Abstract
The past few years have seen a tremendous rise in the number of clinical trials conducted in India. This is been attributed to the huge patient population, genetic diversity, and rich technical pool in our country. However, the economical upsurge in the clinical trial industry has also caused concerns pertaining to the efficiency of the Regulatory Agencies and Ethics Committees (EC). The EC plays an important role in the regulation of clinical research at the local level. However, it is seen that many ECs are oblivious to their roles and responsibilities. It is reported that ECs lack standard operating procedures, do not have a proper composition or adequate representation, thus affecting their functions in regulating clinical research. Moreover, ECs seem to function in isolation, as self-sufficient bodies, having no communication with the regulatory agency or other ECs. This brings forth the need for ECs to come together and share their experiences and observations, with the aim of updating themselves and refining their functions. Efforts also need to be focused on capacity building, centralized registration of ECs, and bringing an oversight mechanism in place. The Ethics Committees in India need to work in close association with forums such as the Forum for Ethics Review Committees in India and the Forum for Ethical Review Committees in Asia Pacific, in an effort towards empowering themselves.
Keywords: Collaborative, capacity building, central registration, ethics committees, oversight mechanism
INTRODUCTION
Clinical Research is a systematic investigation in human beings designed to discover or contribute to a body of generalizable knowledge. As clinical research involves human participants, researchers are ethically obligated to protect them. The two principal protections offered to an individual taking part in clinical research are Written Informed Consent and Ethics Committee (EC) review. The purpose of an EC review of biomedical research is to safeguard the dignity, rights, safety, and well-being of the research participants. The EC review comprises of ethical principles and decision-making to solve actual or anticipated dilemmas in the conduct of human research.[1]
CLINICAL RESEARCH IN INDIA
The last ten years have seen a steep rise in the number of clinical research studies in India. The biopharmaceutical majors world over are turning toward India, given its rich technical resource pool, ease of patient recruitment, and sheer diversity inherent in our country's genetic texture.[2]
However, the exodus of international clinical trial projects to India have also brought concerns about the quality of clinical research, sighting timelines for regulatory approval, deficiencies found in the functioning of the ethics committees, and an unethical approach to the recruitment of trial subjects.[3] The validity of these ethical concerns, although debatable, cannot be ignored. Given the prime responsibility of an EC to regulate clinical research, along with the government's regulatory authority - The Drug Controller General of India (DCGI) the various concerns about EC functions need to be addressed.
ETHICS COMMITTEES IN INDIA
It has been more than 30 years since the establishment of ethics committees in India, as the first official guidelines for the formation of ECs was issued by the Indian Council for Medical Research (ICMR) in February 1980. These guidelines included recommendations for membership criteria and ethical standards for review, which laid down the foundation for the establishment of ECs in India. This was followed by release of the ICMR guidelines in bioethics, which was a guidance document for research in medical, epidemiology, and public health, in the year 2000, which was further revised in 2006.
However, despite the establishment of ethical guidelines since a long time, the ECs in our country are still grappling with basic issues like, inadequate or no standard operating procedures (SOPs) and noncompliance with the Schedule Y recommendations. The EC has the prime responsibility of regulating clinical research and safeguarding the rights and safety of research participants, however, the institutions and hospitals that focus on enhancing their research facilities tend to ignore the EC, which approves their research. ECs have to deal with basic issues such as lack of trained manpower, heavy workload, inadequate space allocated for EC operations, lack of administrative support, and inadequate remuneration offered to members serving on EC boards. These issues culminate into reluctance of trained individuals to serve as members of the EC.[4] ECs also have to cope with problems such as insufficient space allocated to them for operations and archival of records, thus posing problems during audit procedures.
In a study conducted by ICMR it was observed that there were no legal experts on most of the ECs and a lack of clarity in the appointment procedures of EC members. The study also noted that record keeping was poor, and the independence and competence of EC questionable.[5] The scarcity of legal experts and lay persons on the EC quorum, which is mandatory as per Schedule Y specifications, also makes arrangement of regular periodic meetings with the presence of these members a difficult task. It is seen that most often the Head of the Institution's function as chairperson of its EC, thus compromising its independent status.
As per another survey conducted by ICMR, there are more than 200 institutions with functional ECs in India. However, many of the existent ECs do not have their standard operating procedures in place and are not constituted as per Schedule Y recommendations. It is also seen that ECs do not have adequate representation of members during their meetings, which is likely to generate a biased opinion.[6] Added to this is the irregular schedule of meetings, with no process in place for expedited review.
