Abstract
Hepatic Ischemia and Reperfusion Injury (IRI) is a major cause of liver damage during liver surgery and transplantation. Ischemic preconditioning and postconditioning are strategies that can reduce IRI. In this study, different combined types of pre- and postconditioning procedures were tested in a murine warm hepatic IRI model to evaluate their protective effects. Proanthocyanidins derived from grape seed was used before ischemia process as pharmacological preconditioning to combine with technical preconditioning and postconditioning. Three pathways related to IRI, including reactive oxygen species (ROS) generation, pro-inflammatory cytokines release and hypoxia responses were examined in hepatic IRI model. Individual and combined pre- and postconditioning protocols significantly reduce liver injury by decreasing the liver ROS and cytokine levels, as well as enhancing the hypoxia tolerance response. Our data also suggested that in addition to individual preconditioning or postconditioning, the combination of these two treatments could reduce liver ischemia/reperfusion injury more effectively by increasing the activity of ROS scavengers and antioxidants. The utilization of grape seed proanthocyanidins (GSP) could improve the oxidation resistance in combined pre- and postconditioning groups. The combined protocol also further increased the liver HIF-1 alpha protein level, but had no effect on pro-inflammatory cytokines release compared to solo treatment.
Keywords: Preconditioning, Postconditioning, Ischemia, Reperfusion Injury, Proanthocyanidins
INTRODUCTION
Hepatic Ischemia and Reperfusion Injury (IRI), an exogenous antigen-independent inflammatory event, is a major complication in liver surgery, particularly in liver transplantation and hepatic resection 1, 2. IRI has both immediate and long-term effects on the allograft, causing up acute rejection and chronic allograft dysfunction, which in turn significantly contributes to the morbidity and mortality after operation 3.
Increasing evidences have shown that both pro-inflammatory cytokines and the reactive oxygen species (ROS) are key mediators of liver IRI 4, 5. Shortly after the hepatic ischemia and reperfusion injury (1-6 hours), the Kupffer cells are activated and release the pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF α) and interleukin-1 beta (IL-1β). These cytokines have dual role: over range expression of TNFα and IL-1β can induce more production of cytokines and granulocyte colony-stimulating factor, which enhance Kupffer cells activation and promote neutrophil infiltration in microcirculation of liver 6, 7, then aggravate hepatic sterile inflammation after ischemia and reperfusion. On the other hand, TNFα and IL-1 are indispensable for liver regeneration 8.
At the same time, Kupffer cells and hepatocytes also generate ROS, which leading to direct damage on endothelial cells (ECs) and hepatocytes. Due to their important role, ROS levels have to be tightly regulated through different pathways 9, 10. The major regulators are ROS scavengers that include superoxide dismutases (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). These ROS scavengers are responsible for reducing ROS inside the tissues. Another kind of oxidative stress regulation is mediated by nitric oxide (NO), which is created by endothelial nitric oxide synthases (eNOS) and inducible nitric oxide synthases (iNOS) in liver. Nitric oxide can regulate endothelial function and its level can affect blood flow of an organ 11. The reduction of NO level is always associated with IRI.
The whole process of ischemia and reperfusion (I/R) is complicated and the production and release of ROS appears to directly result in hepatocellular injury; at the same time, inflammatory disorder mediated by recruited neutrophils can also result in hepatocellular injury. On the other hand, cells can spontaneously respond to injury by activating defensive mechanisms of themselves. Among them, hypoxia inducible factor-1 alpha (HIF-1α) is a very important nuclear factor act as the oxygen sensor. It plays an important role in the pathogenesis and development of various hypoxic/ischemic diseases 12, 13. It was reported that ischemic preconditioning could attenuate ischemic injury and increase the expression of HIF-1α which involved in oxygen homeostasis responded to diminished oxygen tension 5, 14. HIF-1α can also regulate the expression of the genes such as heme oxygenase-1 (HO-1) and vascular endothelial growth factor (VEGF) 15-20, which can effect on endothelial cells (ECs) proliferation, migration, and cell organization during recovery phases after hepatic microvascular dysfunction by promoting the secretion of growth and survival factors. The stability of HIF-1α protein is tightly regulated by the oxygen level of the tissue. The degradation of HIF-1α is mediated by proline hydroxylases (PHDs), which can catalyse the hydroxylation of specific proline residues in the HIF-1α subunits. The activation of PHDs is oxygen-dependent.
Although the molecular mechanisms underlying IRI remain to be clearly elucidated, during the past several years, many investigations have their focus on the intervention of hepatic IRI 21-23. Ischemic preconditioning (IpreC), defined as brief periods of ischemia and reperfusion before sustained ischemia, is a promising approach to minimize hepatic IRI in animals and humans 1, 2. Direct mechanical preconditioning in target organ has the benefit of reducing ischemia-reperfusion injury (IRI), and resulting in increased tolerance toward organ hypoxia. However, its main disadvantage is trauma to major vessels and stress to the target organ 20, 24. Remote ischemia preconditioning (RIPC) is a recent observation in which brief ischemia of one organ has been shown to confer protection on distant organs without direct stress or trauma to blood vessels of the organ. RIPC was reported for reducing myocardial and renal injury 25- 27. Furthermore, recent studies have proved that several brief cycles of ischemia and reperfusion at the onset of sustained reperfusion after ischemia, termed ischemic postconditioning (IpostC), provided effective cardioprotection on IRI. But most of these studies were focused on hearts and little was known about whether IpostC offered protections on hepatic IRI and the mechanism 28-31. Proanthocyanidins, mainly presenting in the seeds of grapes, has been reported to possess a broad spectrum of biological, pharmacological and therapeutic activities against free radicals and oxidative stress. Vitro studies reported that proanthocyanidins were potent scavengers of peroxyl and hydroxyl radicals that were generated in the reperfusion myocardium after ischemia. However, fewer reports demonstrated their effects on hepatic ischemic and reperfusion injury 32, 33.
In this paper, the protective effects of IpreC, RIPC (we executed remote ischemia preconditioning on hind limbs of mice) and IpostC were evaluated on the warm IRI model of mouse liver. The results show that all these three strategies were effective on protecting liver against ischemia and reperfusion injuries, and providing the preservation of hepatic function. Combined IpreC with IpostC could offer additional protection over the solo treatment. In addition, the underlying mechanisms were examined by monitoring the production of pro-inflammatory cytokines and ROS. We also observed the level of HIF-1α in the IRI liver tissue. The protective effects of individual IpreC and IpostC against hepatic IRI was related with its ability to reduce tissue oxidative stress level by modulating the activities of SOD, GSH-PX, CAT and NOS. Pre- and Post-conditioning procedures could also decrease the cellular injuries and promote cell survival through suppressing cytokine production. Up-regulation of the HIF-1α and VEGF protein level was also evident after IpreC and IpostC treatment.
