Abstract
Operative hysteroscopy has emerged as an effective alternative to hysterectomy and has become standard surgical treatment for varied gynaecological conditions like abnormal uterine bleeding and uterine myomas. This procedure requires distention of the uterine cavity for adequate visualization of the operative field. 1.5% glycine is a widely used distention medium because it has good optical properties and is non-conductive. However, the intraoperative absorption of this electrolyte-free fluid can cause hyponatraemia, hypoosmolality, hyperglycinaemia and volume overload, including pulmonary oedema. We report a case of operative hysteroscopy intravascular absorption (OHIA) syndrome, presenting abruptly during hysteroscopic myomectomy, employing 1.5% glycine as the fluid distention medium. Successful management of the case and prevention strategies are discussed.
Keywords: Dilutional hyponatraemia, glycine fluid overload, operative hysteroscopy intravascular absorption syndrome, transurethral resection of prostate syndrome
INTRODUCTION
Operative hysteroscopy is now routinely employed for endometrial ablation, septum resection, myomectomy and polypectomy. However, it has the potential for serious complications like uterine perforation, haemorrhage, gas or air embolism, sepsis and fluid overload with hyponatraemia.[1]
Liquid distension media like 1.5% glycine are used for optimal visualization during hysteroscopy. Absorption of an excessive amount of this irrigation fluid may rarely lead to fluid overload and attendant toxicity syndrome called Operative Hysteroscopy Intravascular Absorption (OHIA) syndrome.[2] It is similar to the well known entity encountered in endoscopic urology, viz. Transurethral Resection of Prostate (TURP) syndrome; hence, the alternate term gynaecological TURP syndrome.[3]
Although the classic syndrome itself is rare (<1% incidence), its sudden intraoperative presentation is exceptional.[4]
CASE REPORT
A 57-year-old post-menopausal female presented with bleeding per vaginum for 2 months and was posted for hysteroscopic myomectomy under general anaesthesia (GA). She had associated diabetes mellitus Type 2, dyslipidaemia and hypothyroidism and was controlled on medical management. Pre-operative assessment revealed a normally built lady with weight of 58 kg. Routine investigations were normal, with optimized blood glucose levels and normal thyroid profile. She refused regional anaesthesia and was accepted for GA in American Society of Anaesthesiologists grade II.
Premedication consisted of morphine 3 mg intravenous (IV), glycopyrrolate 0.2 mg IV and ondansetron 4 mg IV. Anaesthesia was induced with inj propofol 100 mg slow IV followed by succinylcholine 75 mg IV to facilitate endotracheal intubation employing a 7.5 mm cuffed endotracheal tube (ETT) - Portex. Anaesthesia was maintained with 60% nitrous oxide and 1% isoflurane in oxygen and IV vecuronium. Normal saline solution was given at 250 mL/h, and a total of 500 mL of this was administered. At the beginning of surgery, her intraoperative vitals were stable and she was monitored with electrocardiography (ECG), non-invasive blood pressure (NIBP), end tidal carbon dioxide (EtCO2), pulse oximetry (SpO2) and temperature.
Hysteroscopic examination revealed a large submucous fibroid measuring 5 cm×4 cm arising from the right lateral uterine wall. Resection of the fibroid was started after distending the uterine cavity with 1.5% glycine employing Karl Storz hysteroscope and maximum 80 mmHg intrauterine pressure. After 45 min of surgery (2/3rd of fibroid resected), the SpO2 suddenly fell from 98% to 88–90% (FiO2 1.0). There was concomitant rise in airway pressure, diminished breath sounds, NIBP 90/70 mmHg and pulse rate 100/min. Subsequently, pink frothy secretions appeared in the ETT and crackles were heard throughout both lung fields, substantiating a clinical diagnosis of acute pulmonary oedema. The procedure was stopped after haemostasis. Inj lasix 40 mg IV was given with morphine 3 mg IV. A nasogastric tube was passed to decompress the abdomen. The bladder was catheterized, with a urine output of 100 mL.