As per the Bulletin report of the World Health Organization (WHO) there are less than 40 ECs in our country, which are properly constituted and functioning.[7] This report also raises concerns about the independence of ECs citing that there is no legal requirement for members declaring Conflict of Interest (COI). This is an important issue given the increasing number of privately owned hospitals participating in clinical research. The presence of members with conflicting interests is likely to limit the ability of an impartial review by the EC.
It is also observed that many EC members are ambiguous about their roles and responsibilities, during a review process. ECs members comprise of highly educated and experienced representatives from non-scientific communities, but most of them are silent observers during meeting proceedings and do not participate in scientific or ethical deliberations in the review procedures.[8] Also lack of formal training in bioethics, leads to a limited knowledge of complex ethical issues such as reduced autonomy, distributive justice, subject vulnerability, and subject compensation. EC members see their responsibilities limited to providing approval to research proposals submitted for review and are oblivious to the need for a continuous review. Very rarely do ECs undertake detailed monitoring of studies and scrutinize the informed consent process.
This also advocates the need for an oversight mechanism to be in place during the operations of the EC. To date there is no central registration system for ECs functioning in our country, raising concerns about accountability. Due to lack of visible oversight by the regulatory authorities, there is noncompliance with the recommended ethical standards noted among ECs.
The introduction of the Clinical Trial Registry - India (CTRI) is considered as an ethical imperative for research conducted in India, but this needs to be coupled with a central oversight for ECs approving research studies.[9] Although clinical research in India has increased tremendously in the past years, regulatory reforms and ethical practices have been unable to keep pace with it. The Drug Controller of India (DCGI) has released guidelines for inspection of investigator sites, but there are no recommendations for regulatory inspections to be conducted on ECs. Centralized registration of ECs is a longtime plan, yet to be implemented.
GOVERNMENT INITIATIVES
Although marked by a slow pace, government bodies have taken initiatives toward regulating Indian Ethics Committees relevant to Indian ethos. The ethical guidelines released by the ICMR are elaborate and give a sound direction to biomedical research conducted in the country.
The ICMR has collaborated with the Forum for Ethics Review Committees of Asia Pacific (FERCAP) to develop SOPs for ECs, which are available on their official website. FERCAP is a forum of bioethicists and medical experts who have come together with the objective of fostering an improved understanding and better implementation of the ethical review of biomedical research in Asia and the western Pacific region. It is a project of the World Health Organization (WHO) Special Training and Research Program in Tropical Diseases (TDR), and is a forum under the umbrella of the Strategic Initiative for Developing Capacity in Ethical Review (SIDCER). All the different regional fora for ethics committees from other countries are being brought under a single head of SIDCER, of which ICMR is a founding member. FERCAP works towards facilitating research and education opportunities by acting as a regional collaborator for ECs and various stakeholders in health research.[10]
The Forum for Ethics Committee Review in India (FERCI) has been set up under FERCAP with some members from the bioethics cell in the organizing committee of FERCI.[11] FERCI organized the First National Conference on Research Ethics at the Tata Memorial Hospital, in November 2011. This national conference was a pioneering step toward improving the functioning of ECs in India and getting them together. FERCI, along with WHO and SIDCER, plans to work on the capacity building of ECs in India and impart accreditations to them.
The DCGI has released the revised version of Schedule Y, which describes the roles and responsibilities of EC members as per the ICMR guidelines and provides clarity on the regulatory responsibilities of EC functions. However, these initiatives need to be coupled with strong implementation plans, to ensure stringent regulatory controls to safeguard subject rights.