Our studies also suggested that the synergistic protection by combined pre- and post conditioning was contributed by increased CAT and GSH-Px activities, as well as enhanced hypoxia self-defensive response. However, combined IpreC and IpostC failed to further reduce cytokines release compared to individual treatment. The utilization of grape seed proanthocyanidins in our research showed that GSP could further improve the oxidation resistance in combined pre- and postconditioning groups, and its implementation was more convenient particularly in combined remote preconditioning and postconditioning group. All these results provided experimental evidences to evaluate the protective effects of IpostC/IpreC strategies against hepatic IRI in details and may guide future research on anti-IRI. Furthermore, the combination of GSP, remote ischemic preconditioning and postconditioning strategy was more humane and relatively easier to be operated, especially during the warm liver transplantation: the donor willing to accept the nondestructive preconditioning and postconditioning is convenient to carry out before reopen blood supply on graft in the receptor, this combination protocol might have huge potential to be used in clinical surgery.
MATERIALS AND METHODS
Mice
Male inbred wild-type (WT) C57BL/6 mice weighing 25-30gm were used. The animals were housed in China medical university animal facility under specific pathogen-free conditions and received humane care according to National Institutes of Health guidelines. The mice received GSP preconditioning were injected GSP intraperitoneally 20mg/kg/day for three weeks before using, while the mice in control group were injected the same volume normal saline.
Mouse Hepatic IRI Model
An established mouse warm hepatic IRI model 30 was used with modification. Briefly, mice were injected with heparin (100U/kg), and an atraumatic clip was used to interrupt the arterial and portal venous blood supply to the cephalad lobes of the liver. After 30 minutes of ischemia, the clip was removed, initiating hepatic reperfusion. Mice were sacrificed after 1h to analyze the acute phase of liver IRI 31. GSP (purity>95%) provided by Tianjin Jianfeng Natural Product R&D, Co. Ltd and was diluted by distilled water before using.
Groupings were listed as in Fig. 1A and Fig. 2A.
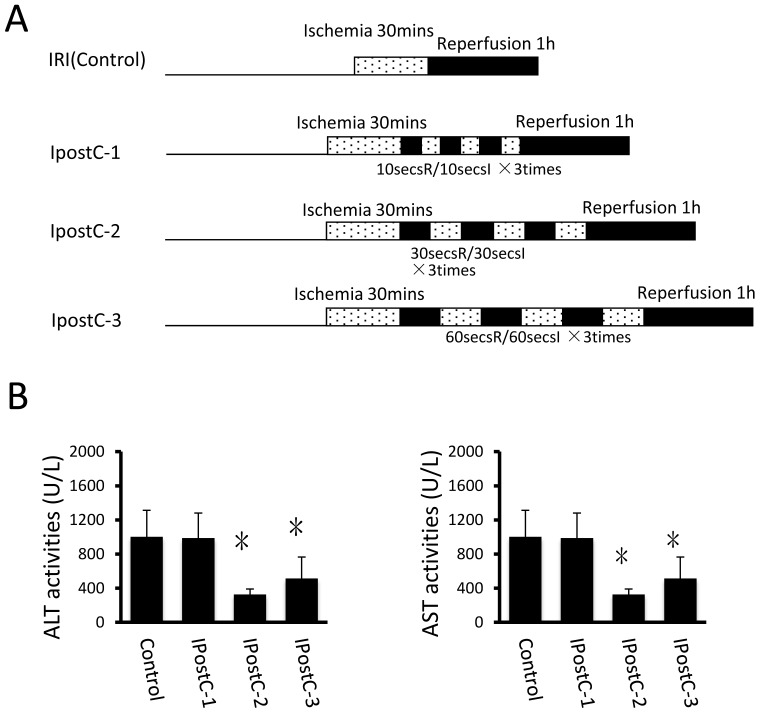
Figure 1.
Effects of ischemic postconditioning on the activities of ALT and AST in serum. Experimental protocols of the IRI (control group) and the ischemic conditioning (pre-, postconditioning groups). IRI: 30 minutes of ischemia followed with 1 hour reperfusion. Dotted areas represent the periods of ischemia; Black areas represent the periods of perfusion. IpostC procedure: 10, 30, 60 seconds of reperfusion followed by 10, 30, 60 seconds of reocclusion for three times at the onset of reperfusion. (B) ALT and A ST activities in mice serum were measured at 1 hour after perfusion. (mean ±s, n=7) Activities of these two enzymes are significantly lower in serum in IpostC-2 and IpostC-3 groups. (* indicate P<0.05 when compared with control group )
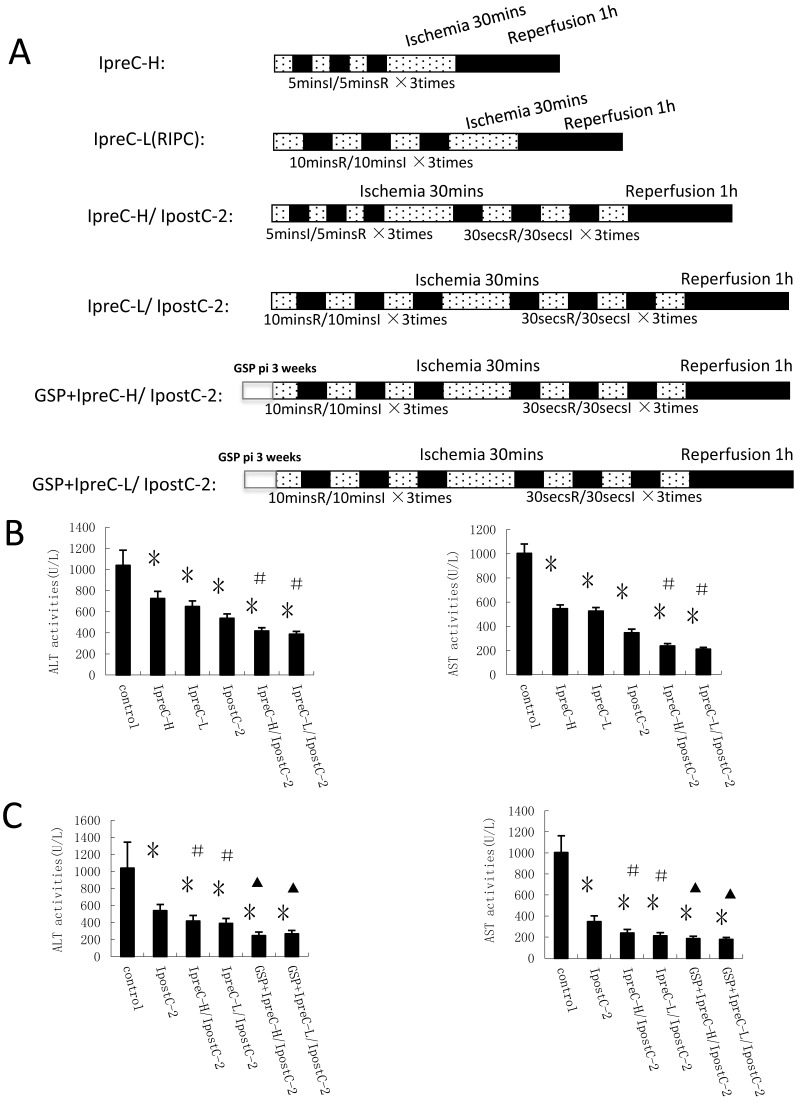
Figure 2.
