Abstract
Background:
Strychnos nux-vomica, commonly known as kuchla, contains strychnine and brucine as main constituents. Minor alkaloids present in the seeds are protostrychnine, vomicine, n-oxystrychnine, pseudostrychnine, isostrychnine, chlorogenic acid, and a glycoside. Seeds are used traditionally to treat diabetes, asthma, aphrodisiac and to improve appetite.
Objective:
The present study was aimed to evaluate the various pharmacognostical characters and antidiabetic activity of S. nux-vomica seed.
Materials and Methods:
Pharmacognostical characters were performed as per the WHO guideline. Extraction was carried out in petroleum ether, chloroform, alcohol, hydroalcoholic, aqueous, and phytochemical constituents present in extracts were detected by different chemical tests. Among these extracts hydroalcoholic, aqueous extracts were evaluated for antidiabetic activity on the basis of extractive yield and phytoconstituents, in alloxan-induced diabetic rats using gliclazide as standard.
Results:
Various analytical values of S. nux-vomica extract were established. Phytoconstituents present in S. nux-vomica extracts were detected.
Conclusion:
S. nux-vomica extracts show antihyperglycemic activity in experimental animals.
Keywords: Antidiabetic activity, alloxan, extract, kuchla
INTRODUCTION
The dried seeds of S. nux-vomica (family Loganiaceae) are commonly known as kuchla. Kuchla contain 2.6%–3% total alkaloids, out of which 1.25%–1.5% is strychnine, 1.7% is brucine, and the rest are vomicine and igasurine.[1] Some other minor alkaloids are α-colubrine, β-colubrine, 3-methoxyicajine, protostrychnine, novacine, n-oxystrychnine, pseudostrychnine, isostrychnine, chlorogenic acid, and glycoside, loganin.[2] Crude drug kuchla is used in the treatment of anemia, lumbago, asthma, bronchitis, constipation, diabetes, malarial fever, skin disease, paralysis, muscle weakness, and appetite loss.[3] Suppressive effect of S. nux-vomica was observed on induction of ovalbumin-specific IgE antibody response in mice.[4] Strychnine was identified and analyzed in detoxified kuchla seeds using liquid chromatography–electrospray mass spectrometry.[5] Strychnine and brucine were separated and quantified in S. nux-vomica by nonaqueous capillary electrophoresis.[6] Matrix-assisted laser desorption/ionization (MALDI) and-time of flight mass spectrometry (TOFMS) analysis of crude and processed S. nux-vomica seeds allowed rapid screening of alkaloid components.[7]
Literature review revealed that S. nux-vomica has been used traditionally for treating diabetes.[1,3,8–11] There has only been one paper reported describing the antidiabetic potential of Strychos nux-vomica in methanolic extract.[12] This study was aimed to establish pharmacognostical parameters and explore antidiabetic potential of aqueous and hydroalcoholic extracts prepared from the seeds of S. nux-vomica. The antidiabetic activity was determined in alloxan-induced diabetic rats, and gliclazide was used as a positive control.
MATERIALS AND METHODS
Plant material
The seeds of S. nux-vomica were purchased from market of Jodhpur. The plant materials were authenticated by Dr. S. N. Mishra, senior scientist, medicinal and aromatic plants project, K.N.K. College of Horticulture, Mandsaur, Madhya Pradesh, India, and deposited at the Department of Pharmacognosy, Jodhpur National University, Jodhpur, India.
Pharmacognostical parameters
Pharmacognostical parameters, namely, powder characteristics, ash values, loss on drying (LOD), extractive values, and fluorescence analysis for S. nux-vomica were estimated as per WHO guidelines.[13]
Extraction and phytochemical investigation
The plant material was ground in mixer. The crude drug powder, passed through sieve no. 10 and retained on sieve no. 60, was used for extraction. The powdered drug was packed in Soxhlet extractor and extracted using solvents like petroleum ether, chloroform, ethanol, ethanol (50%) and water.-[14] Different ash values were determined to find the inorganic content in the sample [Table 1]. Fluorescence analysis of S. nux-vomica seeds powder was done [Table 2]. Solvents of different polarity were used to find out the extractive value for seeds and rhizome of of S. nux-vomica [Table 3]. Phytoconstituents present in extracts were detected by different chemical tests [Table 4].
