Abstract
Many plants have intimate relationships with soil microbes, which improve the plant’s growth and fitness through a variety of mechanisms. Bacillus sp. isolates are natural root-associated bacteria, isolated from Nicotiana attenuata plant roots growing in native soils. A particular isolate B55, was found to have dramatic plant growth promotion (PGP) effects on wild type (WT) and transgenic plants impaired in ethylene (ET) perception (35S-etr1), the genotype from which this bacterium was first isolated. B55 not only improves N. attenuata growth under in vitro, glasshouse, and field conditions, but it also “rescues” many of the deleterious phenotypes associated with ET insensitivity. Most notably, B55 dramatically increases the growth and survival of 35S-etr1 plants under field conditions. To our knowledge, this is the first demonstration of a PGP effect in a native plant–microbe association under natural conditions. Our study demonstrates that this facultative mutualistic plant–microbe interaction should be viewed as part of the plant’s extended phenotype. Possible modalities of recruitment and mechanisms of PGP are discussed.
Keywords: Nicotiana attenuata, Bacillus sp., ethylene-insensitive, plant growth promotion, microbial community, nature, extended phenotype
INTRODUCTION
In addition to the well-studied mutualistic associations that plants have evolved with nitrogen-fixing microbes and mycorrhizae (Franche et al., 2009; Kiers et al., 2011), plants also associate with plant growth promoting bacteria (PGPB), which refer to rhizobacteria and endophytes enhancing their host’s growth and productivity. PGPB have been intensively studied in the context of agricultural practices as means of increasing the productivity of cultivated plants (Maheshwari, 2011). Much less is known about the role of PGPB in an ecological context and whether they increase the growth or fitness of native plants remains unknown. Only a few studies have reported on the root-associated bacterial communities of native plants (Zinniel et al., 2002; Long et al., 2008, 2010), and it is not known if the plant growth promoting (PGP) effects of PGPB occur in nature.
Several mechanisms have been postulated to explain how PGPB stimulate plant growth; broadly categorized as being either direct or indirect (Glick, 1995). Direct mechanisms include the interference with plant hormone homeostasis and increasing nutrient availability to the host by solubilizing inorganic phosphate, or fixing of atmospheric nitrogen (Gamalero and Glick, 2011). Furthermore, in vitro studies have suggested that PGP effects can be mediated by the release of volatile organic compounds (Ryu et al., 2003) which by still unknown mechanisms have PGP effects. PGPB that promote plant growth indirectly by suppressing pathogens and eliciting induced systemic resistance (ISR) are well-known in biological control or defense against insect herbivores (Pineda et al., 2010; Gamalero and Glick, 2011). Bacteria qualify as PGPB when they are able to colonize and elicit positive effects for the plant (Compant et al., 2010). Some bacterial formulations are commercially available for agriculture, even though PGPB often lose their PGP effects when applied under field conditions (reviewed in Kloepper et al., 1989). The inoculation of soil with these microbes may affect the composition and structure of microbial communities, which can result in positive effects on plant growth (Ramos et al., 2003; Jha et al., 2010), but opposite effects have also been reported (Castro-Sowinski et al., 2007; Berg and Zachow, 2011).
The plant hormone ethylene (ET) is known to regulate many different physiological and developmental processes in plants, such as seedling emergence, leaf and flower senescence, and organ abscission, and it is also known to mediate plant responses to abiotic and biotic stresses (Bleecker and Kende, 2000; van Loon et al., 2006). Blocking ET perception with inhibitors such as 1-methylcyclopropene (1-MCP) helps to increase the longevity of flowers, fruits, and ornamental plants (Serek et al., 1995). Ectopically expressing a mutant ET receptor from Arabidopsis (etr1-1) renders plants constitutively insensitive to ET and has revealed the many roles of ET in negotiating (a)biotic stresses (Chang et al., 1993). Transgenic Tetr tobacco plants are unable to withstand attack from common, generally non-pathogenic, opportunistic soil-borne fungal organisms (Knoester et al., 1998; Geraats et al., 2002). Transgenic petunia had poor root development of cuttings, less efficient seed germination and rooting, and delayed seedling growth (Wilkinson et al., 1997; Clark et al., 1999). Several studies found that ET signaling also plays important roles in the communication between plants and mutualistic microbes (Penmetsa, 1997; Iniguez et al., 2005; Camehl et al., 2010; Long et al., 2010). For example, Pseudomonas thivervalensis was originally isolated from 35S-etr1 transformed Nicotiana attenuata plants, and even under stringent conditions (single in vitro inoculation) was only able to colonize these 35S-etr1 plants, suggesting that high levels of ET production (a trait associated with ET insensitivity; von Dahl et al., 2007) coupled with ET insensitivity and its associated changes in metabolism are required for this specific colonization process (Long et al., 2010).
We have developed N. attenuata (coyote tobacco) as a model plant to identify the traits required for the survival of plants in the rough and tumble of their natural environments. To this end we have developed a molecular tool box and a field station so that we transform plants (silenced in the expression of specific genes), fully characterize them under glasshouse conditions and release them into their natural habitat at the field station for detailed characterizations of their ecological performance (Baldwin, 2001). In a previous study (Long et al., 2010), we planted wild type (WT) and isogenic lines transformed to be defective in ET production and perception (ir-aco1 and 35S-etr1) in native Utah soils collected from areas with natural seed banks, to identify the culturable endophytic bacterial community that colonizes N. attenuata seedlings when they germinate from their long-lived seed banks. Isolates of Bacillus sp. strains were found in the roots of the three lines and one particular isolate, dubbed B55, isolated from the roots of 35S-etr1 plants appeared to be beneficial for in vitro seedling growth. Here we explore the PGP potential of this native bacterial isolate, B55, in WT and 35S-etr1 hosts grown in vitro, in the glasshouse, and finally at the field station in Utah.
We had planted 35S-etr1 plants into native habitats of the Great Basin Desert in Utah for four field seasons, and learned that these ET-insensitive plants have many of the same difficulties described above for transformed cultivated tobacco and petunia. The plants have problems establishing strong root growth at the seedling stage, in resisting pathogen attack during rosette-stage growth, and herbivore attack during the flowering stage (Paschold et al., 2007; I. T. Baldwin, unpublished results), difficulties which strongly reduce their survivorship compared to WT plants in the field. Hence these plants were ideal for conducting the first study of the potential PGP effects of a native bacterium (B55) on its host plant in nature. We had fully expected, given what is known about the mechanisms by which PGP comes about (e.g., alterations in ET homeostasis) and the speed with which microbial communities evolve, not to see any PGP effects in nature. However, B55 inoculation not only improved N. attenuata WT’s growth under in vitro, glasshouse, and field conditions, but it also “rescued” many of the deleterious phenotypes of 35S-etr1 plants and dramatically increased their survival under field conditions.
MATERIALS AND METHODS
PLANT MATERIALS AND BACTERIAL STRAIN
The 30th selfed WT line of N. attenuata, originally collected from a native population 35 km upstream from the field plantation, and an isogenic ET-insensitive transgenic line (35S-etr1; A-03-328-8), fully characterized in von Dahl et al. (2007) were used in all experiments. The other transgenic N. attenuata lines used for plant growth promotion assays (Figure 8) were also fully characterized and these lines and their associated references are summarized in Table 2. Seed germination procedures have been described elsewhere (Long et al., 2010). Bacillus sp. B55 (GenBank accession number: JX101913) was isolated from an 35S-etr1 plant grown in native Utah soil (Long et al., 2010). Unless noted otherwise, B55 was routinely cultured on half-strength yeast peptone dextrose agar (YPDA; Sigma, Steinheim, Germany) at 30°C.
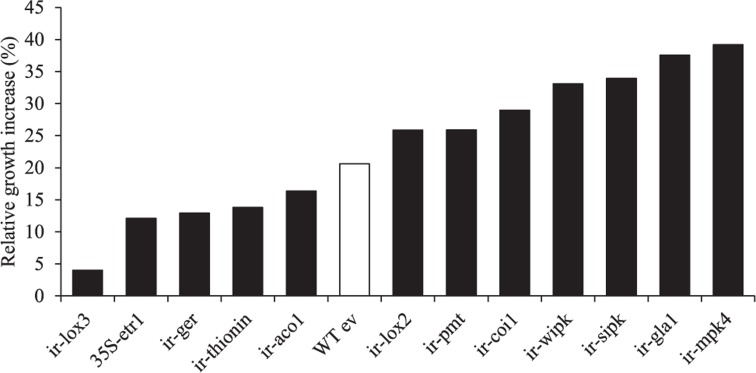
FIGURE 8.