Effects of Ischemic conditioning in solo or combined protocols and GSP on the activities of ALT and AST in serum. (A) Experimental protocols of individual or combined pre- and post- conditioning protocols. Direct mechanical preconditioning (IpreC-H): The portal triad was occluded for 5 minutes followed by reperfusion for 5 minutes, repeated three times before hepatic ischemic, reperfusion procedures. Remote ischemia preconditioning of hind limb (IpreC-L): The hind limbs of the mice were tightly binding by hemostasis clips for 10 minutes followed by reperfusion for 10 minutes, repeated three times before hepatic ischemic, reperfusion procedures. Dotted areas represent the periods of ischemia; Black areas represent the periods of perfusion. GSP preconditioning: The mice received GSP preconditioning were injected GSP intraperitoneally 20mg/kg/per day for three weeks before using. (B) ALT and AST activities in mice serum were measured at 1 hour after perfusion. (mean ±s, n=7). (* indicate P<0.05 when compared with control group; #indicate P<0.05 when compared with IpostC-2 group). GSP could further reduce the activities of ALT and AST in combined remote preconditioning and postconditioning groups. (mean ±s, n=7). (* indicate P<0.05 when compared with control group; #indicate P<0.05 when compared with IpostC-2 group; ▲indicate P<0.05 when compared with IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2).
Hepatocellular Injury Assay
Aspartase aminotransferase (AST) and alanine aminotransferase (ALT) in serum were used as indicators of hepatocyte function. Blood samples were centrifuged immediately at 8000 × g, 4°C for 10 minutes. Serum enzyme levels were measured with a Bayer 1650 automatic biochemical analyzer.
Detection of the total antioxidative capacity and the activities of antioxidative enzymes in hepatocytes
Tissue homogenate was made in PBS buffer and centrifuged at 1800× g 10min at 4°C to precipitate the insoluble material. The supernatant were used for the followed assay. The total antioxidative capacity (T-AOC), malondialdehyde (MDA) and the activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-PX), Nitric Oxide Synthase (NOS) were measured by a commercial testing kit (NanJing JianCheng Bioengineering Institute) according to the manual.
RNA extraction and Quantitative Real-Time PCR
Total RNA was isolated from mouse liver by RNeasy kit (Qiagen) according to the manufacturer's instructions. cDNA was synthesis as suggested by TaKaRa RNA PCR Kit (AMV) Ver3.0. Real-Time PCR was performed using ABI7300 system with the Fermentas SYBR Green PCR pre mix. Primers are listed below:
TNF-α: 5'GTGGGTGAGGAGCACGTAGT3'; and 5'CCCCAAAGGGATGAGAAGTT3'
IL-1: 5'CCTACCTTTGTTCCGCACAT3'; and 5'AAGTGTGTCATCGTGGTGGA3'
HIF-1α: 5'CTGCCACTGCCACCACAACT3'; and 5'CAGGAAAGAGAGTCATAGAA3'
PHD: 5'CCAGCACCTC GGGGTCTGCC3'; and 5'GACTCGTCCC TGTCCCACCT3'
VEGF: 5'GTCGGGTGGGAGTTATGG3'; and 5'GACGAGGTTTGAGGAAGG3'
ELISA and Immunoblotting
Livers were removed from the mice, washed with PBS, and weighed. Livers were homogenized in cold PBS buffer and centrifuged at 1800× g 10mins at 4°C. The supernatant were used in the ELISA assay. TNFα and IL-1 levels were determined by the EBIOSCIENCE Mouse ELISA Ready-SET-Go kit. For immunoblotting assay, mouse liver lysate was made in RIPA buffer and separated on SDS-page gel. The HIF-1 α, PHD, VEGF and GAPDH antibodies were bought from Abcam Ltd (USA).
Statistical Analysis
Statistical analyses were performed by SPSS programs. All data are expressed as means ± standard deviation. Differences between experimental groups were analyzed with an unpaired 2-tailed Student t test. All differences were considered statistically significant at a P value <0.05.
RESULTS
Ischemic preconditioning and postconditioning reduce hepatic ischemia and reperfusion injury
In the first set of experiments, ALT and AST activities were used as markers to evaluate the effects of various ischemic postconditioning strategies against hepatic ischemia and reperfusion injury. Compared to control group, the plasma activities of ALT and AST significantly decreased in IpostC-2 and IpostC-3 groups (P<0.05), decreased more obviously in group IpostC-2, as shown in Figure 1B. No significant alternations of the activities of ALT and AST in plasma were found in IpostC-1 group. Due to the significant protection function of IpostC-2, IpostC-2 was used in the combined Pre-, remote preconditioning and GSP ip procedure in the followed experiments.