Table 1.
Analytical values

Table 2.
Fluorescence analysis of S. nuxvomica seeds powder
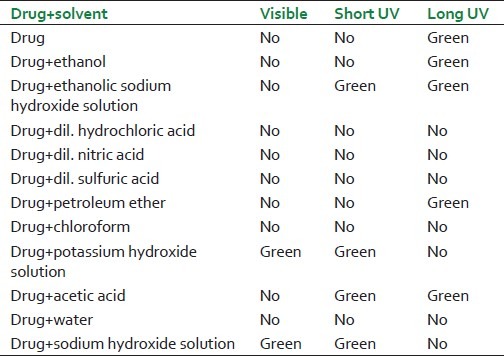
Table 3.
Extractive values of S. nux-vomica seeds and rhizome in different solvents

Table 4.
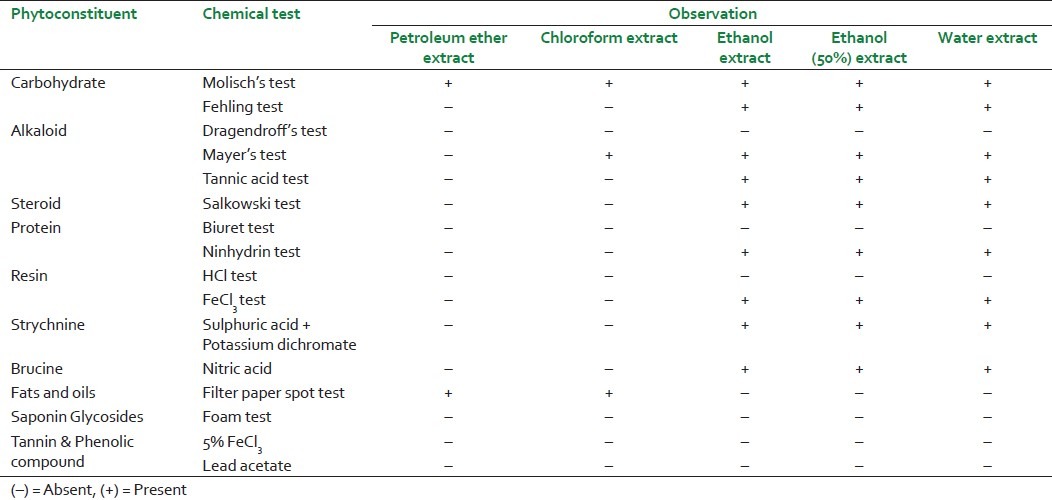
Detection of phytoconstituents present in various extracts of S. nux-vomica
Antihyperglycemic effect of plant extracts
Animals
The study was conducted on healthy albino rats of either sex weighing 150–250 g. Prior approval by institutional animal ethical committee was obtained for conduct of experiments vide protocol number 2009–10/09. The animals were kept in separate cages in animal house under standard conditions.
Induction of diabetes
Diabetes was induced in the rats by administering 110 mg/kg of alloxan intraperitoneally to the rats, which were kept for 24 h fasting prior to administration. After 72 h, the blood samples were collected and analyzed for blood glucose. Albino rats which showed more than 200 mg/dL blood glucose were considered as diabetic and further used in our studies.
Experimental procedure
Healthy albino rats were marked and randomly distributed into 5 groups each consisting of 7 animals in each group. The blood samples were collected randomly to avoid any bias. The groups were divided as following:
Group I: Normal Control
Group II: Diabetic Control
Group III: Standard drug (Gliclazide, 10 mg/kg)
Group IV: Diabetic rats, treated with 50% Ethanolic extract of S. nux-vomica (3.6 mg/kg).