Growth responses of different transgenic N. attenuata lines to a B55 inoculation. Relative B55-associated growth increase of different transgenic N. attenuata lines compared to non-inoculated plants. Twenty day old plants were inoculated with B55 or mock-inoculated (late-stage inoculation). Size of the longest leaf was measured on the day of inoculation and at the end of the experiment (12 or 14 dpi). Growth increase (%) of B55-inoculated plants was calculated relative to non-inoculated plants. See Table 2 for abbreviations of genes silenced by RNAi by expression of inverted repeat (ir) constructs in the transformed lines. dpi, days post infection. n = 10.
Table 2.
Nicotiana attenuata lines used in Figure 8.
| Genotype | Line code | Gene silenced and phenotype | Reference |
|---|---|---|---|
| ir-aco1 | A-03-321-10 | ACC oxidase 1, impaired in ET production | von Dahl et al. (2007) |
| ir-coi1 | A-04-249-1 | Coronatine insensitive 1, impaired in JA perception | Paschold et al. (2007) |
| ir-ger | A-911-10 | Germin-like gene, impaired in responses to (a)biotic stresses | Lou and Baldwin (2006) |
| ir-gla1 | A-09-849-2 | Glycerolipase 1, impaired in oxylipin biosynthesis | Bonaventure et al. (2011) |
| ir-lox2 | A-04-52-2 | Lipoxygenase 2, impaired in traumatin and green leaf volatile production | Allmann et al. (2010) |
| ir-lox3 | A-03-562-2 | Lipoxygenase 3, impaired in JA biosynthesis | Allmann et al. (2010) |
| ir-mpk4 | A-07-119-4 | Mitogen activated protein kinase 4, impaired in stomatal closure in response to (a)biotic stress; elevated chlorophyll contents | Hettenhausen et al. (2012) |
| ir-pmt | A-03-108-3-1 | Putrescine N-methyl transferase, impaired in nicotine production | Steppuhn et al. (2004) |
| ir-sipk | A-06-109-2 | Salicylic acid-induced protein kinase, impaired in MAPK signaling and JA biosynthesis | Meldau et al. (2009) |
| ir-thionin | A-05-96-2 | Pathogenicity-related gene 13, impaired resistance to pathogens | Rayapuram et al. (2008) |
| ir-wipk | A-06-56-1 | Wound-induced protein kinase, impaired in MAPK signaling and JA biosynthesis | Meldau et al. (2009) |
| Ev | A-03-9-1-1 | Empty vector (control) | Zavala et al. (2004) |
All lines used were T3 generation transformed plants harboring a single T-DNA insertion and silenced in the expression of the target gene, and fully characterized in the associated reference publication.
BACTERIAL ACCd, IAA PRODUCTION, AND P SOLUBILIZATION ASSAYS
1-Aminocyclopropane-1-carboxylate (ACC) deaminase (ACCd) activity was determined as described by Glick (1995) by measuring the amount of α-ketobutyrate produced when the enzyme ACCd cleaves ACC. The nanomoles of α-ketobutyrate produced by this reaction were determined by comparing the absorbance at 540 nm of a sample to a standard curve of α-ketobutyrate ranging between 0.1 and 1.0 nmol.
Indole-3-acetic acid (IAA) production was determined as described by Bric et al. (1991) using the colorimetric method. Briefly, DF salt medium supplemented with 5 mM l-tryptophan was inoculated with bacterial isolates and incubated at 30°C. After an incubation period of 48 h on a rotary shaker (200 rpm, 30°C), bacterial cells were removed by centrifugation (4000×g, 10 min). One milliliter of bacterial supernatant was mixed vigorously with 2 mL of Salkowski’s reagent. The mixture was incubated at room temperature for 20 min and the absorbance was measured at 540 nm. Synthetic IAA was used as a positive control.
An inorganic phosphate (P) solubilization assay was carried out after Verma et al. (2001) by inoculating bacterial isolates on Pikovskaya medium. Plates were stabbed using sterile toothpicks. The halo and colony diameters were measured 14 days after the plates were incubated at 30°C.
INOCULATION PROCEDURES
Surface sterilized seeds were incubated over night at room temperature in 3 mL of bacterial suspension in sterile water (OD600 = 1.0); controls were treated with sterile water only. If not otherwise stated, seeds were transferred to Petri dishes containing GB5 medium (Gamborg’s B5 media, Duchefa, Haarlem, The Netherlands) and maintained in a Percival growth chamber (13/11 h day/night cycle, 155 μmol m-2 s-1, 30/28°C). For “late-stage” inoculations, 10-day-old seedlings were transferred to Teku pots (Poeppelmann, Lohne, Germany) filled with sand (0.7–1.2 mm grain size, Raiffeisen, Germany). At day 20, plants were carefully removed from the sand and roots dipped for 2 min into a B55 suspension (OD600 = 1.0). Afterward, plants were placed in 10 cm round pots containing lecaton (Easy Green, Eschborn, Germany and Fibo ExClay, Lahmstedt, Germany) and sand (Figure 4A). Three and six days after transfer, plants were once again inoculated by pouring 50 mL of a B55 suspension (OD600 = 1.0) over the roots.
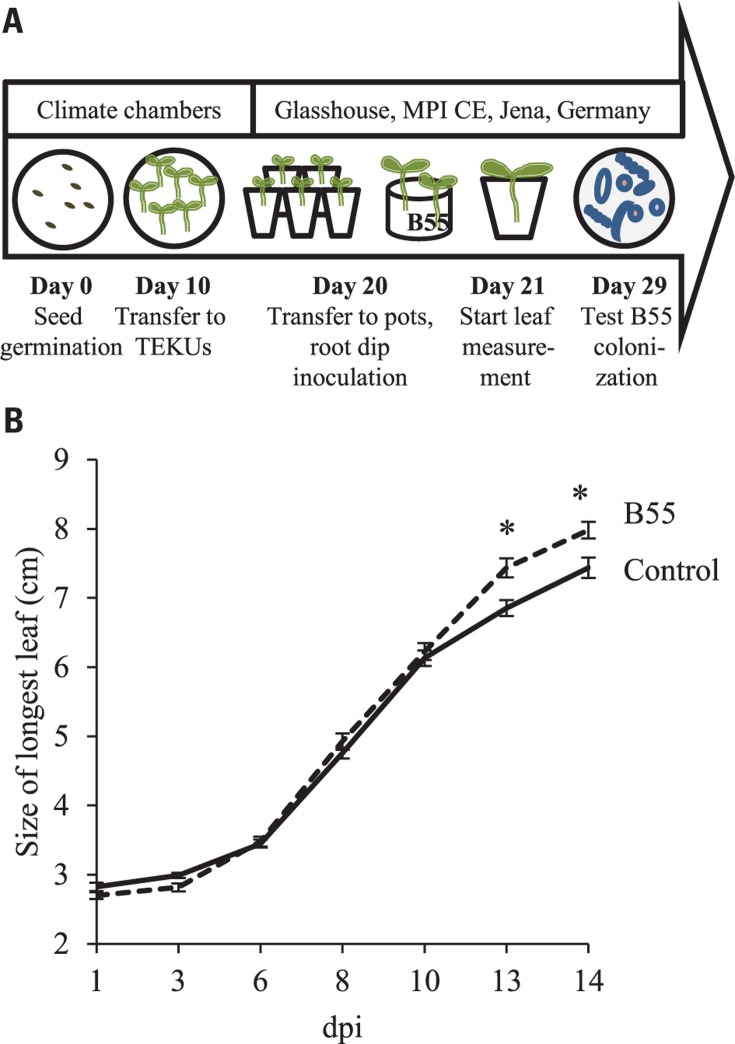
FIGURE 4.
Effects of a “late-stage” B55 inoculation on WT plants. Timeline and experimental design (A). Mean (±SE) length of the longest leaf of mock- or B55-inoculated WT N. attenuata plants cultured in the glasshouse (B). WT plants were grown in a sand-filled Teku pots until day 20, before they were inoculated with B55 and transferred to 10 cm round pots containing lecaton and sand (PLSD test of an ANOVA between mock- and B55-inoculated plants: *P < 0.05). dpi, days post infection. n = 15.
PLANT ET, IAA, AND P CONTENT QUANTIFICATION
Ethylene emissions from B55-inoculated and non-inoculated WT and 35S-etr1 seedlings were measured non-invasively with a photoacoustic spectrometer (INVIVO, Sankt Augustin, Germany) as described by von Dahl et al. (2007). Thirty seeds were germinated in 100 mL three-neck flasks on filter paper and cultivated in a Percival growth chamber (13/11 h day/night cycle, 155 μmol m-2 s-1, 30/28°C). After 12 days, flasks containing the seedlings were subjected to ET measurements (five replicates per treatment). Five empty flasks with filter paper and sterile water served as controls. Two independent experiments were carried out.
