Abstract
Nanotechnology and nanomedicine are complementary disciplines aimed at the betterment of human life. Nanotechnology is an emerging branch of science for designing tools and devices of size 1–100 nm, with unique functions at the cellular, atomic and molecular levels. The concept of using nanotechnology in medical research and clinical practice is known as nanomedicine. Today, nanotechnology and nanoscience approaches to particle design and formulations are beginning to expand the market for many drugs and forming the basis for a highly profitable niche within the industry, but some predicted benefits are hyped. Under many conditions, dermal penetration of nanoparticles may be limited for consumer products such as sunscreens, although additional studies are needed on potential photooxidation products, experimental methods and the effect of skin condition on penetration. Today, zinc oxide and titanium dioxide nanoparticles (20–30 nm) are widely used in several topical skin care products such as sunscreens. Thus, in the present scenario, nanotechnology is spreading its wings to address the key problems in the field of medicine. The benefits of nanoparticles have been shown in several scientific fields, but very little is known about their potential to penetrate the skin. Hence, this review discusses in detail the applications of nanotechnology in medicine with more emphasis on the dermatologic aspects.
Keywords: Nanomedicine, nanoparticles, nanotechnology, silver, skin absorption
Introduction
Nanotechnology is the creation of functional materials, devices and systems through control of matter on the nanometer length scale and exploitation of novel phenomena and properties (physical, chemical, biological, mechanical, electrical) at that length scale. In the area of cosmetics and anti-aging, in particular, as well as in the pharmaceutical arena, nanotechnology has played an important role in delivering active ingredients to the skin, in both patch delivery and timed release application. Nanoparticles/nanospheres sound like futuristic technology[1] The application of nanotechnology concepts to medicine joins two large-scale cross-disciplinary fields with an unprecedented societal and economical potential arising from the natural combination of specific achievements in the respective fields.[2] As the need for the development of new medicines is pressing and given the inherent nanoscale functions of the biological components of living cells, nanotechnology has been applied to diverse medical fields such as oncology and cardiovascular medicine. Indeed, nanotechnology is being used to refine discovery of biomarkers, molecular diagnostics, drug discovery and drug delivery which could be applicable to management of these patients. To achieve these aims, nanotechnology strives to develop and combine new materials by precisely engineering atoms and molecules to yield new molecular assemblies on the scale of individual cells, organelles or even smaller components, providing a personalized medicine.[3,4] Because of increased use of nanotechnology, the risk associated with exposure to nanoparticles, the routes of entry and the molecular mechanisms of any cytotoxicity need to be well understood. In fact, these tiny particles are able to enter the body through the skin, lungs or intestinal tract, depositing in several organs and may cause adverse biological reactions by modifying the physiochemical properties of living matter at the nanolevel.[5–7] Nanotechnology is being applied to biomarker-based proteomics and genomics technologies. Nanoparticles can be used for qualitative or quantitative in vivo or ex vivo diagnosis by concentrating, amplifying and protecting a biomarker from degradation, in order to provide more sensitive analysis.[8] Nanoparticles are currently being tested for molecular imaging to achieve a more precise diagnosis with high-quality images. In fact, contrast agents have been loaded onto nanoparticles for tumor and atherosclerosis diagnosis. Different nanoparticles have been used for molecular imaging with magnetic resonance images (MRI), ultrasound, fluorescence, nuclear, and computed tomography imaging.[9] Nanotechnology is being applied extensively to provide targeted drug therapy, diagnostics, tissue regeneration, cell culture, biosensors and other tools in the field of molecular biology. Various nanotechnology platforms like fullerenes, nanotubes, quantum dots, nanopores, dendrimers, liposomes, magnetic nanoprobes and radio-controlled nanoparticles are being developed.