Combinations of the preconditioning and postditioning procedures provide synergistic protection against the ischemic reperfusion injury
Our hypothesis was that combinations of the preconditioning and postconditoning procedures would show more effective protection effects against hepatic ischemia and reperfusion injury than the individual treatment. To test this hypothesis, we designed different experimental protocols and measured the plasma activities of ALT and AST in serum as shown in Figure 2A. Indeed, the activities of these two enzymes were significantly decreased in IpreC-H, IpreC-L, IpostC-2, IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2 groups (P<0.05) compared to control group (Figure 2B). Remote ischemia preconditioning (IpreC-L) showed similar protective affect against IRI as direct ischemia preconditioning (IpreC-H). More importantly, compared to IpreC-H and IpreC-L groups, the activities of ALT and AST in IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2 groups decreased more obviously (P<0.05). Hence, the combinations of the pre- and post conditioning produce better hepatic protection than individual treatments. Besides, the utilization of GSP in our studies showed that GSP could further reduce the activities of ALT and AST in combined preconditioning and postconditioning groups (P<0.05) compared to IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2 groups (Figure 2C).
TNF-α and Interleukin-1 (IL-1β) levels reduced in the pre- and post- conditioning groups
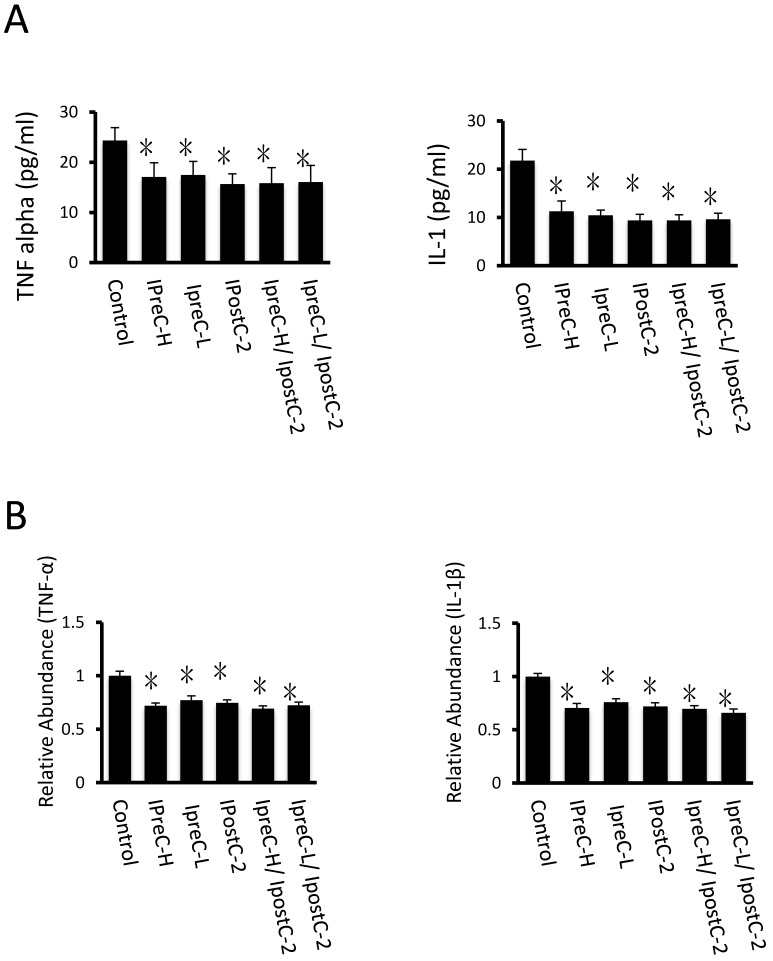
Pro-inflammatory cytokines, including cytokine TNFα and IL-1β, are important mediators of the apoptotic pathway following reperfusion injury. Compared to control samples, the concentration of TNF-α and IL-1β in serum were dramatically decreased in IpreC-H, IpreC-L, IpostC-2, IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2 groups (P<0.05) (Figure 3A). And the pro-inflammatory cytokines reduction was caused by the decreased mRNA expression as shown in Figure 3B. Please note that the combined pre- and postconditioning failed to further reduce the TNFα and IL-1β level in the mouse serum over individual procedure.
Figure 3.
Cytokines level in mouse serum after IpostC, IpreC treatment. TNF-α and IL-1β level were measured in mice serum. (mean ±s, n=7). (* indicate P<0.05 when compared with control group; #indicate P<0.05 when compared with IpostC-2 group). (B) TNF-α and IL-1β mRNA abundance in serum were quantified by RT-PCR. Results obtained with pre-, post- conditioning groups were normalized to results obtained with control group, which were given a value of 1. (mean ±s, n=7). (* indicate P<0.05 when compared with control group; #indicate P<0.05 when compared with IpostC-2 group).