For IAA quantification, 12-day-old B55-inoculated and non-inoculated WT and 35S-etr1 seedlings were harvested and immediately frozen in liquid nitrogen and stored at -80°C until analysis. Approximately 500 mg ground seedling powder was extracted according to the method by Onkokesung et al. (2010) and analyzed on a 1200L quadrupole tandem mass spectrometry system (Varian1).
Dried rosette material of B55-inoculated or control WT (38-day-old) plants was used for total P analysis. Analysis was conducted using a microwave-assisted digestion. Briefly, about 100 mg of sample were dissolved in 3 mL suprapur 65% HNO3 (Merck, Darmstadt, Germany) and digested in a Multiwave® (Anton Paar, Graz, Austria). Subsequently, samples were transferred to 50 mL glass vessels and diluted with ultrapure water (Millipore) and submitted to analyses by ICP-OES (Optima™ 3300 DV, PerkinElmer, Shelton, CT, USA). P was detected at λ = 177.4 nm. SRM 1573 a tomato leaves and SRM 1575 a pine needles (NIST, Gaithersburg, USA) were used as reference material. Total P analysis was carried out at the Max Planck Institute for Biogeochemistry, Jena, Germany.
IN VITRO SEEDLING GROWTH MEASUREMENTS
Length of the primary root and number of lateral roots of vertically (Figure 1A, upper panels) grown B55-inoculated or non-inoculated WT and 35S-etr1 seedlings were determined after 10 days of growth. After 12 days of horizontal growth (Figure 1A, lower panels), secondary leaves were counted and leaf surface area was analyzed (according to the video tutorial by Zach Jarou2) using Adobe Photoshop C5. Chlorophyll a and b contents of 12-day-old seedlings were analyzed spectrophotometrically from an 80% acetone extract using a TECAN plate reader (Tecan, Crailsheim, Germany). Two independent experiments with four replicate Petri dishes containing at least seven (vertical placement) or 20 seeds (horizontal placement) were carried out. In vitro B55 colonization was determined for seedlings grown for 8 days on Whatman No. 1 filter paper amended with 1.5 mL of sterile fertilizer [0.6 g Ca (NO3)2·4H2O and 0.3 g Flory Basisdünger 1 (Euflor, Munich, Germany) per liter]. Two independent experiments were carried out.
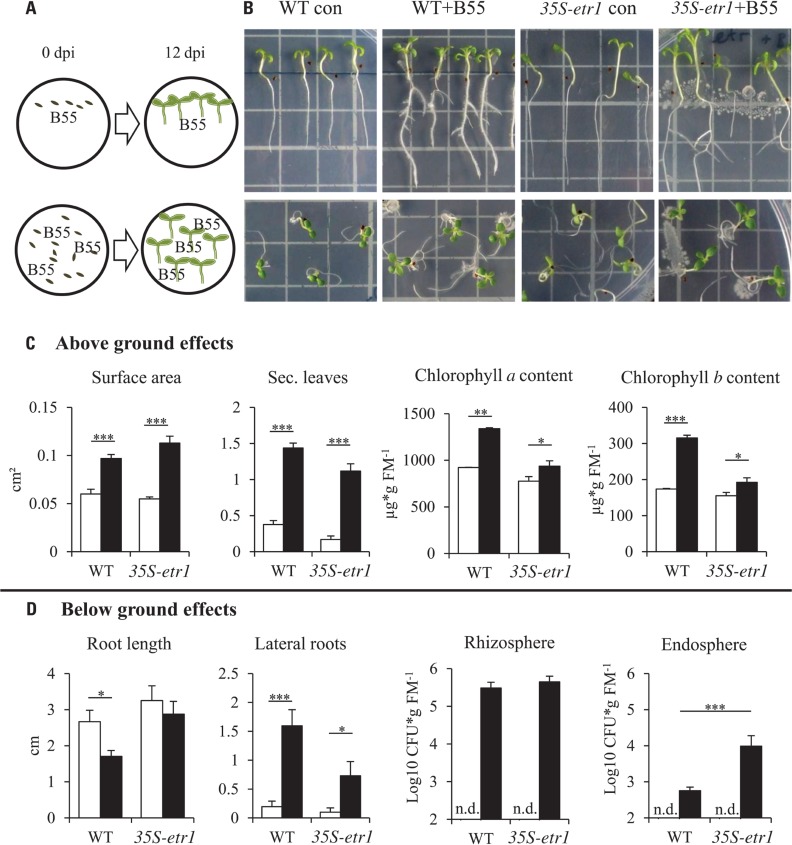
FIGURE 1.
Effects of B55 inoculation on seedlings growth in vitro. Experimental design (A). Effect of a B55 inoculation on WT and 35S-etr1 seedlings, grown vertically and horizontally (B). Mean (±SE) seedling surface area, number of true leaves, chlorophyll a and b content (C), primary root length, number of lateral roots and B55 colonization in the rhizo- and endospheres (D) of mock- or B55-inoculated WT and 35S-etr1 seedlings. White bars represent control treatments, black bars, B55-inoculated seeds (PLSD test of an ANOVA between mock- and B55-inoculated plants: *P < 0.05; **P < 0.01; ***P < 0.001). CFU, colony forming unit; FM, fresh mass; n.d., not detected. n = 4 replicate Petri dishes containing at least 20 or 7 seeds (vertical placement), n = 5 replicate Petri dishes containing at least 20 seeds (for colonization).
BACTERIAL RE-ISOLATION
Rhizoplane bacteria were isolated by vigorously vortexing roots in sterile water for 2 min; appropriate dilutions were mounted on half-strength YPDA and incubated at 30°C. Endophytic bacteria were isolated following the procedure of Long et al. (2010) by removing root epiphytes by surface disinfection. After appropriate surface disinfection, root tissue was cut and titrated in distilled water; dilutions were plated onto half-strength YPDA and incubated at 30°C. After 2–3 days, colony forming units (CFUs) of B55 were counted based on colony morphology. Identity was confirmed by 16S rDNA sequencing (Long et al., 2010). The isolates were identified using the EzTaxon-e server3 (Kim et al., 2012) based on the 16S rRNA sequence data.
PLANT GROWTH PROMOTION EXPERIMENTS IN THE GLASSHOUSE AND FIELD
For glasshouse experiments (Figure 3B), 10-day-old B55-inoculated or non-inoculated seedlings were planted into separate TEKU pots containing sand and lecaton. At 20 dpi, plants were transferred to 10 cm diameter round pots containing lecaton and sand. Pots were placed in a randomized design in the glasshouse (22°C, 65% humidity, 16 h light) in separate bottom containers to avoid cross-contamination. Plants were fertilized every other day with 50 mL distilled water amended with 0.6 g Ca (NO3)2·4H2O L-1 and 0.3 g Flory Basisdünger 1 L-1. Survival of plants was monitored (24 dpi) and length of the longest leaf or stalk height was measured every other day. Total seed capsule number was determined at the end of the experiment (63 dpi). Colonization by B55 was measured 30 dpi; roots were collected and bacterial isolation was performed as described above. Bacterial identity was determined by 16S rDNA sequencing. Two independent experiments were carried out for the analysis of B55 effects on glasshouse-grown plants.
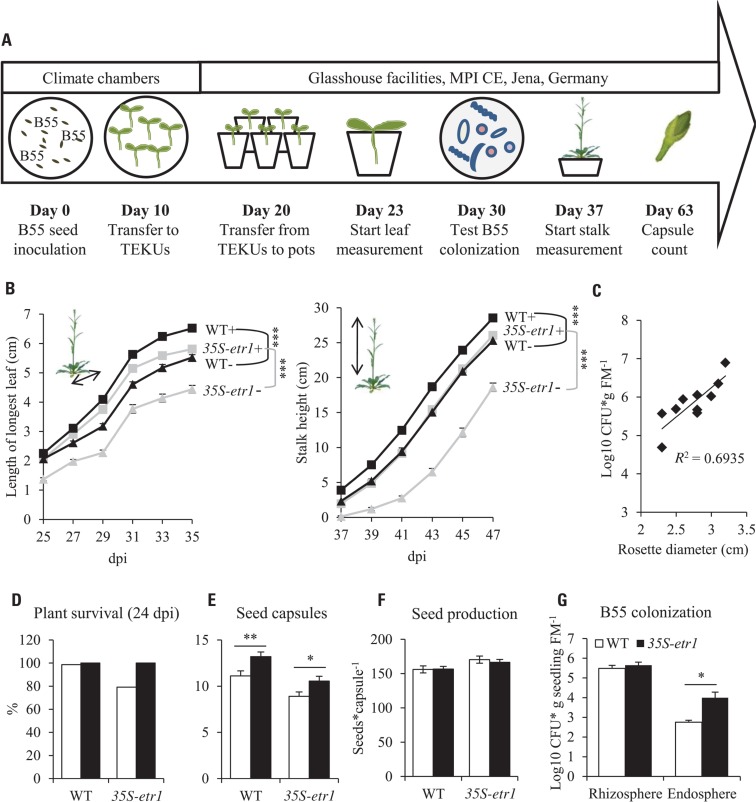
FIGURE 3.