A 12 lead ECG was normal. Arterial blood gas (ABG) revealed pO2: 165.3 mmHg, pCO2: 50.5 mmHg, pH: 7.17, K+: 5.3 mmol/L, Na+: 100 mmol/L, HCO3-: 17.8 mmol/L, BE: –8.2 mmol/L and O2 Sat: 98.6% (FiO2 1.0). A portable chest radiograph revealed perihilar interstitial infiltrates and increase in the pulmonary vasculature, suggestive of pulmonary oedema [Figure 1]. The measured glycine deficit was approx 1 L (glycine used minus efflux via resectoscope, perineal drapes, suction) and clinical deterioration was ascribed to glycine fluid overload in view of glycine deficit, hyponatraemia, metabolic acidosis and pulmonary oedema.
Figure 1.
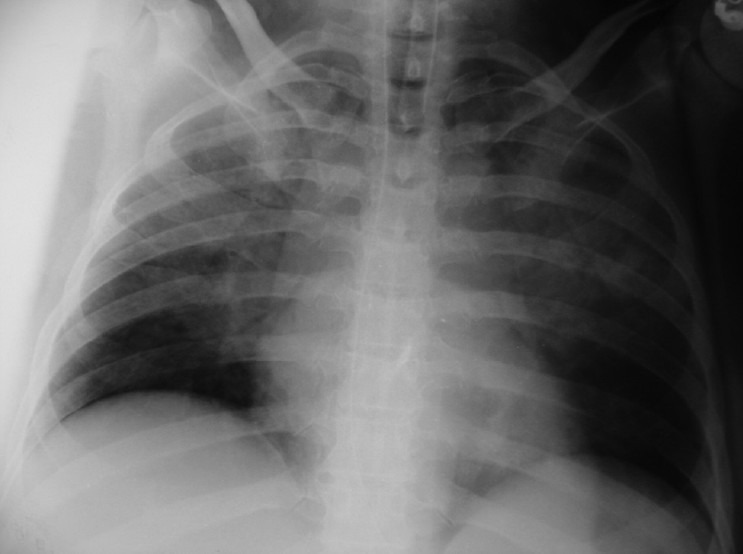
Pulmonary oedema
Right internal jugular vein and left radial artery were cannulated for haemodynamic monitoring and ABG analysis. Initial central venous pressure was 10 cmH2O. The patient was shifted to the intensive care unit for elective mechanical ventilation (SIMV mode, FiO2:1) with IV morphine sedation. Sixty milliliters of 7.5% soda bicarbonate was given slow IV, 3% sodium chloride IV was started at 60 mL/h and inotropes were infused for persistent hypotension. An urgent echocardiogram was normal. Serial ABG were performed to monitor serum Na+ levels and, when the target level of 120 mmol/L was reached (over the next 12 h), 3% NaCl was stopped.
By the evening, the patient developed profuse bleeding per vaginum and endometrial tamponade was done with Foley's catheter. Disseminated intravascular coagulation (DIC) was suspected as she began oozing from other sites, with the nasogastric tube showing a coffee ground aspirate. Her coagulation profile was deranged: Haemoglobin 5 gm%, platelet count 90,000/mm3, prothrombin time 22 s, partial thromboplastin time 68 s, International Normalized Ratio 1.94 and d-dimer positive with hypocalcaemia (7.6 gm/dL). Fresh blood with blood products (fresh frozen plasma, cryoprecipitate) were transfused along with IV calcium.
By day 2, her coagulation profile improved and inotropic support was tapered off. SIMV mode ventilation with pressure support and progressive FiO2 reduction was followed with a continuous positive airway pressure trial for 1 h and uneventful extubation, i.e. within 24 h of initiating ventilation. However, the patient became tachypnoeic on Day 3, with respiratory rate 36/min and bilateral wheeze with SpO2 of 85% (FiO2 1.0). Chest X-ray showed opacities in left lower zone suggestive of pneumonitis [Figure 2]. Fibreoptic bronchoscopy revealed long tubular casts in the left bronchial tree, which were removed. Thereafter, she was managed with asthalin nebulization, chest physiotherapy, BIPAP support and broad-spectrum antibiotics as Pseudomonas aeruginosa was grown on blood culture.
Figure 2.