Response of antioxidative pathways in the pre- and post conditioning process
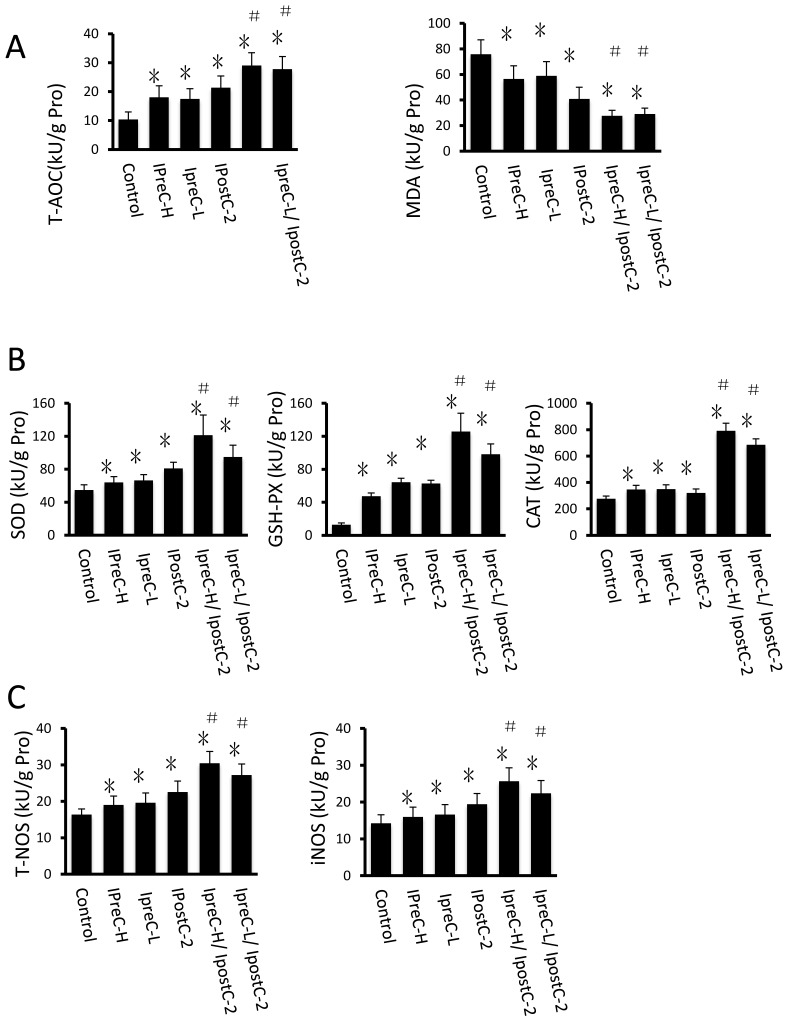
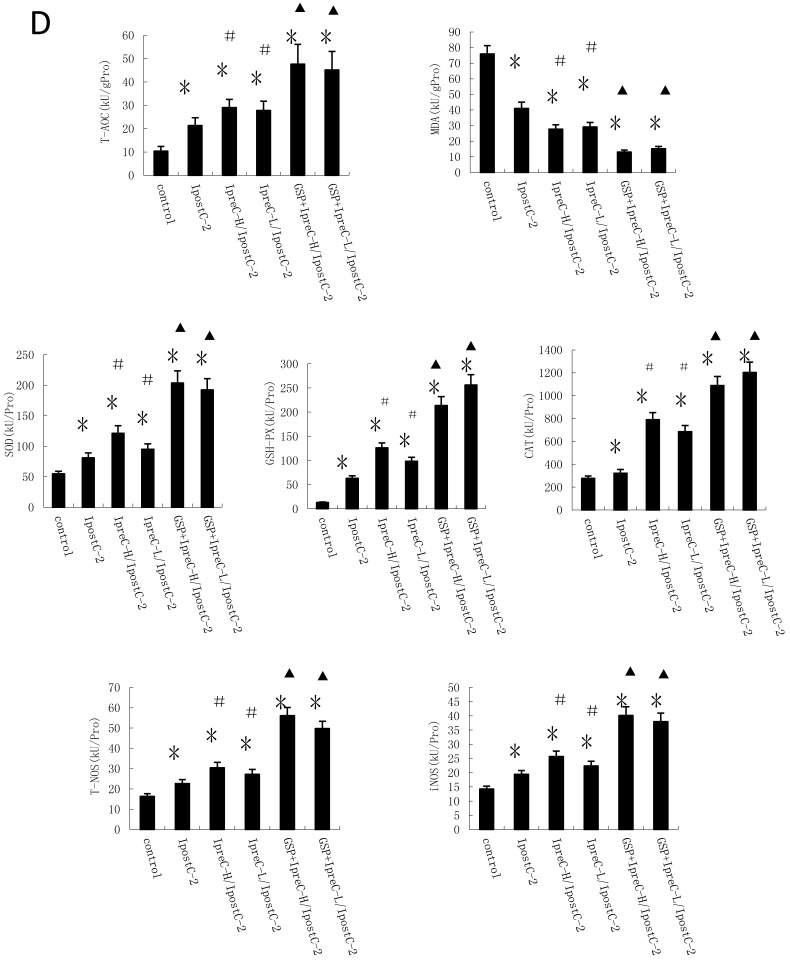
Another important factor causing ischemia and reperfusion injury are reactive oxygen species (ROS) mediated cell apoptosis. To investigate the role of ROS in IR injury, we first measured the total antioxidative capacity (T-AOC) and malondialdehyde (MDA) level that indicate the overall level of the tissue oxidative stress. As shown in Figure 4A, either individual or combinations of IpostC, IpreC protocol could decrease the oxidative stress level indicated by reduced MDA content and elevated T-AOC content. To quantify the contribution of different ROS scavengers, the activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-PX), total nitric oxide synthase (NOS) and iNOS were measured in the mouse serum 1 hour after perfusion. The activities of all these enzymes elevated in IpreC-H, IpreC-L, IpostC-2, IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2 groups at 1h after reperfusion (P<0.05) (Figure 4B and C). Interestingly, only the activities of GSH-PX and CAT showed more dramatic elevation in IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2 groups than those in the individual IPreC and IpostC groups. But in the GSP combined IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2 groups, the activity of all these ROS scavengers and antioxidants were increased (P<0.05) compared to IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2 groups (Figure 4D).
Figure 4.
Effects of Ischemic preconditioning, postcondioning protocols and GSP on hepatic oxidative stress. Total antioxidative capacity (T-AOC) and the malondialdehyde (MDA) contents in mouse liver. (mean ±s, n=7). (* indicate P<0.05 when compared with control group; #indicate P<0.05 when compared with IpostC-2 group). SOD, CAT and GSH-PX enzymatic activities and (C) T-NOS and iNOS activities in mouse liver. (mean ±s, n=7). (* indicate P<0.05 when compared with control group; #indicate P<0.05 when compared with IpostC-2 group). (C) Effect of GSP on the activities of ROS scavengers and antioxidants. (mean ±s, n=7). (* indicate P<0.05 when compared with control group; #indicate P<0.05 when compared with IpostC-2 group; ▲indicate P<0.05 when compared with IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2).)