Nanomedicine
Nanomedicine is the use of nanotechnology to achieve innovative medical breakthroughs. Nanomedicine, with its broad range of ideas, hypotheses, concepts, and undeveloped clinical devices, is still in its early stage. This article outlines the present developments and future prospects for the use of nanotechnology techniques in experimental in vivo and in vitro studies and in engineering nanodevices and biosensors for clinical and investigative use in diagnosis and therapy in the fields of genetics, oncology, cardiology, dermatology and also in the field of medicine, in general, and molecular biology, in particular.[10] The early genesis of the concept of nanomedicine sprang from the visionary idea that tiny nanorobots and related machines could be designed, manufactured, and introduced into the human body to perform cellular repairs at the molecular level. Nanomedicine today has branched out in hundreds of different directions, each of them embodying the key insight that the ability to structure materials and devices at the molecular scale can bring enormous immediate benefits in the research and practice of medicine.[11] Nanomedicine is the application of nanotechnologies in medicine for maintenance and improvement of human life using the knowledge on human organism at a molecular level [Figure 1]. Application of nanoparticles and nanomaterials for the diagnostic and therapeutic purposes is now significantly extended in nanomedicine.[12] Nanomedicine involves utilization of nanotechnology for the benefit of human health and well-being. The use of nanotechnology in various sectors of therapeutics has revolutionized the field of medicine where nanoparticles of dimensions ranging between 1 and 100 nm are designed and used for diagnostics, therapeutics and as biomedical tools for research.[13]
Figure 1.
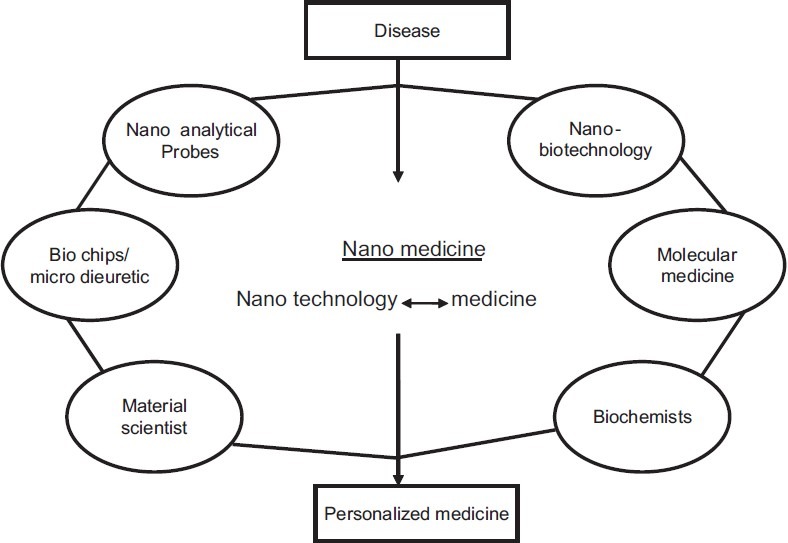
Technologies involved in the field of medicine
Nanotechnology in Dermatology
Today, cosmetic formulation contains nano-sized structures. Nanoemulsions are commonly used in certain cosmetic products such as conditioners or lotions.[14] Many modern cosmetic or sunscreen products contain nano-sized components [Figure 2]. Nanoemulsions are transparent and have unique tactile and texture properties; nanocapsule, nanosome, noisome, or liposome formulations contain small vesicles (range: 50–5000 nm) consisting of traditional cosmetic materials that protect light- or oxygen-sensitive cosmetic ingredients. Transdermal delivery and cosmetic research suggests that vesicle materials may penetrate the stratum corneum (SC) of the human skin, but not into living skin. Modern sunscreens contain insoluble titanium dioxide (TiO2) or zinc oxide (ZnO) nanoparticles (NP), which are colorless and reflect/scatter ultraviolet (UV) more efficiently than larger particles. Also, nanomaterials such as nano-sized vesicles or TiO2 and ZnO nanoparticles which are currently used in cosmetic preparations or sunscreens pose no risk to human skin or human health, although other NP may have properties that warrant safety evaluation on a case-by-case basis before human use.[15] The first generation of lipid nanoparticles was introduced as solid lipid nanoparticles (SLN) and the second, improved generation as nanostructured lipid carriers (NLC). Identical to the liposomes, the lipid nanoparticles appeared as products first on the cosmetic market. The cosmetic benefits of lipid nanoparticles include enhancement of chemical stability of actives, film formation, controlled occlusion, skin hydration, enhanced skin bioavailability and physical stability of the lipid nanoparticles as topical formulations. NLC are on the market as concentrates to be used as cosmetic excipients, special formulation challenges for these products and they also appeared in a number of finished cosmetic products worldwide.[16]
Figure 2.