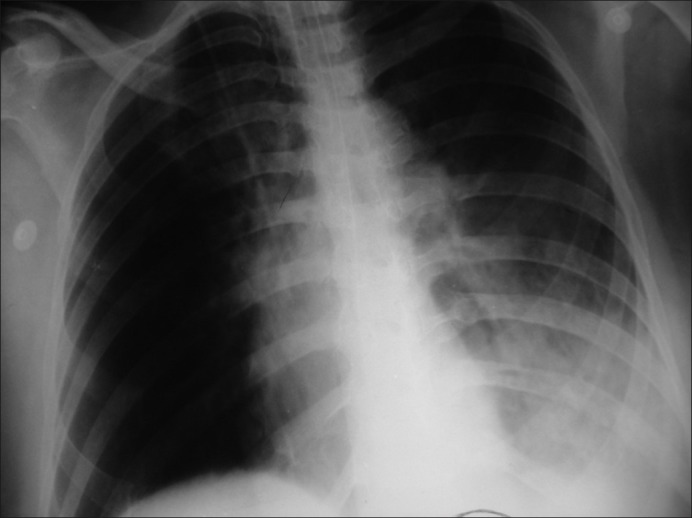
Pneumonitis left
On day 6, the patient developed cramps in both legs with bilateral foot drop. Nerve conduction studies revealed common peroneal nerve palsy, which was successfully managed with physiotherapy. The rest of the hospital stay was uneventful and she was discharged to home on Day 14 and remained asymptomatic on follow-up.
DISCUSSION
There is a spectrum of adverse events associated with operative hysteroscopy. These may relate to patient positioning, uterine perforation, intraoperative bleeding, sepsis, anaesthetic complications and use of distending media (fluid overload, electrolyte disturbance, gas emboli).[5] During gynaecological surgery, fluid absorption occurs more often with resection of fibroid, with pre-menopausal women at higher risk for neurologic sequelae from even modest hyponatraemia, as sex hormones adversely affect the sodium pump in brain cells leading to cerebral oedema.[4,5] The frequency of complications of hysteroscopic surgery is reported to be as low as 0.24%,[6] but may go up to 10%[7] with more complicated surgeries like hysteroscopic myomectomy.
Although OHIA syndrome has a similar pathogenesis as TURP syndrome, the uterine wall is thicker and requires higher distending pressures. The pressure in large venous sinuses in the myometrium is 10–15 mmHg, while the intrauterine pressure for distention and flow is generally kept above 40–60 mmHg. Intracavitary pressure in excess of mean arterial pressure, i.e. 80 mmHg (or 40 inches height of water), can result in rapid intravasation.[3,4] Measurement of fluid absorption however remains capricious. The absorbed volume of fluid employed for uterine distention depends on the extent of transection of vascular beds, intrauterine distention pressures, duration of the procedure and surgical experience.[8] Accurate intake output estimates have also to contend with 5–10% more irrigating fluid in packed containers and “returning” fluid being lost in drapes or on the floor. The present case had measured absorption of about 1 L, but higher amounts were likely absorbed. In fact, absorption of small amounts (1–2 L) occurs in 5–10% of patients with a mild easily overlooked TUR syndrome, while the classic syndrome develops in <1% with intravascular absorption in excess of 2 L. Severe TUR syndrome following 1.5% glycine absorption is associated with a mortality of 25%.[4]
1.5% glycine is commonly used as irrigating fluid as it is non-conductive and non-haemolytic, with a refractive index close to that of water. However, it is hypotonic (osmolarity 200 mOsml/L). The clinical presentation of glycine toxicity and hyponatraemia may be difficult to distinguish from sepsis or DIC, and usually manifests 30–45 min after completion of surgery.[9] Awake patients may initially complain of prickling sensation in face and neck, restlessness, headache, visual disturbances while bradycardia, hypotension and decreasing oxygen saturation are consistent early signs. Pulmonary oedema usually presents after end of surgery, although, rarely, it may occur on the operating table, particularly where the blood loss is not significant.[4] Transient bilateral common peroneal nerve palsy in our patient reinforces the importance of careful lithotomy positioning, especially in prolonged or repeat surgery.