Effect of ischemic postconditioning on the tissue hypoxia response
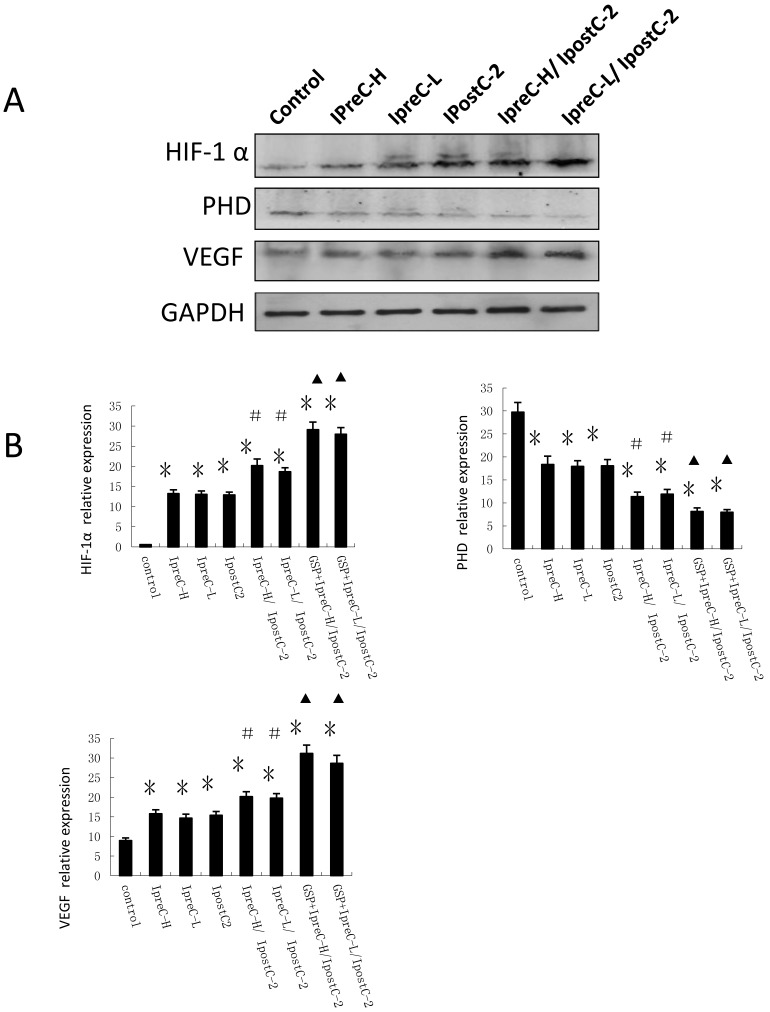
In addition to the cytokine and ROS level, hypoxia was reported as an important environmental alteration in the process of liver IR injury. Adaptation to hypoxia are mediated by hypoxia-inducible factor 1(HIF-1), which is a master transcriptional factors activated by low oxygen tension. HIF-1α and its signaling pathway component PHD level were analyzed by immunoblotting and Real Time PCR. As shown in Figure 5A, the expression of HIF-1α and VEGF were clearly increased in IpreC-H, IpreC-L, IpostC-2, IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2 groups when compared with the control group. The expression of PHD protein only showed mild change, probably because the activity of PHD protein was biochemically regulated by the tissue oxygen level. Compared to IpreC-H and IpreC-L groups, combined IpreC and IpostC could further increase (P<0.05) the expression of HIF-1α and VEGF, particularly in the GSP combined IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2 groups (P<0.05) (Figure 5B).
Figure 5.
Effect of pre- and postconditioning on the expression level of HIF-1 alpha, PHD and VEGF. Whole cell extracts made from mouse liver were separated on SDS- page gel. HIF-1 α, PHD, VEGF and GAPDH protein level were determined by immunblotting with the specific antibodies against the proteins listed on the right. GAPDH was used as a loading control. Effect of GSP on the expression level of HIF-1 alpha, PHD and VEGF. (mean ±s, n=7). HIF-1 α, PHD, VEGF and GAPDH mRNA level were determined by Real-Time PCR. (* indicate P<0.05 when compared with control group; #indicate P<0.05 when compared with IpostC-2 group; ▲indicate P<0.05 when compared with IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2).
DISSCUSSION
Evaluation of the protective effects of pre- and postconditioning against the IRI
Numerous studies have investigated the underlying mechanisms of ischemia and reperfusion injury with the goal of finding therapeutic approaches to enhance ischemia tolerance 2-11. Among them, ischemic conditioning (preconditioning, postconditioning and remote conditioning) was widely used and these strategies could provide tissue-protective effects after ischemia and reperfusion injury. (a) Ischemia preconditioning (IpreC), this strategy has received wide range of research and made great achievements because of its significant protective effects on hepatic ischemia reperfusion injury by stimulating endogenous protective mechanisms. However, IpreC application time is hard to control (hard to implementation before unpredictable long time of ischemia). (b) Remote organ ischemic preconditioning (RIPC): RIPC is brief ischemia and reperfusion of one organ to protect other distant organs from sustained IRI without direct stress to the organ which can overcome some of the limitations of the direct preconditioning. RIPC on limb, gut, mesenteric, or kidney can reduce myocardial infarct size in animal models. In humans, RIPC on skeletal can be used for protecting heart from IRI. (c) Ischemic postconditioning (IpostC): Unlike IpreC, IpostC applies the brief episodes of ischemia at the onset of reperfusion following a prolonged ischemia36. Since IpostC can be easily applied with precisely controlled timing, this approach appears to have greater potential for clinical application. However, most of the current studies are concentrated on the IRI of heart due to the remarkable success of postconditioning, and it remains to be determined whether IpostC can provide similar effective protection on hepatic IRI compared to IpreC.
We are the first to evaluate these strategies side by side in a mouse model using ALT and AST activities as biomarkers. In our study, hepatic IpostC were established by three sets: 10, 30 and 60 seconds of reperfusion followed by 10, 30 and 60 seconds of ischemia for three times at the onset of reperfusion. The results showed that 30 and 60 seconds IpostC could offer effective protection but the 30 seconds protocol could provide the best protection. Our study also found that both direct ischemic preconditioning (IpreC-H) and remote ischemic preconditioning (IpreC-L) were effective interventions to hepatic IRI and the latter one was more convenient for application without the direct damage to blood vessels of the IRI liver. Significantly, IpostC could offer almost the same level of protection as IpreC on hepatic IRI and might be easier for doctors to control the surgery time.
It was the first time that the combined RIPC (IpreC-L) and IpostC procedures was tested on mice hepatic IRI model while most of studies of this field were focused on solo treatment on ischemia and reperfusion organs. Our data suggested that compared with the individual treatment, the combined IpreC and IpostC could offer additional protection. Because the combination of IpreC-L and IpostC-2 was relatively easier to be operated, they could have huge potential to be used in a clinical surgery. However, the significance of our studies in human is still need to be determined in future.