Effects of a B55 inoculation on glasshouse-grown plants. Timeline of experiment (A). Mean (±SE) length of the longest rosette leaf and stalk height (B). Correlation between B55 colonization and WT rosette growth (C). Survivorship (D), seed capsule number (E), seed number per capsule (F) and B55 colonization (G) of B55-inoculated WT and 35S-etr1 plants. Except for (G), white bars represent control treatments, black bars, B55-inoculated plants (PLSD test of an ANOVA between mock- and B55-inoculated plants: *P < 0.05; **P < 0.01; ***P < 0.001). dpi, days post infection; CFU, colony forming unit; FM, fresh mass; n.d., not detected. n = 20 for (B,F); n = 10 for (C); n > 80 for (D); n = 5 for (E).
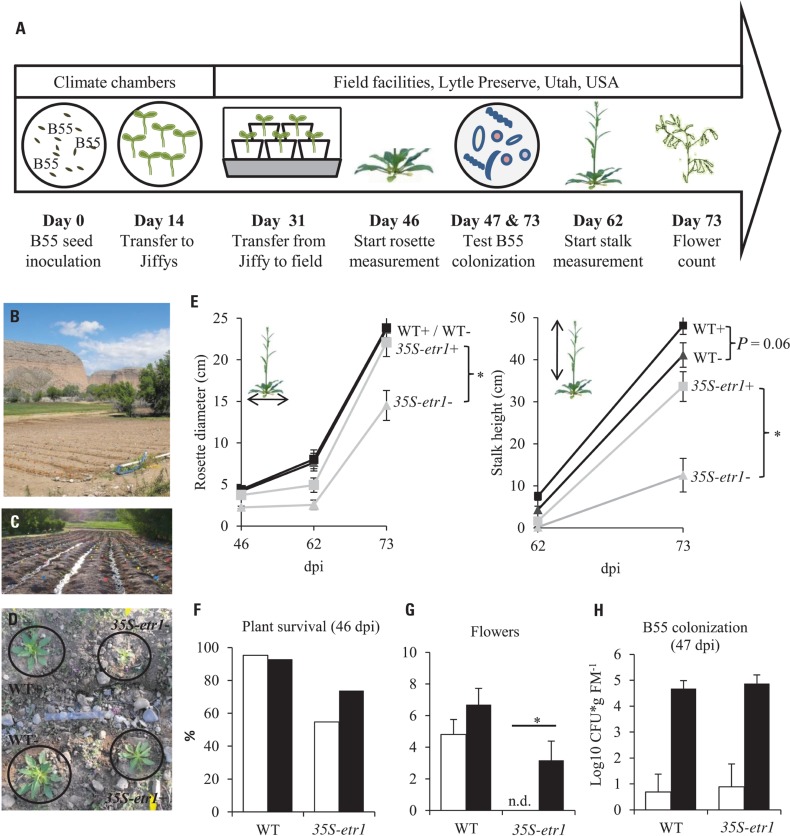
Field experiments were conducted at the at Brigham Young University’s Lytle Ranch Preserve located in the Great Basin Desert, in SW Utah, USA. The release of transgenic plants was carried under APHIS notification 06-242-3r-3a and the seeds were imported under permit number 10-004-105m. Petri dishes containing 3-day-old B55-inoculated or non-inoculated WT and 35S-etr1 seedlings were shipped to the field station. Fourteen days after germination, the seedlings were transferred to pre-hydrated 50 mm peat pellets (Jiffy 7034) and seedlings were gradually adapted to the high light and low relative humidity of the habitat over a 2-week period. Pre-adapted rosette-stage plants were transplanted into an irrigated field plot in size-matched quadruplets (consisting of one B55-inoculated and one non-inoculated WT and 35S-etr1 plant in a randomized design: Figures 5A–D), 31 dpi. Survival of the plants was assessed at 46 dpi, rosette diameters were measured 46, 62, and 73 dpi; stalk height measured at 62 and 73 dpi and the number of flowers was counted at 73 dpi. B55 colonization was quantified at 47 and 73 dpi for five randomly selected quadruplets of plants. Plants were carefully excavated and the loosely attached soil was removed before plants were wrapped in moistened paper towels and sent to the laboratory facility in Jena, Germany. Re-isolation of culturable bacteria was carried out as described, immediately after arrival (2 days after removal from the field). Other culturable, dominant resident bacterial isolates were counted based on colony morphology and the identity of representatives was determined by 16S rDNA sequencing. The experiment was conducted once for 35S-etr1 plants (2009 field season) and twice for WT (2009 and 2010 field seasons).
FIGURE 5.
Effects of a B55 inoculation on field-grown plants (field season 2009). Timeline of experiment (A). Field plot (B). Plants were planted in rows separated by water channels (C), mock- and B55-inoculated WT and 35S-etr1 plants were planted in a quadruplets in a randomized design (D). Mean (±SE) rosette diameter and stalk height (E), survivorship (F), flower number (G), and B55 colonization (H). White bars represent control treatments, black bars, B55-inoculated plants (PLSD test of an ANOVA between mock- and B55-inoculated plants: *P < 0.05). dpi, days post-inoculation; CFU, colony forming unit; FM, fresh mass; n.d., not detected. n = 40 for WT–, WT+, and 35S-etr1+; n = 23 for 35S-etr1 at start of measurements (46 dpi); n = 5 for (H).
DATA ANALYSIS
Data analysis was carried out with the StatView software package (SAS Institute) with a completely randomized analysis of variance. One-way and two-way ANOVAs followed by Fisher’s PLSD test or t-test were used to compare differences among treatments. Correlation analysis was performed with simple regression tests.
RESULTS
BACILLUS SP. B55 CHARACTERISTICS AND IN VITRO PGP
Bacillus sp. B55 was isolated from the endosphere of an ET-insensitive 35S-etr1 N. attenuata plant grown in native Utah soil (Long et al., 2010). Re-isolation experiments have shown that this Gram-positive bacterium is able to colonize the endosphere and rhizoplane of N. attenuata roots; with the rhizoplane typically harboring 103 more bacteria than the endosphere (data not shown).
Inoculation of WT and 35S-etr1 seeds with B55 revealed dramatic PGP effects on the in vitro growth of WT and especially 35S-etr1 seedlings. The ET-insensitive transgenic line produces few root hairs and lateral roots (Long et al., 2010) and tends to grow poorly on Petri dishes (Figure 1B). B55 inoculation enhanced WT and 35S-etr1 seedling growth significantly as clearly seen in seedling leaf surface area (62 and 105% increase, respectively) and in the production of true leaves (three and six times more true leaves per seedling, respectively). Seedling chlorophyll a content increased by 45 and 20% and chlorophyll b content by 80 and 24% for WT and 35S-etr1, respectively (Figure 1C). Although primary root length of WT and 35S-etr1 seedlings tended to decrease by 36 and 11% after B55 inoculation, lateral root number increased significantly. On average, B55-inoculated WT and 35S-etr1 roots had eight and seven times more lateral roots, respectively (Figure 1D, left panels). Interestingly, B55 colonization of the endosphere of 35S-etr1 seedlings was more than 10 times higher than in WT, while rhizosphere colonization was similar (Figure 1D, right panels).
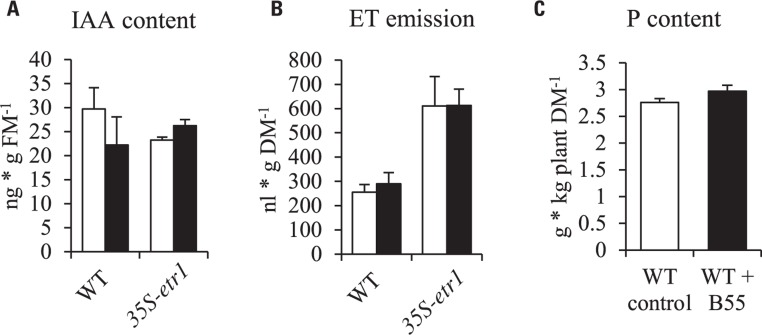
In order to understand the underlying mechanisms of the PGP effect, B55 was tested for known PGP traits. In vitro cultures of B55 produced 0.303 ± 0.026 μM α-ketobutyrate ⋆ (mg protein⋆ h)-1 ACCd (an enzyme that decreases ET production by the cleavage of the ET precursor, ACC) and 9.478 ± 2.522 μg ⋆ mL-1 IAA, an auxin analog. Furthermore, qualitative enzyme tests revealed that B55 is able to solubilize phosphate. However, seedling ET emissions and IAA contents were not significantly changed by B55 inoculation and WT rosette plant P contents were not altered by B55 inoculation (Figure 2).