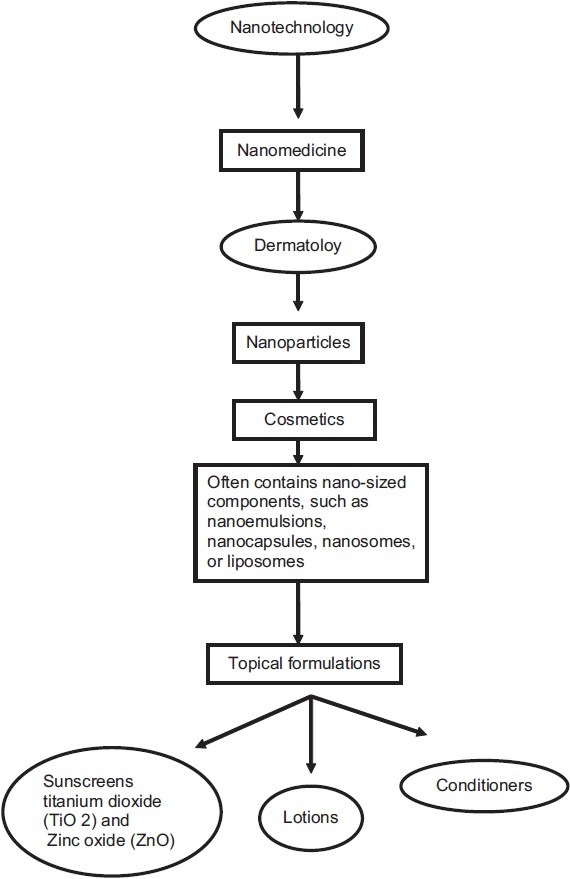
Nanotechnology in dermatology
Due to the lower risk of systemic side effects, topical treatment of skin disease appears favorable, yet the SC counteracts the penetration of xenobiotics into viable skin. Particulate carrier systems may mean an option to improve dermal penetration. Since epidermal lipids are found in high amounts within the penetration barrier, lipid carriers attaching themselves to the skin surface and allowing lipid exchange between the outermost layers of the SC and the carrier appear promising. Besides liposomes, SLN and NLC have been studied intensively.[17] Today, SLN and NLC exhibit many features for dermal application of cosmetics and pharmaceutics, i.e. controlled release of actives, drug targeting, occlusion and penetration enhancement and also increase of skin hydration associated with it [Figure 3].[18] The potential of topically applied NP to penetrate human skin and produce a systemic health risk has been suggested in reviews by Hoet et al.[19]
Figure 3.