The DCGI designates the EC as an important regulator of ethical research; however, both the bodies seem to work in isolation. There is no proper communication network between the ECs functional in the country and the DCGI. Both the ICMR, which has issued Guidelines in Bioethics for research conducted in our country and DCGI, which is the primary authority for research in India, do not have any autonomy over the research reviewed and approved by the ECs in our country. There is no central public authority that is responsible for supervising the proper and competent functioning of ECs. This result in ECs functioning as self-sufficient bodies concerned with approval of research conducted in their institutes, with no accountability whatsoever. The implications of lack of central autonomy are evident in the findings of a recent study, sighting the ethical concerns in India. The study reported ethical issues observed during the conduct of clinical trials, such as, violation of ICMR guidelines, failure to report to regulatory authority/EC, if a study is rejected by some other EC, and lack of training procedures for EC members.[12]
Furthermore, the ICMR guidelines are not legislated, hence, the ECs cannot act against those who violate the prescribed guidelines. The role of the EC is merely restricted to being an advisory or to facilitate research. The DCGI has given ECs the power to reject trials not conforming to the recommended ethical standards. In addition, the DCGI provides clearance only to those trials that have been reviewed and approved by the concerned EC. Thus, apart from governing the ethical aspects of research, the EC also plays a significant role of an ethical regulator for the DCGI. However, the lack of a national ethics body, with a strong regulatory control, has further hampered the establishment of a legal ethics policy.[13]
ETHICS COMMITTEES: FACING THE CHALLENGES
A recent survey conducted to evaluate the competence of the ethics committees in subject protection seems to be an eye-opener in this case.[14] The survey studied 11 ECs in India for their knowledge of the ethical guidelines, and the attitudes and practices followed by them. The results revealed lack of knowledge of Schedule Y norms among the EC members, coupled with inadequate training in Good Clinical Practice (GCP), inability to enlist the essential documents for EC review, and failure to realize the important role of EC approval in clinical research. The study also had some assuring findings, as all the ECs had written SOPs and one-third of them had conducted internal audits, to ensure the quality of their operations. Although the results were encouraging, as compared to ICMR-WHO surveys conducted in 2003 and 2007, the study suggested that ECs in India had a challenging task ahead.
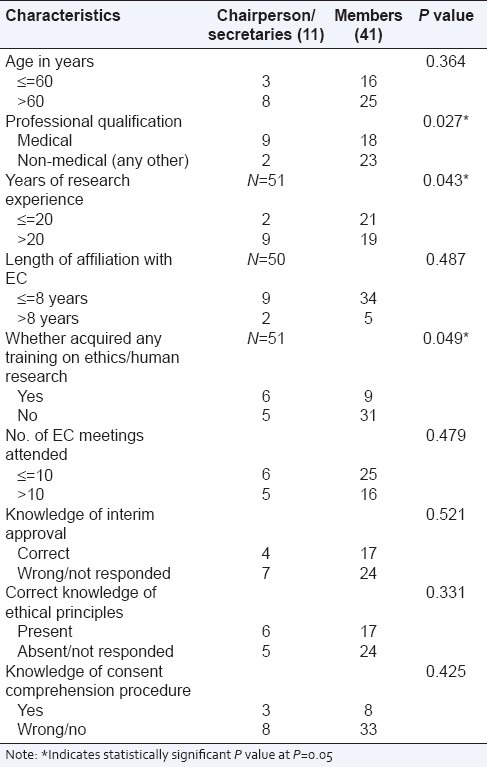
Another study conducted in 2009, to study the profile and roles of EC members in Pune, reported similar findings. The study noted that most EC members were senior in age, highly educated, and well experienced in research, however, only half of them had correct knowledge of the ethical principles. Even with an appropriate EC constitution, the members had suboptimal understanding of the ethical issues and principles. The study also pointed out that a majority of them felt that a formal training in ethics was essential and that there should be networking among various ECs, to share thoughts and experiences [Table 1].[15]
Table 1.
EC office bearers and other members: Good Clinical Practices (GCP). Comparison of profiles, knowledge and level of participation[15]
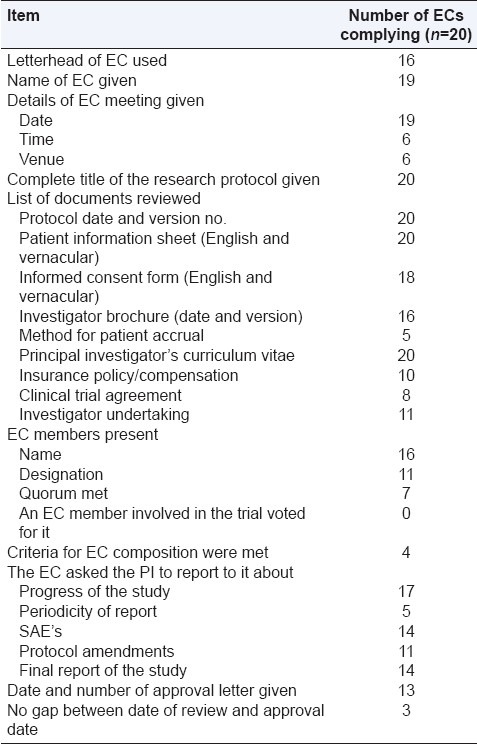
A recent survey conducted on the compliance of issued EC approval letters to the Schedule Y/ICMR guidelines found visible noncompliance in the EC review process to recommended regulatory guidelines. The study reported absence of legal experts and social scientists in the EC approval meetings, lack of quorum requirement as per Schedule Y, and failure to review essential documents such as the clinical trial agreement and the insurance policy [Table 2].[16] In order to overcome the challenges faced by the ethics committees, as cited in the above-mentioned studies, one needs to constructively look into the deficiencies that have emerged in the past surveys.
