Abstract
Obesity is increasingly a public health problem due to its high risk of developing insulin resistance, diabetes, atherosclerosis, hypertension, chronic kidney disease, and increased cardiovascular morbidity and mortality. In particular, the association of obesity and hypertension is well recognized; however, the underlying mechanisms are not fully understood. This article reviews recent advancements of cellular and molecular mechanisms by which adipocyte dysfunction and obesity contribute to hypertension through endocrine and paracrine effects of the adipose tissue-derived adipokines on the function of vascular endothelial cells, smooth muscle cells and macrophages.
Keywords: Obesity, adipocyte, adipokine, vascular function, hypertension
Introduction
Obesity is increasingly a public health problem due to its high risk of developing insulin resistance, diabetes, atherosclerosis, hypertension, chronic kidney disease, and increased cardiovascular morbidity and mortality [1-4]. In particular, the relationship between obesity and hypertension is well recognized; some studies suggest that 65-75% of the risk for hypertension is attributed to excess body weight [5-7]. Blood pressure can be lowered when body weight control is effective by using caloric restriction, aerobic exercise, weight loss drugs, or bariatric surgery [8-10]. On the other hand, it has been shown that activation of sympathetic nervous system (SNS) and renin-angiotensin system (RAS) and impairments of pressure natriuresis play an important role in the development of obesity-associated hypertension [11-16]. Now it is clear that adipose tissue is an active endocrine and paracrine organ, which releases a variety of cytokines and hormones (adipokines) to influence not only energy homeostasis but also blood pressure regulation [17-19]. This review summarizes recent advancements that link adipocyte dysfunction and obesity to vascular deregulation and hypertension through endocrine and paracrine mechanisms of adipose tissue-secreted factors. We will specifically focus on the role of adipokines in the regulation of vascular endothelial and smooth muscle cell function and inflammation.
Adipose tissue
Adipose tissue consists of white adipose tissue (WAT) and brown adipose tissue (BAT). WAT makes up to 20% to 25% of total body weight; it acts mainly as an energy store (in the form of triglycerides and free fatty acid [FFA]) however expands during obesity. WAT accumulation is responsible for many adverse outcomes of obesity such as hypertension, insulin resistance, atherosclerosis and chronic kidney disease [17-19]. The role of BAT is to maintain body temperature during the exposure to a cold environment, but not to maintain a normal body weight in the obesogenic environment [20]. The major cell type in an adipose tissue is adipocytes, which are mixed with endothelial cells, vascular smooth muscle cells (VSMCs), macrophages, and fibroblasts. Adipose tissue is predominantly located around internal organs (i.e., visceral adipose tissue), underneath the skin (i.e., subcutaneous adipose tissue), and around blood vessels (i.e., perivascular adipose tissue, PVAT).
In the last few years, a large amount of scientific knowledge about adipose tissue has been learnt. Adipose tissue is no longer considered solely a fat deposit, but one of the largest endocrine organs, producing a variety of bioactive factors termed adipokines including cytokines (e.g., tumor necrosis factor-α [TNF-α], interleukin-6 [IL-6], plasminogen activator inhibitor-1 [PAI-1], fibrinogen, Visfatin and Omentin) and hormones (e.g., leptin, angiotensinogen, agouti-related peptide, resistin and adiponectin). Adipokines act in either paracrine or endocrine manner to regulate energy homeostasis, glucose and lipid metabolism, and cardiovascular function. Bioactive factors secreted from the adipose tissue can easily enter the systemic circulation and exert their effects on other peripheral organs or central nervous system through the brain-blood barrier. In particular, PVAT has direct contact to the adventitia of vessels without an anatomical barrier; bioactive factors secreted by PVAT may readily gain access into the blood vessel wall and function in a paracrine fashion to transduce metabolic signals to blood vessels. The identification of adipokine receptors in vascular endothelia and smooth muscles suggests that adipose tissue derived bioactive factors might have direct vasoactive properties and participate in the regulation of vascular function and peripheral resistance [21].
Adipokines and vascular function
The cross-talk between adipose tissue and the vasculature appears to play an important role in maintaining vascular homeostasis [22,23]. Both WAT and BAT, particularly PVAT, modulate vascular reactivity, inflammation and remodeling through the release of a variety of adipokines, which target adipocytes, endothelial cells, VSMCs and macrophages. Adipocyte dysfunction in obesity can lead to dysregulation of glucose and lipid metabolism, coagulation and inflammation [24,25]. Obese adipose tissue is characterized by adipocyte hypertrophy and hyperplasia and excessive infiltration of macrophages and lymphocytes, leading to elevated production of pro-inflammatory adipokines and vasoactivators that result in endothelial dysfunction, VSMCs proliferation and migration, and vascular inflammation. Unlike most adipokines with detrimental effects on the vasculature, adiponectin exerts beneficial effects on vascular disorders through its vasodilator, anti-inflammatory and anti-oxidative activities in vascular cells [26,27].
Adipokines and endothelial function
Endothelial cells form the chief physical barrier between blood and vessel wall. For vascular homeostasis, endothelial cells play a critical role in the regulation of vasomotion, coagulation and inflammation by producing a variety of mediators such as prostacyclin, endothelium derived relaxing factor (EDRF)/nitric oxide (NO), angiotensin II, and endothelin-1 (ET-1) [23,28]. Insulin induced vasodilation, an effect mediated by the NO release, is impaired in obese individuals who display insulin resistance [29]. Adiponectin directly exerts effects on endothelial function through eNOS-dependent and COX-2-dependent regulatory mechanisms, respectively [30-32] (Figure 1). Resistin promotes endothelial cell activation through the release of ET-1 and upregulation of vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) [33]; TNF-α inhibits eNOS, therefore causing a reduction in NO bioavailability [34].