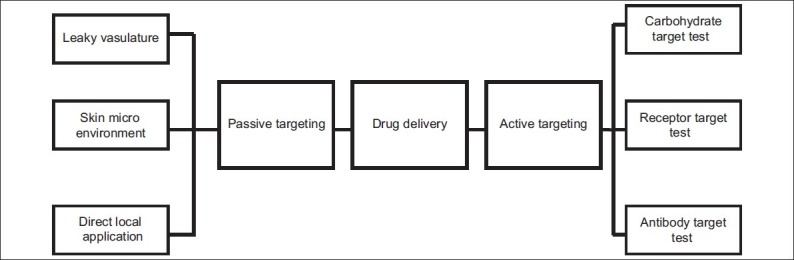
Mechanism of nanoparticle drug delivery via two main mechanisms – passive and active targeting
With advances in nanotechnology, pure silver has been recently engineered into nanometer-sized particles (diameter <100 nm) for use in the treatment of wounds. In conjunction with other studies, it was demonstrated that the topical application of silver nanoparticles (AgNPs) can promote wound healing through the modulation of cytokines. Nonetheless, the question as to whether AgNPs can affect various skin cell types – keratinocytes and fibroblasts – during the wound-healing process still remains.[20] A study was conducted for evaluating in vitro skin penetration of AgNPs and the results showed that AgNPs’ absorption through intact and damaged skin was very low but detectable, and that in case of damaged skin it was possible to increase permeation of silver when applied as nanoparticles. Moreover, AgNPs could be detected in the SC and the outermost surface of the epidermis by electron microscopy, and silver applied as nanoparticles coated with polyvinylpirrolidone is able to permeate the damaged skin in an in vitro diffusion cell system.[21] The topical administration of the lyotropic liquid crystal nanocube to SC rapidly broke down the lipid lamella structure which would be recognized as a wound without organ change and the observations suggested that a structural change in lipid can stimulate and trigger recognition of a slight injury in the wound defense and a repair response as homeostasis and this actually succeeded in improving photo-induced hyperpigmentation on human face.[22]
Skin can be exposed to solid nanoscale particles through either intentional or non-intentional means. Intentional dermal exposure to nanoscale materials may include the application of lotions or creams containing nanoscale TiO2 or ZnO as a sunscreen component or fibrous materials coated with nanoscale substances for water- or stain-repellent properties. Non-intentional exposure could involve dermal contact with anthropomorphic substances generated during nanomaterial manufacture or combustion.[23] Tinkle et al. provided compelling evidence that nanoparticles could penetrate the skin in demonstrating epidermal and dermal penetration by fluorescent microspheres (0.5–1.0 μm) in human skin in an in vitro flexed skin model.[23]
Dermal Exposure to Nanoparticles
The interaction of nanoparticles with skin has received significant attention recently because of the increasing use of nanoscale particles in stain-resistant clothing, cosmetics, and sunscreens. The dermal route of exposure is also important because of the tendency of agglomerated airborne nanoparticles to settle on surfaces and the difficulties in preventing dermal contact with these settled particles. Several studies have been conducted examining the ability of nanoscale TiO2, used as an ultraviolet (UV)-absorbing component in sunscreens, to penetrate the epidermis in human volunteers and animal and in vitro models.[24–28] The primary TiO2 particles used in these studies ranged in size from 10 to 60 nm, with larger-sized aggregates present. In all of these studies, nanoparticles did not appear to penetrate past the SC of the epidermis, though in some cases, accumulation in the hair follicles was observed.[7] Studies have found 20-nm, negatively charged polystyrene spheres to behave similarly to nanoscale TiO2 with regard to their follicular localization and lack of SC penetration.[29]
In contrast to the studies of nanoscale TiO2 and polystyrene, a study examining the dermal penetration of quantum dots has shown limited dermal penetration.[30] This study[30] utilized scanning fluorescent confocal microscopy to examine the influence of size, shape and surface charge on quantum dot penetration in an in vitro, flow-through diffusion porcine skin model. A small fraction of the applied dose for some quantum dot species was shown to penetrate the SC, with an even smaller fraction escaping the epidermis and accumulating within the dermis. This penetration was dependent upon size, shape and surface charge of the quantum dots, with smaller, spherical particles demonstrating greater penetration than larger, ellipsoidal particles. None of the quantum dot species were shown to pass through the entire skin thickness to the opposing perfusate side of the diffusion cell. This limited dermal penetration has also been shown recently for <10-nm maghemite and iron nanoparticles using an in vitro human skin model,[31] suggesting that “smaller” nanoparticles may have this ability. Though studies have yet to examine the effect of skin condition on nanoparticle absorption, damaged skin would be expected to provide less of a barrier, and this issue may be important in certain situations such as the application of nanoparticle-containing sunscreens to sun-damaged skin. In support of this idea, the flexing of skin in vitro has been shown to increase dermal penetration of micron-sized dextran particles and derivatized fullerenes.[23,32] Together, the available data suggest that healthy, intact skin is a significant barrier to certain nanomaterials.