FIGURE 2.
Effects of B55 inoculation on N. attenuata IAA content, ET emission and P content. Mean (±SE) IAA content (A) and ethylene emission (B) of N. attenuata WT and 35S-etr1 seedlings. White bars represent mock-inoculated seedlings; black bars represent seedlings inoculated with B55. Total P content of 38-day-old WT rosette plants (C). FM, fresh mass; DM, dry mass. n = 3 for IAA; 5 for ET; n = 6 for P.
PGP EFFECTS IN THE GLASSHOUSE
In the glasshouse, B55 inoculation increased the growth of both WT and 35S-etr1 plants (Figure 3). At 35 dpi, length of the longest rosette leaf of B55-inoculated WT and 35S-etr1 plants was almost 20% and more than 30% increased compared to non-inoculated plants, respectively (Figure 3B). Stalk heights of B55-inoculated WT and 35S-etr1 plants were 13 and 40% taller, respectively, compared to controls at 47 dpi (Figure 3B). Furthermore, rosette diameters of B55-inoculated WT plants correlated positively with B55 colonization (Figure 3C). The survival of B55-inoculated 35S-etr1 plants was increased by 20% compared to control 35S-etr1 plants; survival rate of WT plants, which was already close to 100%, was not affected (Figure 3D). B55 inoculation also influenced the production of reproductive structures: B55-inoculated WT and 35S-etr1 plants yielded on average 2 and 1.5 more seed capsules, respectively, than control plants (Figure 3E). The seed production (number of seeds per capsule) was not affected by B55 (Figure 3F). B55-inoculated WT and 35S-etr1 glasshouse plants were similarly well-colonized at 30 dpi (Figure 3G).
For these in vitro and glasshouse experiments, B55 treatment prior to germination resulted in strong PGP effects. To determine if B55 could similarly influence plant growth when plants were inoculated at a later stage of development, 20-day-old N. attenuata WT plants were inoculated by root-dipping. At 13 dpi, the length of the longest rosette leaf of B55-inoculated WT plants was significantly larger compared to control; even though the 14% relative growth increase (Figure 4B) was less pronounced than the effects measured when seeds were inoculated prior to germination. Plants were colonized by ca. 1.8 × 102 and 106 log10 CFU⋆g FM-1 in the endosphere and rhizosphere, respectively.
PGP EFFECTS IN THE FIELD
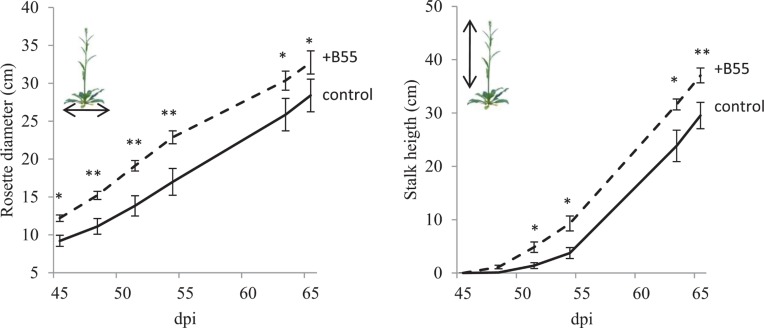
The consistency of PGP effects observed in vitro and in the glasshouse has rarely been tested under field conditions and the PGP effects of a native root-associated bacterium had not been tested in its native host in the field. To conduct such a test, we examined the effect of B55 inoculation (of seeds) on the growth of WT plants, during two field seasons, and 35S-etr1 plants during one field season in their native habitat in SW Utah, USA. B55 inoculation strongly enhanced the survival and growth of the ET-insensitive 35S-etr1 plants, whereas during the first field season (2009), effects on WT plants were barely detectable (Figure 5). At the end of the first field season experiment (73 dpi), 35S-etr1 rosette diameters and stalk heights were significantly increased by about 52 and 170%, respectively, compared to control 35S-etr1 plants (Figure 5E). During the first field season (2009), WT rosette growth did not benefit from B55 inoculation, however, stalks of B55-inoculated plants tended to grow faster than control plants (P = 0.06; Figure 5E, right panel). During the second field season (2010), B55-inoculated WT plants exhibited significant increases in both rosette diameter and stalk height compared to controls (Figure 6).
FIGURE 6.
Effects of a B55 inoculation on field-grown WT plants (field season 2010). Mean (±SE) rosette diameter and stalk height of B55-inoculated and mock-inoculated plants. Plants were grown in a paired design. Only pairs in which both plants survived until the end of the experiment were included in the analysis. Asterisk indicates a statistically significant difference between treatments as determined by a paired t-test (*P < 0.05; **P < 0.01). dpi, days post infection. n = 11 pairs.
The effect of B55 inoculation on the survival of 35S-etr1 plants was dramatic, increasing plant survival by almost 20% (Figure 5F). The number of flowers was evaluated 62 dpi; and no significant difference was found between B55-inoculated and non-inoculated WT plants. Interestingly, the non-inoculated 35S-etr1 plants did not produce any flowers until the end of the experiment, while the B55-inoculated 35S-etr1 plants produced almost as many as the WT non-inoculated plants (Figure 5G). Re-isolation experiments revealed that with 4.7 and 4.9 log10 CFU⋆g FM-1, B55-inoculated WT and 35S-etr1 plants were well-colonized by B55 even after 47 days of growth in the field. Surprisingly, B55-like colonies were also identified from some roots of non-inoculated WT and 35S-etr1 plants (Figure 5H), suggesting that Bacillus sp. isolates were either resident in the field plot, or easily moved between inoculated and non-inoculated plants, perhaps via the watering channels of the field plot (see Figures 5C,D.
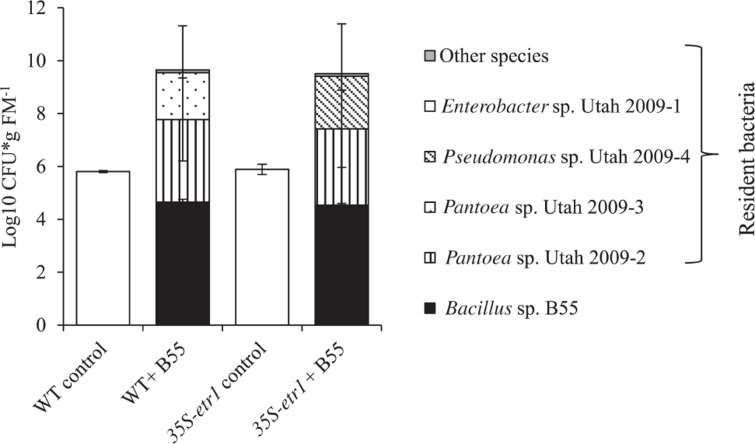
EFFECTS OF B55 ON THE RESIDENT CULTURABLE BACTERIAL COMMUNITY
We examined the influence of a B55 inoculation on the culturable, endophytic, naturally associated plant microbial community of plants after 73 days of growth in the field. B55 inoculation strongly affected the most abundant culturable bacterial taxa associated with WT and 35S-etr1 roots. The analysis focused on bacterial strains found to colonize roots to the same degree as the introduced B55. B55-inoculated plants harbored twice as many bacteria of a greater diversity of bacterial taxa, compared to controls (Figure 7). In addition to B55, inoculated WT roots harbored Pantoea sp. Utah 2009-2 and Pantoea sp. Utah 2009-3 and 35S-etr1 roots harbored Pantoea sp. Utah 2009-2 and Pseudomonas sp. Utah 2009-4 (for accession numbers see Table 1). These bacterial genera were not detected in roots of control plants. No difference in colonization by B55 of the inoculated plants was observed, and B55 was not found amongst the dominant bacterial taxa of non-inoculated plants. The culturable bacterial communities of WT and 35S-etr1 control plants were dominated by one isolate: Enterobacter sp. Utah 2009-1. There was no difference in the extent of colonization by Enterobacter sp. Utah 2009-1 of the non-inoculated WT and the 35S-etr1 plants (Figure 7).
FIGURE 7.