Table 2.
Compliance with ICMR guidelines and Schedule Y requirements[16]
CAPACITY BUILDING FOR ETHICS COMMITTEES
The strong need for ECs in our country is to focus on capacity building. Members of ECs should be trained in the principles of bioethics, local regulatory guidelines, and Good Clinical Practices (GCP).
Training programs should be conducted keeping the social and cultural scenario in perspective and aim at community welfare and protection.[17]
Government agencies like the ICMR along with the FERCI need to take forefront initiatives in organizing training programs for EC members. Sponsors being the major stakeholders in clinical research need to invest in EC training, an area which has unfortunately being ignored by them.
Training of EC members is essential as they come from varied academic and research backgrounds and may not be aware of the ethical principles and technical requirements of the EC review process.
A national training program in bioethics needs to be introduced and made mandatory for every functional EC member. This should be coupled with strategic workshops, organized by the local ethics committees and research institutes. Training programs should emphasize on the codes of ethical conduct, principles of GCP, compliance with applicable regulatory guidelines, developing SOPs, composition of ECs, roles and responsibilities of the members, and review procedures. The EC members should also be trained on complex, but important, topics in clinical research, such as, the rights of vulnerable populations, therapeutic misconception, informed consent process, and issues related to subject compensation and insurance.[18]
The content of the programs should be designed keeping the nonscientific EC members in perspective, and thus, equip them to discharge their responsibilities efficiently. Bioethics should be introduced as a subject in medical, life sciences, pharmacy, and other relevant curriculums to sensitize our future researchers to this topic. The ICMR and FERCI should strongly undertake the process of imparting accreditations to the ECs achieving competency in their procedures, thus encouraging and motivating their efforts. There seems to be no argument on the strong focus needed on capacity building to empower ECs, the important regulators of research.
NEED FOR CENTRAL REGISTRATION OF ETHICS COMMITTEES
The decentralization of autonomy over research conducted in our country is highly critical, as it adversely affects the obligations of both the EC and the DCGI towards the regulation of ethics in research. An important initiative that needs to be taken in this direction is the establishment of a Central Registration System for ECs in India at par with the CTRI. This should be an important step towards introducing regulatory control in the proceedings of the ECs. The DCGI should make it mandatory for all ECs functional in our country to be registered, without which an EC approval should not be considered valid. The central registry should maintain record of the EC details like the affiliated institutes, member details along with their qualifications, training records, registered personal account number (PAN) details, official address, and information about EC reviewed trial approval status. Establishment of a central registry should bring about the much needed transparency and accountability in the regulation of clinical research in India. This would also help strengthen the efforts to curb occurrences of any malpractices in EC operations. These steps in increasing accountability of ECs would further enable them to refine their procedures.
The proposed Bill to the Health Ministry on Biomedical Research on Human subjects (Regulation, Control and safeguard) will be a turning point in this direction. The bill envisages creation of National Biomedical Research Authority along with setting up of National Ethics committee on Human Research. The bill also includes central registration of institutional ethics committees. Once in place the bill will ensure regulation of biomedical research on human subjects, restrict unscrupulous clinical trials and provide legislative powers to the ICMR ethical guidelines.[19]
OVERSIGHT MECHANISMS
An essential task that needs to go in hand with developing the central registry for ECs is bringing an efficient oversight mechanism in place. It is essential that regulatory and ethical reforms are introduced in pace with the ever-increasing clinical research activities in India. DCGI has issued the trial inspection guidelines for investigator sites, but have not revealed any inspection plans for ECs. The DCGI should take an initiative in conducting random inspections and ensure that ECs comply with the recommended standards. ECs by themselves should take active steps in ensuring the competency of their procedures. Periodic internal audits can be conducted by independent auditors, to ensure that SOPs and review-related documents are in place.