Conclusion
Nanotechnology is a fast-expanding area of science. This area of research is anticipated to lead to the development of novel, sophisticated, multifunctional applications which can recognize cancer cells, deliver drugs to target tissue, aid in reporting outcome of therapy, provide real-time assessment of therapeutic and surgical efficacy, and most importantly, monitor intracellular changes to help prevent precancerous cells from becoming malignant. Although the expectations from nanotechnology in medicine are high and the potential benefits are endlessly enlisted, the safety of nanomedicine is not yet fully defined. Use of nanotechnology in medical therapeutics needs adequate evaluation of its risk and safety factors. However, it is possible that nanomedicine in future would play a crucial role in treatment of human diseases and also in enhancement of normal human physiology. With concurrent application of nanotechnology in other fields, its utility is likely to extend further into diagnostics, molecular research techniques and tools.
Future of Nanotechnology
Nanotechnology has a bright future with multiple applications in many areas including engineering, optics, energy, and consumer products. Nanomedicine will develop applications for novel and superior diagnostic, therapeutic and preventive measures. Exciting achievements based on nanotechnology and nanomedicine await us in the future. Yet, there are as many challenges to get it right, and recognize and avoid potential risks associated with these new developments. Essential for the successful present and future development is a multidisciplinary team approach involving material scientists, physicians and toxicologists who work closely together.
We will also need to push the existing knowledge as far as it will go in the service of protecting people. Where existing knowledge fails, new research is needed to fill the gaps. This must be administered strategically and targeted to addressing specific issues in a timely manner. Failing to take these steps will ultimately lead to people's health being endangered and emerging nanotechnologies floundering. But with foresight, sound science and strategic research, we have the opportunity to ensure that emerging nanotechnologies are as safe as possible, while reaching their full potential.
Ongoing efforts by scientists, researchers, and medical personnel can sincerely ensure to “do big things using the very small.” But as such, the applications of nanotechnology in dermatology are still a developing and challenging field to dermatologists.
Future of Nanomedicine
Nanotechnology is beginning to change the scale and methods of vascular imaging and drug delivery. Indeed, the National Institutes of Health (NIH) Roadmap's “Nanomedicine Initiatives” envisage that nanoscale technologies will begin yielding more medical benefits within the next 10 years. This includes the development of nanoscale laboratory-based diagnostic and drug discovery platform devices such as nanoscale cantilevers for chemical force microscopes, microchip devices, nanopore sequencing, etc. The National Cancer Institute has related programs too, with the goal of producing nanometer scale multifunctional entities that can diagnose and deliver therapeutic agents and monitor cancer treatment progress. These include design and engineering of targeted contrast agents that improve the resolution of cancer cells to the single cell level and nanodevices capable of addressing the biological and evolutionary diversity of the multiple cancer cells that make up a tumor within an individual. Thus, for the full in vivo potential of nanotechnology in targeted imaging and drug delivery to be realized, nanocarriers have to get smarter. Pertinent to realizing this promise is a clear understanding of both physicochemical and physiological processes. These form the basis of complex interactions inherent to the fingerprint of a nanovehicle and its microenvironment, the examples of which include carrier stability, extracellular and intracellular drug release rates in different pathologies, interaction with biological milieu, such as opsonization and other barriers en route to the target site, be it anatomical, physiological, immunological or biochemical, and exploitation of opportunities offered by disease states (e.g. tissue-specific receptor expression and escape routes from the vasculature). Inherently, carrier design and targeting strategies may vary in relation to the type, developmental stage, and location of the disease. Toxicity issues are of particular concern but are often ignored. Therefore, it is essential that fundamental research be carried out to address these issues if successful efficient application of these technologies has to be achieved. The future of nanomedicine will depend on rational design of nanotechnology materials and tools based on a detailed and thorough understanding of biological processes rather than forcing applications for some materials currently in vogue.