Effects of a B55 inoculation on the resident culturable bacterial community of field-grown plants. Colony forming units of B55 and resident isolates at 73 dpi; see Table 1 for accession numbers of isolates. dpi, days post infection; CFU, colony forming units; FM, fresh mass. n = 3.
Table 1.
Accession numbers of bacterial isolates used in this study.
| Bacterial isolate | GenBank accession no. | Closest type strain neighbor | Reference |
|---|---|---|---|
| B55 | JX101913 | Bacillus aryabhattai B8W22 | This study |
| Utah 2009-1 | JQ172772 | Enterobacter cloacae sub sp. dissolvens LMG 2683 | This study |
| Utah 2009-2 | JQ172773 | Pantoea gaviniae A18/07 | This study |
| Utah 2009-3 | JQ172774 | Pantoea agglomerans DSM 3493 | This study |
| Utah 2009-4 | JQ172775 | Pseudomonas lurida DSM 15835 | This study |
PGP EFFECTS OF B55 ON OTHER TRANSGENIC N. ATTENUATA LINES
B55’s dramatic PGP effects on the ET-insensitive genotype 35S-etr1 motivated us to compare PGP effects on other transgenic N. attenuata lines silenced in phytohormone signaling or defenses against herbivores or pathogens (Table 2). All lines inoculated with B55 (late-stage inoculation) showed positive growth responses. Figure 8 shows the increase in growth of each line compared to non-inoculated plants. While the JA-deficient N. attenuata line, ir-lox3, was barely affected by B55 inoculation, ir-mpk4 (impaired in herbivore-elicited phytohormone signaling) and ir-gla1 (impaired in oxylipin synthesis) benefited the most from the interaction with B55. Interestingly, and in contrast to the results from the seed inoculation procedure, 35S-etr1 plants benefited less from the interaction compared to WTev, when inoculated at a later stage of development (20 days).
DISCUSSION
Bacillus sp. isolates are natural root-associated bacteria of N. attenuata (Long et al., 2010) and the particular isolate that we have named “B55,” has dramatic PGP effects on WT and 35S-etr1 plants (a transgenic line impaired in ET perception), the line from which this bacteria was first isolated. Here, we show that this native PGPB not only improved N. attenuata WT’s growth under in vitro, glasshouse, and field conditions, but it also “rescued” many of the deleterious phenotypes of 35S-etr1 plants and dramatically increased the survival of this plant under field conditions, demonstrating PGP effect of a PGPB on its native host in nature.
Numerous studies have reported on bacterial-mediated PGP effects in vitro and in the glasshouse, however, PGPB frequently fail under field conditions, probably due to their inability to colonize roots properly in a competitive environment (Compant et al., 2010; Smyth et al., 2011). Furthermore, bacterial formulations, previously reported to promote plant growth, often exhibited negative effects when applied in a natural environment. Particular physical and chemical properties of the soil are thought to interfere with the “targeted” plant species or the indigenous microbial community (reviewed in Aeron et al., 2011). If PGPB were selected from and hence adapted to the host plant’s native habitat, the PGP effects might be made more consistent.
Our in vitro experiments demonstrated that many parameters associated with PGP (e.g., plant size, chlorophyll content, root branching) were increased in B55-inoculated seedlings. 35S-etr1 seedlings, which grow poorly compared to WT, benefited dramatically from the inoculation: seedling surface area doubled and the characteristic “unbranched” roots gave rise to seven times more lateral roots compared to control seedlings. These observations are consistent with those of López-Bucio et al. (2007), who found that a PGP Bacillus megaterium strain increased root hair and lateral root formation of Arabidopsis thaliana auxin (aux1–7) and ethylene (eir1) mutants, which tend to produce fewer root hairs and lateral roots compared to WT. Furthermore, chlorophyll a and b contents, previously reported to be lower in ET-insensitive plants (Grbic and Bleecker, 1995) were restored to WT control levels by B55 inoculation. B55’s effects on 35S-etr1 seedling performance were associated with higher endosphere colonization: 35S-etr1 seedlings were found to be 10 times higher colonized than WT. Two types of explanations could account for the greater endosphere colonization despite similar rhizosphere colonization (Figure 1D): B55 was originally isolated from a 35S-etr1 plant and hence this genotype may have phenotypes that reflect its natural host. Native N. attenuata populations are known to be highly genetically diverse (Bahulikar et al., 2004) and recent work has found that populations of N. attenuata harbor natural jasmonate-deficient ecotypes (Wu et al., 2008; I. T. Baldwin, unpublished results) and it would not be surprising if natural ET-deficient ecotypes, similar to those of the 35S-etr1 also occurred in these populations. Similar host-specific associations have recently been reported by Weyens et al. (2011), demonstrating that the endophyte Pseudomonas putida W619 exerted positive effects only on the poplar accession it was originally extracted from. Second, N. attenuata 35S-etr1 seedlings do not appear to restrict microbial entry and growth in their roots, pursuing an “open immigration policy” with regard to their endophytic microbial community (Long et al., 2010). Similar results were found in an ET-insensitive Medicago truncatula line, which was hypercolonized by rhizobia or Klebsiella pneumoniae 342, respectively (Penmetsa, 1997; Iniguez et al., 2005). Interestingly, the rhizospheres of WT and 35S-etr1 plants were similarly colonized by B55. Hence the greater apparent benefit obtained by 35S-etr1 plants compared to WT may simply have resulted from the greater endosphere B55 colonization.
Glasshouse experiments with B55-inoculated and non-inoculated WT and 35S-etr1 plants were consistent with our in vitro findings. Inoculated 35S-etr1 plants grew similarly to non-inoculated WT plants and B55-inoculated plants yielded more seed capsules than their respective controls. Again, 35S-etr1 plants gained a greater growth benefit from B55 inoculation than WT plants did. Surprisingly in these glasshouse experiments, colonization of 35S-etr1 roots was similar to that of WT plants. We propose that higher colonization levels during seedling emergence and establishment (which are likely the most critical growth stages in nature) might be responsible for the observed durable PGP effects that lasted through all stages of development.
The 2009 field study was the most “successful” field planting of N. attenuata 35S-etr1 plants to date. In the previous four field attempts usually more than 50% of the 35S-etr1 plants died before stem elongation and flowering, most probably due to high pathogen susceptibility and poor root development. Studies on the field performance of ET-sensing defective plants are rare, likely due to above-mentioned reasons. Bent et al. (2006) reported that field-grown ET-insensitive soybean were also highly susceptible to fungal pathogens. Geraats et al. (2007) analyzed the effect of bacterial antagonists on disease susceptibility of transgenic, ET-insensitive N. tabacum Tetr plants (which are also highly susceptible to fungal pathogens); here, bacterial antagonists were unable to alleviate infection, suggesting that one of the indirect PGP mechanisms, namely the suppression of soil-borne diseases and/or ISR (Lugtenberg and Kamilova, 2009; Gamalero and Glick, 2011) are not likely involved.
Our results suggest that impaired ET perception augments the beneficial effects of B55 inoculation. Similar effects were observed by López-Bucio et al. (2007) who concluded from their experiments that the PGP associated with a B. megaterium strain was independent of ET and auxin signaling. Recently, however, Camehl et al. (2010) reported that the beneficial interaction outcome between a PGP fungus (Piriformospora indica) and A. thaliana required functional ET perception and signaling; while WT plants benefited from the interaction, growth of etr1, ein2, and ein3/eil1 mutants was not promoted or even inhibited by the fungus. Similar results were found with Glomus intraradices’s parasitic interaction with N. attenuata plants (Riedel et al., 2008) and is also likely the case with P. indica’s PGP interaction with N. attenuata (Barazani et al., 2005). The multiple functions of ET signaling and the specific interactions that occur among different partners (bacteria vs. fungi) are likely to account for these different results (Broekaert et al., 2006; Hardoim et al., 2008).
B55’s dramatic effects on 35S-etr1 plant growth prompted us to test its effect on other transgenic N. attenuata lines. A late-stage B55 inoculation enhanced growth of all lines tested (Figure 8) leading to the conclusion that the interaction of B55 with N. attenuata is independent of the (phytohormone) signaling pathways tested so far, including ET perception, and is likely a response of the particular accession that was used to produce all transformed lines. Interestingly, however, benefits gained by 35S-etr1 plants by a late-stage inoculation (12% growth increase) were lower than for WT (ca. 20%) and much reduced compared to that obtained by seed inoculation (30%). The influence of B55 on 35S-etr1 seedlings’ root structure seems to strengthen the young plant, an effect that lasts for the plant’s life. This finding highlights the likely importance of a seedling’s selection of PGPB into its rhizosphere at the onset of germination.