The mechanisms by which that pre- and postconditioning decreasing the liver injury
The mechanisms involved in IpreC and IpostC on hepatic IRI also had been investigated in our study in detail. Our results suggested that the protective effects of IpreC and IpostC against hepatic IRI were related with their roles in reducing the tissue oxidative stress level 35, 36. Both pre- and postconditioning could regulate the activities of SOD, GSH-PX, NOS and CAT. Combined ischemic preconditioning with ischemic postconditioning could offer additive protection by increase the GSH-PX and CAT activities. IpreC and IpostC could also decrease the cellular injuries and promote cell survival through suppressing cytokines release. But combined IpreC and IpostC could not further change the cytokines level. As reported, IpreC and IpostC have similar physiological and cellular protection mechanism: they have the same catalytic substrate-- adenosine; they transport bioinformation through PI3K/Akt pathway; they can decrease cytokines release, etc 20. All these suggested that individual application IpreC or IpostC might reach the limit of decreasing cytokines release in mice, then the combinations of pre- and postconditioning failed to further reduce the TNFα and IL-1β production. Recent studies have hypothesized a role of TNFα and IL-1β in liver regeneration: TNFα and IL-1 are indispensable for liver regeneration. So, our data confirmed that maintain the concentration of TNFα and IL-1 at certain level might help liver function recovery in liver regeneration process. The expression of HIF-1α and VEGF were increased in IpreC-H, IpreC-L, IpostC-2, IpreC-H/ IpostC-2 and IpreC-L/ IpostC-2 groups compared with control which would help illustrating the speed of cells regeneration and the recover status of hepatic function.
Grape seed proanthocyanidins (GSP) could further improve the oxidation resistance in combined pre- and postconditioning groups
The therapeutic approaches to enhance ischemia tolerance, besides ischemic conditioning, covered metabolic strategies, therapeutic gases (NO, H2S), nucleotide and microRNAs, et al. Some botanical extracts such as trans-resveratrol was recently used in hepatic IRI model and showed its benefits on hepatocyte protection 37. Grape seed proanthocyanidins has been reported to possess a broad spectrum of pharmacological and medicinal properties against oxidative stress. This study first showed that GSP exerted an antioxidant effect on hepatic ischemic and reperfusion injury: GSP could improve the activities of ROS scavengers and oxidation resistance in combined pre- and postconditioning groups. More important, the utilization of GSP was a well-accepted, noninvasive way to be operated as pharmacologic preconditioning during clinical liver transplantation and had minimal negative effects for patients to take without aggravating liver metabolic burden.
In summary, the comparation between these three strategies IpreC/RIPC/IpostC was associated with their comparable protection on mouse livers against warm IRI. Combined IpreC with IpostC could offer additive protection and the protocol of remote preconditioning combined with IpostC-2 was more convenient for clinical application. The utilization of grape seed proanthocyanidins (GSP) in our research showed that GSP could further improve the oxidation resistance in combined pre- and postconditioning groups. We investigated the generation of reactive oxygen species and pro-inflammatory cytokines in mice after hepatic ischemia reperfusion, which helped us find the best strategy suitable for decreasing the damage of warm IRI. And we also observed the alternations of the hepatic tissues response to hypoxia: it described the hepatic own defensive condition and the mechanism of these strategies promoting the organ fit for the oxygen-deficient environment. Further studies are necessary to determine other mechanisms involved in IpreC and IpostC, as well as the development of novel drugs, which can mimic the function of pre- and postconditioning.
Acknowledgments
The authors would like to thank Rui Ye for critical comments on the manuscript. This study was supported by Natural Science Foundation of China (NO.81130042 and NO.31171323) and University Innovation Team support plan of Liaoning (NO.LT2011011).
Authorship: XS, NZ and HZ: designed and performed research/study. XS, HX and LC: wrote the paper.
References
- 1.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284(1):G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 2.Rougemont O, Lehmann K, Clavien PA. Preconditioning, organ preservation, and postconditioning to prevent ischemia-reperfusion injury to the liver. Liver Transpl. 2009;15(10):1172–1182. doi: 10.1002/lt.21876. [DOI] [PubMed] [Google Scholar]
- 3.Ambros JT, Herrero-Fresneda I, Borau OG, Boira JM. Ischemic preconditioning in solid organ transplantation: from experimental to clinics. Transpl Int. 2007;20(3):219–229. doi: 10.1111/j.1432-2277.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhou G, Dada LA, Wu M, Kelly A, Trejo H, Zhou Q. et al. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am J Physiol Lung Cell Mol Physiol. 2009;297(6):1120–1130. doi: 10.1152/ajplung.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penna C, Mancardi D, Rastaldo R, Pagliaro P. Cardioprotection: A radical view Free radicals in pre and postconditioning. Biochim Biophys Acta. 2009;1787(7):781–793. doi: 10.1016/j.bbabio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Sínay L, Kürthy M, Horváth S, Arató E, Shafiei M, Lantos J. et al. Ischemic postconditioning reduces peroxide formation, cytokine expression and leukocyte activation in reperfusion injury after abdominal aortic surgery in rat model. Clin Hemorheol Microcirc. 2008;40(2):133–142. [PubMed] [Google Scholar]
- 7.Kin H, Wang NP, Mykytenko J, Reeves J, Deneve J, Jiang R. et al. Inhibition of myocardial apoptosis by postconditioning is associated with attenuation of oxidative stress-mediated nuclear factor-kappa B translocation and TNF alpha release. Shock. 2008;29(6):761–768. doi: 10.1097/SHK.0b013e31815cfd5a. [DOI] [PubMed] [Google Scholar]
- 8.Jia C. Advances in the regulation of liver regeneration. Expert Rev Gastroenterol Hepatol. 2011;5(1):105–121. doi: 10.1586/egh.10.87. [DOI] [PubMed] [Google Scholar]
- 9.Lauzier B, Delemasure S, Debin R, Collin B, Sicard P, Acar N. et al. Beneficial effects of myocardial postconditioning are associated with reduced oxidative stress in a senescent mouse model. Transplantation. 2008;85(12):1802–1808. doi: 10.1097/TP.0b013e3181775367. [DOI] [PubMed] [Google Scholar]