The ECs need to adapt innovative procedures to ensure an oversight mechanism for clinical studies approved by them. It is essential for ECs to understand that protecting the rights and safety of trial participants does not end with granting trial approval, but has to continue throughout the conduct of the study.[20] The ECs need to conduct random audits on investigator sites, to ensure that research trials approved by them are conducted in an ethical manner. There are international programs such as the SIDCER Recognition Program and the Accreditation of Human Research Protection Program (AAHRP), which give recognition and accreditation to the ECs.[21] The ECs in India need to constructively work toward attaining such recognitions.
NEED FOR A NATIONAL FORUM
The isolated existence of the ECs and the lack of communication framework between the ECs and the regulatory bodies make it essential for ECs in our country to come together and work in a collaborative manner to develop a uniform code of conduct. The members of various ethics committees need to reflect on their roles and responsibilities and come up with solutions for issues faced during the EC review process. Ethics committees need to interact with each other and share their experiences and observations with an aim to update themselves and refine their functions. The EC members need to understand that their responsibilities are not merely restricted to the ethical review of research, but toward the well-being of the community they represent. This advocates the need for ECs to come together and form a collaborative network, initially at a local level, and then expand to the regional and national levels. There is a need to form joint forums of ECs, which can serve as platforms to address the issues and dilemmas faced by them.
Addressing the above-mentioned need, an experimental initiative (Pune Model) was taken by the Independent Ethics Committee (IEC) associated with the Chest Research Foundation (CRF) Pune, wherein a Scientific Meeting in Human Ethics was organized. This meeting was an effort to bring together all the ECs functional in Pune and create a joint forum to interact and communicate with each other. The meeting comprised of a talk by Dr. Urmila Thatte regarding the ‘Challenges faced by Ethics Committees’ followed by a discussion, wherein all the attending EC members put forth their questions on EC operations and shared their experiences. The proposal of having a joint forum of ECs in Pune was also put forth by Dr. S M Karandikar (Chairperson IEC-CRF). This meeting provided clarity on the ethical issues encountered by EC members related to subject compensation, trial insurance, and informed consent.
The Pune Model was a pioneering step towards forming a collaborative network of ECs across India. This was the first step in creating a platform for contemplating ethical and operational issues faced by the ethics committees at a local level. The local forum will also serve as a base for strategic plans in capacity building, an essential requisite for EC members today. The forum can periodically organize workshops for training in bioethical issues, in collaboration with FERCI and ICMR. It can also be utilized as a channel for updating members about new regulations and guidelines issued by the regulatory authorities.
A challenging task for the Pune Model will be its sustainability. However, we are very optimistic about it, with the second meeting of this forum held within the same year. This meeting was organized by the EC associated with Lupin Research Center (Pune) and was attended by over 50 EC members in Pune. The highlights of this meeting were article presentations by legal members of ECs on subject compensation and trial insurance. The ECs in Pune forum have mutually decided to host periodic meetings among themselves and we are looking forward to the third meeting of this forum, which is to be organized soon. Given the increasing awareness among EC members to train themselves, and recent audits conducted by DCGI at some ECs, sustainability of the Pune Model is imperative.
We propose to expand the joint local forum of ECs (The Pune Model) [Figure 1] to the regional level (Maharashtra) and then to a national level (India).The intent of the Pune Model is to be closely associated with the ICMR and FERCI in their efforts towards empowerment of ECs in India.
Figure 1.