To analyze the mechanism behind B55’s remarkable PGP effects, we tested B55 for known PGP traits. The ability of bacteria to produce IAA and ACCd is often considered a pre-requisite for PGP. For example, Long et al. (2008) found that ACCd production by endophytic bacteria was negatively correlated with plant ET production and positively with seedling root growth. Also IAA excretion into the rhizosphere is thought to promote root growth (Gamalero and Glick, 2011; Helman et al., 2011). Even though B55 was found to produce substantially high concentrations of ACCd and IAA, WT and 35S-etr1 seedling IAA content and ET production were not affected by a B55 inoculation (Figures 2A,B). Furthermore, the high levels of ET production by 35S-etr1 seedlings were not changed, hence restoring ET sensing as possible PGP mechanism can be excluded (Figure 2B). Neither was the P content of WT plants changed by a B55 inoculation (Figure 2C). These findings point to other, unexplored PGP mechanisms.
B55 inoculation significantly changed the quantity and quality of the resident bacterial community of WT and 35S-etr1 plants. Even though we analyzed only the culturable bacterial community (which represents just a minute proportion of microbes interacting with plants), our results point toward the often underestimated importance of microbe–microbe interactions in facilitating PGP (reviewed by Berg and Zachow, 2011). While B55-inoculated plants harbored several bacterial isolates, non-inoculated plants only housed Enterobacter sp. Utah 2009-1. Interestingly, an E. cloacae sp. isolate has been described as having PGP effects on several plant species (reviewed in Jha et al., 2011); however, as for many plant–microbe interactions, host specificity and PGP occurs in a species-specific manner and hence determine the outcome of the interaction (Long et al., 2008; Berg and Smalla, 2009). Further experiments will be carried out to unravel B55’s antagonistic and synergistic actions on microbial communities and plant growth.
That B55 could alleviate some of the negative effects associated with the 35S-etr1 plant’s inability to perceive ET, leads to the hypothesis that plants that harbor various mutations (such as phytohormone mutants) might be able to recruit particular microbes to help them compensate for their fitness deficiencies. As such, these facultative mutualistic associations with microbes could be viewed as part of the plant’s extended phenotype, as proposed by Partida-Martinez and Heil (2011), a phenotype that deserves much more additional work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Brigham Young University for use of their field station, the Lytle Ranch Preserve; C. Diezel, D. Kessler, A. Weinhold, and M. Heinrich for help with field work, A. Wissgott, C. Lembke, F. Wenig, S. Hilck, and S. Ghimire for experimental assistance, R. Santhanam for 16S rRNA sequencing of B55, S. Meldau for helpful comments, Dr. M. Raessler (MPI for Biogeochemistry, Jena) for analysis of total P, and the Max Planck Society for financial support. D. G. Meldau was supported by the Jena School of Microbial Communication (JSMC) and H. H. Long by the International Leibniz Research School (ILRS).
Footnotes
REFERENCES
- Aeron A., Kumar S., Pandey P., Maheshwari D. K. (2011). “Emerging role of plant growth promoting rhizobacteria in agrobiology,” in Bacteria in Agrobiology: Crop Ecosystems ed. Maheshwari D. K. (Berlin: Springer; ), 1–36 [Google Scholar]
- Allmann S., Halitschke R., Schuurink R. C., Baldwin I. T. (2010). Oxylipin channelling in Nicotiana attenuata: lipoxygenase 2 supplies substrates for green leaf volatile production. Plant Cell Environ. 33 2028–2040 [DOI] [PubMed] [Google Scholar]
- Bahulikar R. A., Stanculescu D., Preston C. A., Baldwin I. T. (2004). ISSR and AFLP analysis of the temporal and spatial population structure of the post-fire annual, Nicotiana attenuata, in SW Utah. BMC Ecol. 4 12 10.1186/1472-6785-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin I. T. (2001). An ecologically motivated analysis of plant–herbivore interactions in native tobacco. Plant Physiol. 127 1449–1458 [PMC free article] [PubMed] [Google Scholar]
- Barazani O., Benderoth M., Groten K., Kuhlemeier C., Baldwin I. T. (2005). Piriformospora indica and Sebacina vermifera increase growth performance at the expense of herbivore resistance in Nicotiana attenuata. Oecologia 146 234–243 [DOI] [PubMed] [Google Scholar]
- Bent A. F., Hoffman T. K., Schmidt J. S., Hartman G. L., Hoffman D. D., Xue P., Tucker M. L. (2006). Disease- and performance-related traits of ethylene-insensitive soybean. Crop Sci. 46 893–901 [Google Scholar]
- Berg G., Smalla K. (2009). Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 68 1–13 [DOI] [PubMed] [Google Scholar]
- Berg G., Zachow C. (2011). “PGPR interplay with rhizosphere communities and effect on plant growth and health,” in Bacteria in Agrobiology: Crop Ecosystems ed. Maheshwari D. K. (Berlin: Springer; ), 97–109 [Google Scholar]
- Bleecker A. B., Kende H. (2000). Ethylene: a gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 16 1–18 [DOI] [PubMed] [Google Scholar]
- Bonaventure G., Schuck S., Baldwin I. T. (2011). Revealing complexity and specificity in the activation of lipase-mediated oxylipin biosynthesis: a specific role of the Nicotiana attenuata GLA1 lipase in the activation of JA biosynthesis in leaves and roots. Plant Cell Environ. 34 1507–1520 [DOI] [PubMed] [Google Scholar]
- Bric J. M., Bostock R. M., Silverstone S. E. (1991). Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 57 535–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert W. F., Delaure S. L., De Bolle M. F., Cammue B. P. (2006). The role of ethylene in host–pathogen interactions. Annu. Rev. Phytopathol. 44 393–416 [DOI] [PubMed] [Google Scholar]
- Camehl I., Sherameti I., Venus Y., Bethke G., Varma A., Lee J., Oelmueller R. (2010). Ethylene signalling and ethylene-targeted transcription factors are required to balance beneficial and nonbeneficial traits in the symbiosis between the endophytic fungus Piriformospora indica and Arabidopsis thaliana. New Phytol. 185 1062–1073 [DOI] [PubMed] [Google Scholar]
- Castro-Sowinski S., Herschkovitz Y., Okon Y., Jurkevitch E. (2007). Effects of inoculation with plant growth-promoting rhizobacteria on resident rhizosphere microorganisms. FEMS Microbiol. Lett. 276 1–11 [DOI] [PubMed] [Google Scholar]
- Chang C., Kwok S. F., Bleecker A. B., Meyerowitz E. M. (1993). Arabidopsis ethylene-response gene ETR1 – similarity of product to 2-component regulators. Science 262 539–544 [DOI] [PubMed] [Google Scholar]
- Clark D. G., Gubrium E. K., Barrett J. E., Nell T. A., Klee H. J. (1999). Root formation in ethylene-insensitive plants. Plant Physiol. 121 53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compant S., Clément C., Sessitsch A. (2010). Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 42 669–678 [Google Scholar]
- Franche C., Lindstrom K., Elmerich C. (2009). Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 321 35–59 [Google Scholar]
- Gamalero E., Glick B. R. (2011). “Mechanisms used by plant growth-promoting bacteria,” in Bacteria in Agrobiology: Plant Nutrient Management ed. Maheshwari D. K. (Heidelberg: Springer; ), 17–46 [Google Scholar]
- Geraats B. P. J., Bakker P. A. H. M., Linthorst H. J. M., Hoekstra J., Van Loon L. C. (2007). The enhanced disease susceptibility phenotype of ethylene-insensitive tobacco cannot be counteracted by inducing resistance or application of bacterial antagonists. Physiol. Mol. Plant Pathol. 70 77–87 [Google Scholar]
- Geraats B. P. J., Bakker P. A. H. M., Van Loon L. C. (2002). Ethylene insensitivity impairs resistance to soilborne pathogens in tobacco and Arabidopsis thaliana. Mol. Plant Microbe Interact. 15 1078–1085 [DOI] [PubMed] [Google Scholar]
- Glick B. R. (1995). The enhancement of plant-growth by free-living bacteria. Can. J. Microbiol. 41 109–117 [Google Scholar]
- Grbic V., Bleecker A. B. (1995). Ethylene regulates the timing of leaf senescence. Plant J. 8 8 [Google Scholar]
- Hardoim P. R., Van Overbeek L. S., Elsas J. D. (2008). Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 16 463–471 [DOI] [PubMed] [Google Scholar]