- 10.Raat NJ, Shiva S, Gladwin MT. Effects of nitrite on modulating ROS generation following ischemia and reperfusion. Adv Drug Deliv Rev. 2009;61(4):339–350. doi: 10.1016/j.addr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Zaouali MA, Ben Mosbah I, Boncompagni E, Ben Abdennebi H, Mitjavila MT, Bartrons R. et al. Hypoxia inducible factor-1alpha accumulation in steatotic liver preservation: role of nitric oxide. World J Gastroenterol. 2010;16(28):3499–3509. doi: 10.3748/wjg.v16.i28.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koshikawa N, Hayashi J, Nakagawara A, Takenaga K. Reactive oxygen species -generating mitochondrial DNA mutation up-regulates hypoxia-inducible factor -1alpha gene transcription via phosphatidylinositol 3-kinase-Akt/protein kinase C/histone deacetylase pathway. J Biol Chem. 2009;284(48):33185–33194. doi: 10.1074/jbc.M109.054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molitoris KH, Kazi AA, Koos RD. Inhibition of oxygen-induced hypoxia-inducible factor-1alpha degradation unmasks estradiol induction of vascular endothelial growth factor expression in ECC-1 cancer cells in vitro. Endocrinology. 2009;150(12):5405–5414. doi: 10.1210/en.2009-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paddenberg R, Howold N, Hoger C, Janssen H, Grau V, Kummer W. Organ preservation solutions attenuate accumulation and nuclear translocation of hypoxia-inducible factor-1alpha in the hepatoma cell line HepG2. Cell Biochem Funct. 2009;27(8):516–525. doi: 10.1002/cbf.1608. [DOI] [PubMed] [Google Scholar]
- 15.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD. et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104(11):1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law AY, Wong CK. Stanniocalcin-2 is a HIF-1 target gene that promotes cell proliferation in hypoxia. Exp Cell Res. 2010;316(3):466–476. doi: 10.1016/j.yexcr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Lemus ML, Flores ME, Cervantes R, Torres BM, Gudiño G, Chaparro V. et al. Expression of HIF-1 alpha, VEGF and EPO in peripheral blood from patients with two cardiac abnormalities associated with hypoxia. Clin Biochem. 2010;43(3):234–239. doi: 10.1016/j.clinbiochem.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Gessi S, Fogli E, Sacchetto V, Merighi S, Varani K, Preti D. et al. Adenosine modulates HIF-1{alpha}, VEGF, IL-8, and foam cell formation in a human model of hypoxic foam cells. Arterioscler Thromb Vasc Biol. 2010;30(1):90–97. doi: 10.1161/ATVBAHA.109.194902. [DOI] [PubMed] [Google Scholar]
- 19.Bockhorn M, Goralski M, Prokofiev D, Dammann P, Grünewald P, Trippler M. et al. VEGF is important for early liver regeneration after partial hepatectomy. J Surg Res. 2007;138(2):291–299. doi: 10.1016/j.jss.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Hausenloy Derek J, Yellon Derek M. Preconditioning and postconditioning: Underlying mechanisms and clinical application. Atherosclerosis. 2009;204(2):334–341. doi: 10.1016/j.atherosclerosis.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nature Medicine. 2011;17(11):1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Rougemont O, Dutkowski P, Clavien P.A. Biological modulation of liver ischemia-reperfusion injury. Curr Opin Organ Transplant. 2010;15(2):183–189. doi: 10.1097/MOT.0b013e3283373ced. [DOI] [PubMed] [Google Scholar]
- 23.De Rougemont O, Lehmann K, Clavien P.A. Preconditioning, Organ Preservation, and Postconditioning to Prevent Ischemia-Reperfusion Injury to the Liver. Liver Transpl. 2009;15(2):1172–1182. doi: 10.1002/lt.21876. [DOI] [PubMed] [Google Scholar]
- 24.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten J. Inhibition of myocardial injury by ischemic posteonditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):579–588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 25.Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote postconditioning: A novel protective method from ischemia reperfusion injury - A review. J Surg Res. 2008;150(2):304–330. doi: 10.1016/j.jss.2007.12.747. [DOI] [PubMed] [Google Scholar]
- 26.Kanoria S, Jalan R, Seifalian AM, Williams R, Davidson BR. Protocols and mechanisms for remote ischemic preconditioning: a novel method for reducing ischemia reperfusion injury. Transplantation. 2007;84(4):445–458. doi: 10.1097/01.tp.0000228235.55419.e8. [DOI] [PubMed] [Google Scholar]
- 27.Saxena P, Newman MA, Shehatha JS, Redington AN, Konstantinov IE. Remote ischemic conditioning: evolution of the concept, mechanisms, and clinical application. J Card Surg. 2010;25(1):127–134. doi: 10.1111/j.1540-8191.2009.00820.x. [DOI] [PubMed] [Google Scholar]
- 28.Thibault Helene, Piot Christophe, Staat Patrick, Bontemps L, Sportouch C, Rioufol G. et al. Long-term benefit of postconditioning. Circulation. 2008;117(8):1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- 29.Granfeldt Asger, Lefer David J, Vinten-Johansen J. Protective ischaemia in patients: preconditioning and postconditioning. Cardiovascular Research. 2009;83(2):234–246. doi: 10.1093/cvr/cvp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav SS, Sindram D, Perry DK, Clavien PA. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30(5):1223–1231. doi: 10.1002/hep.510300513. [DOI] [PubMed] [Google Scholar]
- 31.Ferrera R, Benhabbouche S, Bopassa JC, Li B, Ovize M. One hour reperfusion is enough to assess function and infarct size with TTC staining in Langendorff rat model. Cardiovasc Drugs Ther. 2009;23(4):327–331. doi: 10.1007/s10557-009-6176-5. [DOI] [PubMed] [Google Scholar]
- 32.Guler A, Sahin MA, Yucel O, Yokusoglu M, Gamsizkan M, Ozal E. et al. Proanthocyanidin prevents myocardial ischemic injury in adult rats. Med Sci Monit. 2011;17(11):BR326–331. doi: 10.12659/MSM.882042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang CZ, Mehendale SR, Calway T, Yuan CS. Botanical flavonoids on coronary heart disease. Am J Chin Med. 2011;39(4):661–671. doi: 10.1142/S0192415X1100910X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song QH, Carl Atkinson, Fei Qiao, Cianflone K, Chen X, Tomlinson S. A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J Clin Invest. 2009;119(8):2304–2316. doi: 10.1172/JCI38289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abas F, Alkan T, Goren B, Taskapilioglu O, Sarandol E, Tolunay S. Neuroprotective effects of postconditioning on lipid peroxidation and apoptosis after focal cerebral ischemia/reperfusion injury in rats. Turk Neurosurg. 2010;20(1):1–8. [PubMed] [Google Scholar]
- 36.Li S, Wu J, Watanabe M, Li C, Okada T. Protective effects of ischemic postconditioning against hypoxia-reoxygenation injury and hydrogen peroxide-induced damage in isolated rat hearts. Exp Clin Cardiol. 2006;11(4):280–285. [PMC free article] [PubMed] [Google Scholar]
- 37.Sahar HK, Cottart CH, Wendum D, Vibert F, Clot JP, Savouret JF. et al. Postischemic treatment by trans-resveratrol in rat liver ischemia-reperfusion: A possible strategy in liver surgery. Liver Transpl. 2008;14(4):451–459. doi: 10.1002/lt.21405. [DOI] [PubMed] [Google Scholar]