Treatment of glycine intoxication and fluid overload should start early. Besides supportive measures, IV hypertonic saline (commonly 3%) should be given for Na+ <120 mmol/L, keeping the rate of sodium replacement <1 mmol/L/h as rapid correction may result in pontine myelinolysis. The main indication of IV furosemide is to treat acute pulmonary oedema or induce diuresis where this does not occur spontaneously.[4]
Prevention of fluid overload is of prime importance. Volumetric fluid balance and gravimetry are traditional methods for assessment of fluid absorption during resectoscopic surgery, and deficits should be calculated at least every 10 min. While manual closed systems of fluid balance may not always be practical or precise, the newer automated fluid management systems may prove to be more accurate, especially for advanced resections like myomectomy, and 5% mannitol is probably safer than other distending media.[5] As with the TURP syndrome, it may be advantageous to use regional anaesthesia because awake patients are likely to display precursor symptoms (akin to a “miner's canary”) that portend impending brain or cardiac dysfunction. During GA, it is advisable to adhere to a pre-determined schedule for sampling patient blood for serum sodium concentration. The parotid area sign[10] (intraoperative increase in philtrum-mastoid prominence distance) and the ethanol breath test[11] employing 1.5% glycine with added 1% ethanol for irrigation have been proposed as early signs of fluid overload during resectoscopic surgery. Bipolar resectoscopes employing normal saline as distending medium and vaporization techniques are being explored as safer alternatives for the future.
CONCLUSION
This report points out the potential for sudden development of glycine fluid overload in hysteroscopic surgery. GA during such surgical procedures precludes the use of patient's mental status as an indicator of glycine intoxication and dilutional hyponatraemia. Anaesthetic management of these otherwise healthy women should underline the prevention of OHIA syndrome and its attendant morbidity and possible mortality.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Musambi MC, Williamson K. Anaesthetic considerations for hysteroscopic surgery. Best Pract Res Clin Anaesthesiol. 2002;16:35–52. doi: 10.1053/bean.2002.0206. [DOI] [PubMed] [Google Scholar]
- 2.Jackson S, Lampe G. Operative hysteroscopy intravascular absorption (OHIA) syndrome. West J Med. 1995;162:53–4. [PMC free article] [PubMed] [Google Scholar]
- 3.Serocki G, Hanss R, Bauer M, Scholz J, Bein B. The gynaecological TURP syndrome.Severe hyponatremia and pulmonary edema during hysteroscopy. Anaesthesist. 2009;58:30–4. doi: 10.1007/s00101-008-1446-3. [DOI] [PubMed] [Google Scholar]
- 4.Hahn RG. Fluid absorption in endoscopic surgery. Br J Anaesth. 2006;96:8–20. doi: 10.1093/bja/aei279. [DOI] [PubMed] [Google Scholar]
- 5.Munro MG. Complications of hysteroscopic and uterine resectoscopic surgery. Obstet Gynecol Clin North Am. 2010;37:399–425. doi: 10.1016/j.ogc.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Aydeniz B, Gruber IV, Schauf B, Kurek R, Meyer A, Wallwiener D. A multicenter survey of complications associated with 21,676 operative hysteroscopies. Eur J Obstet Gynecol Reprod Biol. 2002;104:160–4. doi: 10.1016/s0301-2115(02)00106-9. [DOI] [PubMed] [Google Scholar]
- 7.Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: Predicting patients at risk. Obstet Gynecol. 2000;96:517–20. doi: 10.1016/s0029-7844(00)00958-3. [DOI] [PubMed] [Google Scholar]
- 8.Paschopoulos M, Polyzos NP, Lavasidis LG, Vrekoussis T, Dalkalitsis N, Paraskevaidis E. Safety issues of hysteroscopic surgery. Ann N Y Acad Sci. 2006;1092:229–34. doi: 10.1196/annals.1365.019. [DOI] [PubMed] [Google Scholar]
- 9.Yende S, Wunderink R. An 87-year-old man with hypotension and confusion after cystoscopy. Chest. 1999;115:1449–51. doi: 10.1378/chest.115.5.1449. [DOI] [PubMed] [Google Scholar]
- 10.Sinha M, Hegde A, Sinha R, Goel S. Parotid area sign: A clinical test for the diagnosis of fluid overload in hysteroscopic surgery. J Minim Invasive Gynecol. 2007;14:161–8. doi: 10.1016/j.jmig.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Hall JM, Tingey WR, Gillmer MD. The use of glycine-ethanol irrigating fluid for the early detection of fluid absorption during endometrial resection. Gynaecol Endosc. 2003;6:39–44. [Google Scholar]