Group V: Diabetic rats, treated with aqueous extract of S. nux-vomica (3.6 mg/kg).
Blood sampling and biochemical analysis
Preparation and administration of drug.
Distilled water was used as vehicle used for administration of aqueous and 50% ethanolic extract of S. nux-vomica. Drug was administered using oral feeding needle.
Blood samples were collected at day 0, 4, and 10 from animals by retro-orbital plexus of rats with the help of microcapillary tubes. The blood samples were collected in microcentrifuge tubes (Eppendorf tubes) and centrifuged for 10 min at 10,000 rpm. Blood glucose was estimated by GOD–PAP (glucose oxidase/phenol/4-amino-antipyrine) method using Elitech glucose SL kit with autoanalyzer (EC-5 plus V2 model, Erba, Mannheim, Germany) at filter range 500–550 nm. The blood glucose level was expressed as mg/dL of blood.
Statistical analysis
The data obtained in the studies was subjected to one-way analysis of variance (ANOVA) for comparison among different groups. P value <0.05 was considered significant and results were expressed as mean±SD [Figure 1].
Figure 1.
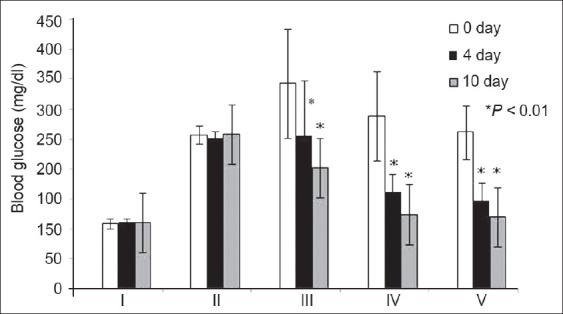
Blood glucose levels in different groups on different days of treatment. Notations on x axis: I: Normal control; II: Diabetic control; III: Standard drug; IV: Strychnos nux-vomica ethanolic (50%) extract; V: Strychnos nux-vomica aqueous extract. Blood glucose values are presented as mean±SD; *represents significant percentage reduction of blood glucose level when compared with day 0 at P<0.01
RESULTS
Results of pharmacognostical, extraction and phytochemical investigations of S. nux-vomica
The powder characteristics show the presence of lignified trichomes, aleurone grains, oil globules, and endosperm with plasmodesma [Figure 2].
Figure 2.

Powder characteristics. (a) Aleurone grain; (b) vessel; (c) Fiber; (d) Starch grains; (e) Endosperm
Various analytical values and fluorescence analysis are presented in [Table 1 and 2] respectively. Extractive values and extractive yields of S. nux-vomica seeds in solvents of different polarity are reported in [Table 3]. Carbohydrate, protein, oil, steroid, alkaloid, resin, strychnine and brucine were detected in phytochemical screening [Table 4].
Thin-layer chromatography and high-performance liquid chromatography analysis with chemical controls may be conducted in ongoing studies for thorough phytochemical investigations.
Antihyperglycemic effect of plant extracts
The hydroalcoholic and aqueous extract of S. nux-vomica (3.6 mg/kg) had shown significant reduction in blood glucose level in alloxan-induced diabetic rats [Figure 1]. The percent reduction of blood glucose levels in groups III, IV, and V at day 4 and 10 showed significant difference (P<0.01) when compared with percentage of blood glucose reduction at day 0 within the same group. Groups IV and V showed a significant difference in reducing blood glucose level when compared with standard control group III at P<0.05.
DISCUSSION
Medicinal plants have been widely claimed useful and found effective in the treatment of diabetes mellitus in various traditional systems of medicine. Various species of Strychnos have been reported traditionally for the treatment of diabetes.[1,3,8] The other species of the same genus, that is, S. potatorum, was used in the treatment of diabetes and it was proved effective.[15,16] In a recent published report, methanolic extract of S. nux-vomica has been proved as having a potential antidiabetic activity and antioxidant property. Considering these facts, the present study deals with the evaluation of diabetic activity of the aqueous and hydroalcoholic (50% ethanol) extract of seed powder of S. nux-vomica in alloxan-induced diabetic rats.
