagnostic fibreoptic bronchoscopy helps to identify the problem and plan the management.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sverzellati N, Rastelli A, Chetta A, Schembri V, Fasano L, Pacilli AM, et al. Airway malacia in chronic obstructive pulmonary disease: Prevalence, morphology and relationship with emphysema, bronchiectasis and bronchial wall thickening. Eur Radiol. 2009;19:1669–78. doi: 10.1007/s00330-009-1306-9. [DOI] [PubMed] [Google Scholar]
- 2.Jokinen K, Palva T, Sutinen S, Nuutinen J. Acquired tracheobronchomalacia. Ann Clin Res. 1977;9:52–7. [PubMed] [Google Scholar]
- 3.Jokinen K, Palva T, Nuutinen J. Chronic bronchitis: A bronchologic evaluation. ORL J Otorhinolaryngol Relat Spec. 1976;38:178–86. doi: 10.1159/000275273. [DOI] [PubMed] [Google Scholar]
- 4.Kandaswamy C, Balasubramanian V. Review of adult tracheomalacia and its relationship with chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2009;15:113–9. doi: 10.1097/MCP.0b013e328321832d. [DOI] [PubMed] [Google Scholar]
- 5.Epstein SK. Late complications of tracheostomy. Respir Care. 2005;50:542–9. [PubMed] [Google Scholar]
- 6.Finder JD. Bronchomalacia and Tracheomalacia. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson Textbook of Pediatrics. 18th ed. Philadelphia, PA: Saunders, Elsevier; 2008. pp. 1771–2. [Google Scholar]
- 7.Wright CD. Tracheomalacia. Chest Surg Clin N Am. 2003;13:349–57. doi: 10.1016/s1052-3359(03)00036-x. [DOI] [PubMed] [Google Scholar]
- 8.Fergusson GT, Benoist J. Nasal continuous positive airway pressure in the treatment of tracheobronchomalacia. Am Rev Respir Dis. 1993;147:457–61. doi: 10.1164/ajrccm/147.2.457. [DOI] [PubMed] [Google Scholar]
- 9.Sommer D, Forte V. Advances in the management of major airway collapse: The use of airway stents. Otolaryngol Clin North Am. 2000;33:163–77. doi: 10.1016/s0030-6665(05)70213-9. [DOI] [PubMed] [Google Scholar]
- 10.Murgu SD, Colt HG. Treatment of adult tracheobronchomalacia and excessive dynamic airway collapse: An update. Treat Respir Med. 2006;5:103–15. doi: 10.2165/00151829-200605020-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ho EC, Kapila A, Colquhoun-Flannery W. Tracheal ring fracture and early tracheomalacia following percutaneous dilatational tracheostomy. BMC Ear Nose Throat Disord. 2005;5:6. doi: 10.1186/1472-6815-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Athanassiou L, Charissi N. Tracheal tube obstruction in a case of concealed tracheomalacia–a case report. Middle East J Anesthesiol. 2005;18:401–5. [PubMed] [Google Scholar]
- 13.van Heurn LW, Theunissen PH, Ramsay G, Brink PR. Pathologic changes of the trachea after percutaneous dilatational tracheotomy. Chest. 1996;109:1466–9. doi: 10.1378/chest.109.6.1466. [DOI] [PubMed] [Google Scholar]
- 14.Sun M, Ernst A, Boiselle PM. MDCT of the central airways: Comparison with bronchoscopy in the evaluation of complications of endotracheal and tracheostomy tubes. J Thorac Imaging. 2007;22:136–42. doi: 10.1097/01.rti.0000213579.24527.6c. [DOI] [PubMed] [Google Scholar]
- 15.Baroni RH, Ashiku S, Boiselle PM. Dynamic CT evaluation of the central airways in patients undergoing tracheoplasty for tracheobronchomalacia. AJR Am J Roentgenol. 2005;184:1444–9. doi: 10.2214/ajr.184.5.01841444. [DOI] [PubMed] [Google Scholar]