Multiple Choice Questions
- Nanotechnology is an emerging branch of science for designing tools and devices of size:
-
a)1–100 mm
-
b)1–100 nm
-
c)1–100 cm
-
d)1–100 m
-
a)
-
Nanotechnology is the creation of:
-
a)Functional materials
-
b)Control on the nanometer length scale
-
c)Exploitation of properties
-
d)All of the above
-
a)
-
Tiny particles enter the body through:
-
a)Skin
-
b)Lungs or intestinal tract
-
c)a and b
-
d)Eyes
-
a)
-
Nanotechnology is being applied extensively to provide targeted:
-
a)Drug therapy
-
b)Diagnostics
-
c)Tissue degeneration
-
d)All of the above
-
a)
-
Early genesis of the concept of nanomedicine sprang from the visionary idea:
-
a)Endoscope
-
b)Nanorobots and related machines
-
c)Magnetic resonance images
-
d)Genetic engineering
-
a)
-
Nanoparticles are:
-
a)Transparent
-
b)Opaque
-
c)Turbid
-
d)Greasy
-
a)
-
Stratum corneum counteracts the penetration of:
-
a)Antibiotics
-
b)Xenobiotics
-
c)Deodorants
-
d)Conditioners
-
a)
-
Silver nanoparticles can promote wound healing through the modulation of:
-
a)Lymphocytes
-
b)Leukocytes
-
c)Platelets
-
d)Cytokines
-
a)
-
Increasing use of nanoscale particles in:
-
a)Stain resistant clothing
-
b)Cosmetics
-
c)Sunscreens
-
d)All of the above
-
a)
-
Scientists, researchers and medical personnel can sincerely ensure to do:
-
a)Small things using the very big
-
b)Big things using the very small
-
c)Both of the above
-
d)None of the above
-
a)
Answers:
Acknowledgement
Dr. K H Basavaraj thanks Professor Suresh, Vice Chancellor, JSS University. Mysore, India, and the Principal, JSS Medical College, India, for their support and encouragement.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Kaur IP, Agrawal R. Nanotechnology: A new paradigm in cosmeceuticals. Recent Pat Drug Deliv Formul. 2007;1:171–82. doi: 10.2174/187221107780831888. [DOI] [PubMed] [Google Scholar]
- 2.Angew Chem Nanomedicine-challenge and perspectives. Int Ed Engl. 2009;48:872–97. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain KK. Role of nanobiotechnology in developing personalized medicine for cancer. Technol Cancer Res Treat. 2005;4:645–50. doi: 10.1177/153303460500400608. [DOI] [PubMed] [Google Scholar]
- 4.Jain KK. The role of nanobiotechnology in drug discovery. Drug Discov Today. 2005;10:1435–42. doi: 10.1016/S1359-6446(05)03573-7. [DOI] [PubMed] [Google Scholar]
- 5.Service RF. Nanotoxicology: Nanotechnology grows up. Science. 2004;304:1732–4. doi: 10.1126/science.304.5678.1732. [DOI] [PubMed] [Google Scholar]
- 6.Oberdorster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geho DH, Lahar N, Ferrari M, Petricoin EF, Liotta LA. Opportunities for nanotechnology-based innovation in tissue proteomics. Biomed Microdevices. 2004;6:231–9. doi: 10.1023/B:BMMD.0000042053.51016.b4. [DOI] [PubMed] [Google Scholar]
- 9.Wickline SA, Lanza GM. Nanotechnology for molecular imaging and targeted therapy. Circulation. 2003;107:1092. doi: 10.1161/01.cir.0000059651.17045.77. [DOI] [PubMed] [Google Scholar]
- 10.Zuo L, Wei W, Morris M, Wei J, Gorbounov M, Wei C. New technology and clinical applications of nanomedicine. Med Clin North Am. 2007;91:845–62. doi: 10.1016/j.mcna.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Freitas RA., Jr What is nanomedicine? Nanomedicine. 2005;1:2–9. doi: 10.1016/j.nano.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Medvedeva NV, Ipatova OM, Ivanov ID. Nanobiotechnology and nanomedicine: Biochemistry (Moscow) supplemental series B. Biomed Chem. 2007;1:114–24. [Google Scholar]