Alloxan, a toxic glucose analogue, selectively destroys β-cells when administered to rodents and many other animal species, resulting in insulin-dependent diabetes mellitus in these animals, with characteristics similar to diabetes in humans.[17] Alloxan, in the presence of intracellular thiols, generates reactive oxygen species in a cyclic reaction with its reduction product, dialuric acid.[18] The β-cell toxic action of alloxan is initiated by free radicals formed in this redox reaction. Increased food consumption and decreased body weight were observed in alloxan-induced diabetic rats, which occurred due to excessive breakdown of tissue proteins.[12]
Earlier reported literature suggests that the glycosides, flavonoids, steroids, tannins, and alkaloids are responsible for antidiabetic activity.[19] Preliminary phytochemical studies revealed the presence of steroids, alkaloids, and glycosides in hydroalcoholic and aqueous extracts of S. nux-vomica (strychnine, brucine, vomicine, loganin, and others). Thus, the antidiabetic effect produced by the extract of S. nux-vomica may be due to the presence of these active ingredients. In our studies, significant reduction of blood glucose was observed on day 4 and 10 of treatment in groups III, IV, and V, which proved significant antidiabetic activity with the gliclazide, 50% ethanolic extract of S. nux-vomica, and aqueous extract of S. nux-vomica when compared with group II. Both the extracts were found effective and significantly (P<0.05) superior in reducing the blood glucose when compared with standard (gliclazide) control drug on day 10.
There was a significant increase in the superoxide dismutase and catalase levels and a significant decrease in the lipid peroxide level after the administration of methanolic seed extract of S. nux-vomica. Thus S. nux-vomia possesses antioxidant property, which prevents lipid peroxidation that leads to protein modification and damage.[12]
The hypoglycemic activity of ethanol extract of S. potatorum was related to the effect of ethanol extract on diabetic-induced rat liver alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase levels, which significantly reduced to almost normal level after 15 and 30 days of treatment.[16] The serum insulin and cholesterol levels were not significantly modified upon treatment with the plant extract, suggesting that the hypoglycemic activity of S. potatorum is independent of insulin secretion.
Therefore, it is anticipated that lipid peroxidation may provide scientific rationale for the use of S. nux-vomica as an antidiabetic plant.
CONCLUSIONS
Pharmacognostic parameters for S. nux-vomica seeds were estimated, which are helpful in identification and standardization. The aqueous and hydroalcoholic extracts showed the presence of steroids, alkaloids, and glycosides in S. nux-vomica seeds. Aqueous and hydroalcoholic extracts were superior to positive diabetic control in controlling blood glucose level on day 10. In conclusion, the present study indicates that hydroalcoholic and aqueous S. nux-vomica seed extracts, administered per os, are effective in controlling diabetes.