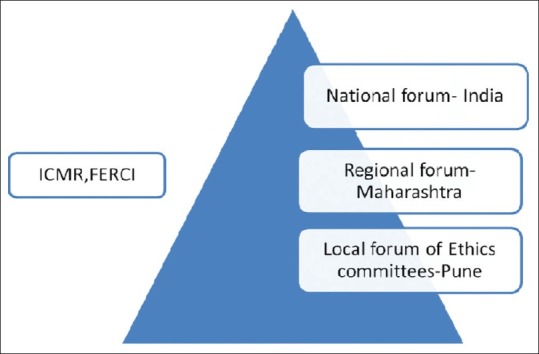
Proposed collaboration of Pune Model with ICMR and FERCI
This will enable ECs in our country to come together and develop a communication network among them. We recommend collaborative efforts between all the stakeholders in biomedical research — the regulatory agencies — DCGI, ICMR, FERCI, sponsors, investigators, and members of ECs, to strive toward enhancing the clinical research scenario in India.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Thatte UM. Ethical issues in clinical research. Indian J Plast Surg. 2007;40:2. [Google Scholar]
- 2.Maiti R, Raghavendra M. Clinical trials in India. Pharmacol Res. 2007;56:1–10. doi: 10.1016/j.phrs.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Gulhati CM. Needed: Closer scrutiny of clinical trials. Indian J Med Ethics. 2004;1:4–5. doi: 10.20529/IJME.2004.002. [DOI] [PubMed] [Google Scholar]
- 4.Thatte U, Bavdekar S. Clinical research in India: Great expectations? J Postgrad Med. 2008;54:3. doi: 10.4103/0022-3859.43517. [DOI] [PubMed] [Google Scholar]
- 5.Kumar N. Bioethics Activities in India under ICMR. [Last accessed on 2011 Nov 11]. http://icmr.nic.in/bioethics/cc_biothics/presentations/haryana/activity.pdf .
- 6.Bhatt A. Clinical Trials in India: Pangs of globalization. Indian J Pharmacol. 2004;36:207–8. [Google Scholar]
- 7.Chatterjee P. Clinical Trials in India: Ethical concerns. Bull World Health Organ. 2008;86:581–2. doi: 10.2471/BLT.08.010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas G. Institutional Ethics Committees: Critical gaps. Indian J Med Ethics. 2011;8:200. doi: 10.20529/IJME.2011.080. [DOI] [PubMed] [Google Scholar]
- 9.Tharyan P. Ethics Commitees and clinical Trials registration in India: Opportunities, obligations, challenges and solutions. Indian J Med Ethics. 2011;8:168–9. doi: 10.20529/IJME.2007.066. [DOI] [PubMed] [Google Scholar]
- 10. [Last accessed on 2011 Nov 11]. Available from: http://www.fercap-sidcer.org .
- 11. [Last accessed on 2011 Nov 11]. Available from: http://icmr.nic.in/bioethics/bioethics%20cell/intenational .
- 12.Srinivasan S, Nikarge S. Ethical concerns in clinical trials in India: An investigation. [Last accessed on 2011 July 25]. Available from: http://www.cser.in/Publication .
- 13.Jesani A. Ethics in ethics committees: Time to share experiences, discuss challenges and do a better job. Indian J Med Ethics. 2009;6:62–3. doi: 10.20529/IJME.2009.022. [DOI] [PubMed] [Google Scholar]
- 14.Nadig P, Joshi M, Uthappa A. Competence of ethics committees in patient protection in clinical trials. Indian J Med Ethics. 2011;8:151–3. doi: 10.20529/IJME.2011.061. [DOI] [PubMed] [Google Scholar]
- 15.Bramhe R, Mehendale S. Profile and role of the members of ethics committees in hospitals and research organizations in Pune, India. Indian J Med Ethics. 2009;6:78–84. doi: 10.20529/IJME.2009.026. [DOI] [PubMed] [Google Scholar]
- 16.Taur S, Bavdekar S, Thatte U. Survey of ethics committee protocol Approval letters: Compliance with Schedule Y/ICMR guidelines 2006. Indian J Med Ethics. 2011;8:214–5. doi: 10.20529/IJME.2011.083. [DOI] [PubMed] [Google Scholar]
- 17.Jesani A. Can ethics committees address society's concerns about standards in research? Indian J Med Ethics. 2011;8:134. doi: 10.20529/IJME.2011.056. [DOI] [PubMed] [Google Scholar]
- 18.Pandiya A. Quality of independent review board/ethics committee oversight in clinical trials in India. Perspect Clin Res. 2011;2:45–7. doi: 10.4103/2229-3485.80364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthuswamy V. Legislating the ethical guidelines –the Indian Model. [Last accessed on 2011 Nov 11]. Available from: http://www.knepk.litbang.depkes.go.id/knepk/kegiatan/conference/VM India model.ppt .
- 20.Silverman H. Ethical Issues during conduct of clinical trials. Proc Am Thorac Soc. 2007;4:180–4. doi: 10.1513/pats.200701-010GC. [DOI] [PubMed] [Google Scholar]
- 21. [Last accessed on 2011 Nov 11]. Available from: http://www.aahrpp.org/www.aspx .