- 13.Medina C, Santos-Martinez MJ, Radomski A, Corrigan OI, Radomski MW. Nanoparticles: Pharmacological and toxicological significance. Br J Pharmacol. 2007;150:552–8. doi: 10.1038/sj.bjp.0707130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonneville-Aubrun O, Simonnet JT, L’Alloret F. Nanoemulsions: A new vehicle for skincare products. Adv Colloid Interface Sci. 2004;108/109:145–9. doi: 10.1016/j.cis.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Nohynek GJ, Lademann J, Ribaud C, Roberts MS. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 2007;37:279–85. doi: 10.1080/10408440601177780. [DOI] [PubMed] [Google Scholar]
- 16.Müller RH, Peterson RD, Hommoss A, Pardeike J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv Drug Deliv Rev. 2007;59:522–30. doi: 10.1016/j.addr.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Schäfer-Korting M, Mehnert W, Korting HC. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv Drug Deliv Rev. 2007;59:427–43. doi: 10.1016/j.addr.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366:170–84. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Hoet PH, Bruske-Hohlfeld I, Sata OV. Nanoparticles-known and unknown health risks. J Nanotechnol. 2004;2:1–15. doi: 10.1186/1477-3155-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Lee PY, Ho CM, Lui VC, Chen Y, Che CM, et al. Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing. Chem Med Chem. 2010;5:468–75. doi: 10.1002/cmdc.200900502. [DOI] [PubMed] [Google Scholar]
- 21.Larese FF, D’Agostin F, Crosera M, Adami G, Renzi N, Bovenzi M, et al. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology. 2009;255:33–7. doi: 10.1016/j.tox.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi Y, Nagasawa T, Kitagawa A, Nakamura N, Matsumoto K, Uchiwa H, et al. New nanotechnology for the guided tissue regeneration of skin-potential of lyotropic liquid crystals. Pharmazie. 2006;61:112–6. [PubMed] [Google Scholar]
- 23.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gamer AO, Leibold E, van Ravenzwaay B. The in vitro absorption of microfine zinc oxide and titanium dioxide through porcine skin. Toxicol in vitro. 2006;20:301–7. doi: 10.1016/j.tiv.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Lademann J, Wiegmann H, Rickmeyer C, Barthelmes H, Schaefer H, Mueller G, et al. Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice. Skin Pharmacol Appl Skin Physiol. 1999;12:247–56. doi: 10.1159/000066249. [DOI] [PubMed] [Google Scholar]
- 26.Mavon A, Miquel C, Lejeune O, Payre B, Moretto P. In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen. Skin Pharmacol Physiol. 2007;20:10–20. doi: 10.1159/000096167. [DOI] [PubMed] [Google Scholar]
- 27.Pflucker F, Wendel V, Hohenberg H, Gärtner E, Will T, Pfeiffer S, et al. The human stratum corneum layer: An effective barrier against dermal uptake of different forms of topically applied micronised titanium dioxide. Skin Pharmacol Appl Skin Physiol. 2001;14:92–7. doi: 10.1159/000056396. [DOI] [PubMed] [Google Scholar]
- 28.Schultz J, Hohenberg H, Pflucker F, Gärtner E, Will T, Pfeiffer S, et al. Distribution of sunscreens on skin. Adv Drug Deliv Rev. 2002;54:S157–63. doi: 10.1016/s0169-409x(02)00120-5. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez-Roman R, Naik A, Kalia YN, Guy RH, Fessi H. Skin penetration and distribution of polymeric nanoparticles. J Control Release. 2004;99:53–62. doi: 10.1016/j.jconrel.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicol Sci. 2006;91:159–65. doi: 10.1093/toxsci/kfj122. [DOI] [PubMed] [Google Scholar]
- 31.Baroli B, Ennas MG, Loffredo F, Isola M, Pinna R, López-Quintela MA. Penetration of metallic nanoparticles in human full-thickness skin. J Invest Dermatol. 2007;127:1701–12. doi: 10.1038/sj.jid.5700733. [DOI] [PubMed] [Google Scholar]
- 32.Rouse JG, Yang J, Ryman-Rasmussen JP, Barron AR, Monteiro-Riviere NA. Effects of mechanical flexion on the penetration of fullerene amino acid-derivatized peptide nanoparticles through skin. Nano Lett. 2007;7:155–60. doi: 10.1021/nl062464m. [DOI] [PubMed] [Google Scholar]