ACKNOWLEDGMENT
The authors are thankful to Dr. S. N. Mishra, senior scientist, medicinal and aromatic plants project, K.N.K. College of Horticulture, Mandsaur, Madhya Pradesh, India, for authentication of the plant material.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Nadkarni KM. 3rd ed. Vol. 2. Mumbai: Popular Prakashan; 1954. Dr. K.M. Nadkarni's Indian materia medica; p. 1179. [Google Scholar]
- 2.Trease GE, Evans WC. 15th ed. Philadelphia: W.B. Saunders Company Ltd; 2000. Trease and Evans’ Pharmacognosy; p. 378. [Google Scholar]
- 3.Gruenwald J. 2nd ed. Montvale: Thomson Healthcare; 2000. PDR for herbal medicines; p. 548. [Google Scholar]
- 4.Duddukuri GR, Brahmam AN, Rao DN. Suppressive effect of Strychnos nux-vomica on induction of ovalbumin-specific IgE antibody response in mice. Indian J Biochem Biophys. 2008;45:341–4. [PubMed] [Google Scholar]
- 5.Young H, You-min S, Chul YK, Kwan YO, Jinwoong K. Analysis of strychnine from detoxified Strychnos nux-vomica seeds using liquid chromatography-electrospray mass spectrometry. J Ethanopharmacol. 2004;93:109–12. doi: 10.1016/j.jep.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, He X, Qi S, Gao W, Chen X, Hu Z. Separation and determination of strychnine and brucine in Strychnos nux-vomica L.and its preparation by nonaqueous capillary electrophoresis. J Pharma Biomed Anal. 2006;41:400–7. doi: 10.1016/j.jpba.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 7.Wu W, Qiao C, Liang Z, Xu H, Zhao Z, Cai Z. Alkaloid profiling in crude and processed Strychnos nux-vomica seeds by matrix-assisted laser desorption/ionization-time of flight mass spectrometry. J Pharm Biomed Anal. 2007;45:430–6. doi: 10.1016/j.jpba.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Gupta SK, Sabir G, Gupta MK, Seth PK. A database for anti-diabetic plants with clinical/experimental trials. Bioinformation. 2009;4:263–8. doi: 10.6026/97320630004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao PS, Ramanadham M, Prasad MN. Anti-proliferative and cytotoxic effects of Strychnos nux-vomica root extract on human multiple myeloma cell line - RPMI 8226. Food Chem Toxicol. 2009;47:283–8. doi: 10.1016/j.fct.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Yin W, Wang T-S, Yin F-Z, Cai B-C. Analgesic and anti-inflammatory properties of brucine and brucine N-oxide extracted from seeds of Strychnos nux-vomica. J Ethnopharmacol. 2003;88:205–14. doi: 10.1016/s0378-8741(03)00224-1. [DOI] [PubMed] [Google Scholar]
- 11.Warrier PK, Nambiar VP, Ramankutty C. Vol. 5. Hyderabad: Orient Longman; 1996. Indian medicinal plants: A compendium of 500 species; p. 202. [Google Scholar]
- 12.Chitra V, Varma PV, Raju KA, Prakash KJ. Study of antidiabetic and free radical scavenging activity of the seed extract of Strychnos nuxvomica. Int J Pharm Pharm Sci. 2010;2(Suppl 1):106–10. [Google Scholar]
- 13.Geneva: World Health Organization; 1998. WHO. Quality control methods for medicinal plant materials; pp. 10–34. [Google Scholar]
- 14.Mukherjee PK. 1st ed. New Delhi: Business Horizons; 2002. Quality control of herbal drugs: An approach to evaluation of botanicals; pp. 114–24. [Google Scholar]
- 15.Mathuram LN, Samanna HC, Ramasamy VM, Natarajan R. Studies on the hypoglycemic effects of Strychnos potatorum and Acacia arabica on alloxan diabetes in rabbits. Cheiron. 1981;10:1–5. [Google Scholar]
- 16.Dhasarathan P, Theriappan P. Evaluation of anti-diabetic activity of Strychnous potatorum in alloxan induced diabetic rats. J Med Med Sci. 2011;2:670–4. [Google Scholar]
- 17.Tyrberg B, Andersson A, Hakan Borg LA. Species differences in susceptibility of transplanted and cultured pancreatic islets to the beta-cell toxin alloxan. Gen Comp Endocrinol. 2001;122:238–51. doi: 10.1006/gcen.2001.7638. [DOI] [PubMed] [Google Scholar]
- 18.Elsner M, Gurgul-Convey E, Lenzen S. Importance of cellular uptake and reactive oxygen species for the toxicity of alloxan and dialuric acid to insulin-producing cells. Free Radical Biol Med. 2006;41:825–34. doi: 10.1016/j.freeradbiomed.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Koti BC, Biradar SM, Karadi RV, Taranalli AD, Benade VS. Effect of Bauhinia variegate bark extract on blood glucose level in normal and alloxanised diabetic rats. J Nat Remedies. 2009;9:27–34. [Google Scholar]