- Helman Y., Burdman S., Okon Y. (2011). “Plant growth promotion by rhizosphere bacteria through direct effects,” in Beneficial Microorganisms in Multicellular Life Form eds Rosenberg E., Gophna U. (Heidelberg: Springer; ), 89–103 [Google Scholar]
- Hettenhausen C., Baldwin I. T., Wu J. (2012). Silencing MPK4 in Nicotiana attenuata enhances photosynthesis and seed production but compromises abscisic acid-induced stomatal closure and guard cell-mediated resistance to Pseudomonas syringae pv tomato DC3000. Plant Physiol. 158 759–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez A. L., Dong Y., Carter H. D., Ahmer B. M., Stone J. M., Triplett E. W. (2005). Regulation of enteric endophytic bacterial colonization by plant defenses. Mol. Plant Microbe Interact. 18 169–178 [DOI] [PubMed] [Google Scholar]
- Jha C. K., Aeron A., Patel B. V., Maheshwari D. K., Saraf M. (2011). “Enterobacter: role in plant growth promotion,” in Bacteria in Agrobiology: Plant Growth Responses ed. Maheshwari D. K. (Heidelberg: Springer; ), 159–182 [Google Scholar]
- Jha Y., Subramanian R. B., Patel S. (2010). Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol. Plant. 33 797–802 [Google Scholar]
- Kiers E. T., Duhamel M., Beesetty Y., Mensah J. A., Franken O., Verbruggen E., Fellbaum C. R., Kowalchuk G. A., Hart M. M., Bago A., Palmer T. M., West S. A., Vandenkoornhuyse P., Jansa J., Buecking H. (2011). Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333 880–882 [DOI] [PubMed] [Google Scholar]
- Kim O.-S., Cho Y.-J., Lee K., Yoon S.-H., Kim M., Na H., Park S.-C., Jeon Y. S., Lee J.-H., Yi H., Won S., Chun J. (2012). Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 62 716–721 [DOI] [PubMed] [Google Scholar]
- Kloepper J. W., Lifshitz R., Zablotowicz R. M. (1989). Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol. 7 39–44 [Google Scholar]
- Knoester M., Van Loon L. C., Van Den Heuvel J., Hennig J., Bol J. F., Linthorst H. J. M. (1998). Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc. Natl. Acad. Sci. U.S.A. 95 1933–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H. H., Schmidt D. D., Baldwin I. T. (2008). Native bacterial endophytes promote host growth in a species-specific manner; phytohormone manipulations do not result in common growth responses. PLoS ONE 3 e2702 10.1371/journal.pone.0002702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H. H., Sonntag D. G., Schmidt D. D., Baldwin I. T. (2010). The structure of the culturable root bacterial endophyte community of Nicotiana attenuata is organized by soil composition and host plant ethylene production and perception. New Phytol. 185 554–567 [DOI] [PubMed] [Google Scholar]
- López-Bucio J., Campos-Cuevas J. C., Hernández-Calderón E., Valásquez-Becerra C., Farias-Rodriguez R., Macías-Rodriguez L. I., Valencia-Cantero E. (2007). Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol. Plant Microbe Interact. 20 207–217 [DOI] [PubMed] [Google Scholar]
- Lou Y. G., Baldwin I. T. (2006). Silencing of a Germin-like gene in Nicotiana attenuata improves performance of native herbivores. Plant Physiol. 140 1126–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Kamilova F. (2009). Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 63 541–556 [DOI] [PubMed] [Google Scholar]
- Maheshwari D. K. (ed.). (2011). Bacteria in Agrobiology: Crop Ecosystems. Heidelberg: Springer [Google Scholar]
- Meldau S., Wu J., Baldwin I. T. (2009). Silencing two herbivory-activated MAP kinases, SIPK and WIPK, does not increase Nicotiana attenuata’s susceptibility to herbivores in the glasshouse and in nature. New Phytol. 181 161–173 [DOI] [PubMed] [Google Scholar]
- Onkokesung N., Galis I., Von Dahl C. C., Matsuoka K., Saluz H. P., Baldwin I. T. (2010). Jasmonic acid and ethylene modulate local responses to wounding and simulated herbivory in Nicotiana attenuata leaves. Plant Physiol. 153 785–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida-Martinez L. P., Heil M. (2011). The microbe-free plant: fact or artifact? Front. Plant Sci. 2:100 10.3389/fpls.2011.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschold A., Halitschke R., Baldwin I. T. (2007). Co(i)-ordinating defenses: NaCOI1 mediates herbivore-induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J. 51 79–91 [DOI] [PubMed] [Google Scholar]
- Penmetsa R. V. (1997). A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275 527–530 [DOI] [PubMed] [Google Scholar]
- Pineda A., Zheng S. J., Van Loon J. J., Pieterse C. M., Dicke M. (2010). Helping plants to deal with insects: the role of beneficial soil-borne microbes. Trends Plant Sci. 15 507–514 [DOI] [PubMed] [Google Scholar]
- Ramos B., Garcia J. A. L., Probanza A., Barrientos M. L., Manero F. J. G. (2003). Alterations in the rhizobacterial community associated with European alder growth when inoculated with PGPR strain Bacillus licheniformis. Environ. Exp. Bot. 49 61–68 [Google Scholar]
- Rayapuram C., Wu J., Haas C., Baldwin I. T. (2008). PR-13/Thionin but not PR-1 mediates bacterial resistance in Nicotiana attenuata in nature, and neither influences herbivore resistance. Mol. Plant Microbe Interact. 21 988–1000 [DOI] [PubMed] [Google Scholar]
- Riedel T., Groten K., Baldwin I. T. (2008). Symbiosis between Nicotiana attenuata and Glomus intraradices: ethylene plays a role, jasmonic acid does not. Plant Cell Environ. 31 1203–1213 [DOI] [PubMed] [Google Scholar]
- Ryu C. M., Farag M. A., Hu C. H., Reddy M. S., Wei H. X., Pare P. W., Kloepper J. W. (2003). Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 100 4927–4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serek M., Sisler E. C., Reid M. S. (1995). Effects of 1-MPC on the vase life and ethylene response of cut flowers. Plant Growth Regul. 16 93–97 [Google Scholar]
- Smyth E. M., Mccarthy J., Nevin R., Khan M. R., Dow J. M., O’Gara F., Doohan F. M. (2011). In vitro analyses are not reliable predictors of the plant growth promotion capability of bacteria; a Pseudomonas fluorescens strain that promotes the growth and yield of wheat. J. Appl. Microbiol. 111 683–692 [DOI] [PubMed] [Google Scholar]
- Steppuhn A., Gase K., Krock B., Halitschke R., Baldwin I. T. (2004). Nicotine’s defensive function in nature. PLoS Biol. 2 1074–1080 10.1371/journal.pbio.0020217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon L. C., Geraats B. P., Linthorst H. J. (2006). Ethylene as a modulator of disease resistance in plants. Trends Plant Sci. 11 184–191 [DOI] [PubMed] [Google Scholar]
- Verma S. C., Ladha J. K., Tripathi A. K. (2001). Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J. Biotechnol. 91 127–141 [DOI] [PubMed] [Google Scholar]
- von Dahl C. C., Winz R. A., Halitschke R., Kuehnemann F., Gase K., Baldwin I. T. (2007). Tuning the herbivore-induced ethylene burst: the role of transcript accumulation and ethylene perception in Nicotiana attenuata. Plant J. 51 293–307 [DOI] [PubMed] [Google Scholar]
- Weyens N., Boulet J., Adriaensen D., Timmermans J.-P., Prinsen E., Oevelen S., D’Haen J., Smeets K., Lelie D., Taghavi S., Vangronsveld J. (2011). Contrasting colonization and plant growth promoting capacity between wild type and a gfp-derative of the endophyte Pseudomonas putida W619 in hybrid poplar. Plant Soil. 10.1007/s11104-011-0831-x [Epub ahead of print] [DOI] [Google Scholar]
- Wilkinson J. Q., Lanahan M. B., Clark D. G., Bleecker A. B., Chang C., Meyerowitz E. M., Klee H. J. (1997). A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat. Biotechnol. 15 444–447 [DOI] [PubMed] [Google Scholar]
- Wu J., Hettenhausen C., Schuman M. C., Baldwin I. T. (2008). A comparison of two Nicotiana attenuata accessions reveals large differences in signaling induced by oral secretions of the specialist herbivore Manduca sexta. Plant Physiol. 146 927–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala J. A., Patankar A. G., Gase K., Baldwin I. T. (2004). Constitutive and inducible trypsin proteinase inhibitor production incurs large fitness costs in Nicotiana attenuata. Proc. Natl. Acad. Sci. U.S.A. 101 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinniel D. K., Lambrecht P., Harris N. B., Feng Z., Kuczmarski D., Higley P., Ishimaru C. A., Arunakumari A., Barletta R. G., Vidaver A. K. (2002). Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl. Environ. Microbiol. 68 2198–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]