Figure 1.
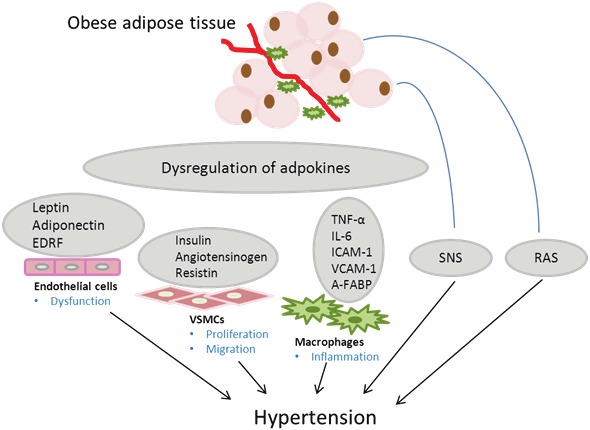
Obesity-associated adipocyte dysfunction contributes to hypertension by endocrine and paracrine effects of adipokines on endothelial cells, vascular smooth muscle cells (VSMCs) and macrophages. EDRF, Endothelium derived relaxing factor; TNF-α, Tumor necrosis factor-α; IL-6, Interleukin-6; VCAM-1, Vascular cell adhesion molecule-1; ICAM-1, Intercellular adhesion molecule-1; A-FABP, Adipocyte fatty acid binding protein; SNS, Sympathetic nervous system; RAS, Renin-angiotensin system.
Adipokines and smooth muscle cell function
VSMCs form middle layers within the vessel wall and control blood flow by contraction and relaxation in response to external stimuli. VSMCs do not proliferate under normal physiological condition; however, after injury, aberrant proliferation and migration of VSMCs can lead to pathologic changes in the vessel wall. Adipose tissue at physiological state has been shown to preserve normal VSMC function. Removal of periadventitial fat enhances neointima formation after endovascular injury, which can be attenuated by transplantation of subcutaneous adipose tissue from mice fed on regular chow [35]. In patients with coronary artery bypass surgery, harvesting the saphenous vein with preserved surrounding tissue (mainly PVAT) provides better short- and long-term patency rates [36]. However, during obesity, accumulation of visceral adipose tissues promotes synthesis of proinflammatory adipokines which cause an increase in the level of reactive oxygen species (ROS) derived from NADPH oxidase. ROS are important signaling molecules or second messengers that regulate the proliferation, hypertrophy, and migration of VSMCs [37,38]. The vascular-protective effects of adiponectin have been largely attributed to its anti-oxidant actions [26]. Adiponectin exerts an inhibitory effect on the proliferation and migration of VSMCs by interacting with various atherogenic growth factors, including heparin-binding epidermal growth factor-like growth factor, platelet-derived growth factor and basic fibroblast growth factor, thereby blocking the binding of these growth factors to their respective membrane receptors and subsequent ERK1/2 activation and AMPK phosphorylation [39]. Adiponectin-deficient mice display enhanced proliferation of VSMC and increased neointimal thickening after mechanical injury, and these changes are reversed by adenovirus-mediated expression of adiponectin [26]. In contrast, Resistin induces human aortic SMC proliferation by ERK1/2 and Akt signaling pathways [40]. In human VSMCs, insulin inactivates the small GTPase RhoA and its target, Rho kinase, thereby leading to decreased phosphorylation of myosin light chain and subsequent vasodilation [41] (Figure 1).
Adipokines and macrophage function
Recent evidence suggests that the infiltration of macrophages in adipose tissue play an important role in the chronic inflammatory state and metabolic dysfunction associated with obesity [42]. The activity of macrophages in adipose tissue perpetuates a vicious cycle of increased macrophage recruitment, production of inflammatory cytokines, and impaired adipocyte function. Adipokines play multiple roles in this inflammatory process. TNF-α increased expression of ICAM-1 and VCAM-1 in vascular tissue by the activation of the nuclear factor-kappa B (NF-kB) signaling pathway, thereby leading to the enhancement of monocyte adhesion to the vessel wall and up-regulation of inducible nitric oxide synthase (iNOS), interleukins, superoxide dismutase, etc [43]. Leptin, especially in the presence of high glucose, stimulates macrophages to accumulate cholesterol [44]. Resistin has been shown to induce pentraxin 3, an inflammatory mediator in human endothelial cells [45]. Adipocyte fatty acid binding protein (A-FABP) potentiates toxic lipids-induced inflammation in macrophages by inducing endoplasmic reticulum stress, thereby leading to the activation of JNK and NF-kB signaling pathways [46]. Frizzled-related protein 5 (Sfrp5) secretion by adipocytes exerts salutary effects on metabolic dysfunction by controlling inflammatory cells within adipose tissue [47] (Figure 1).
Adipocyte dysfunction and hypertension
Normal adipose tissue maintains a specifically balanced adipokine profile, which modulates vascular homeostasis via endocrine and paracrine pathways [22,23]. Excessive accumulation of adipose tissue may alter the expression of the adipokine profile and consequently the physiological effects of adipokines on the vessels. Excessive weight gain contributes to increased blood pressure in most patients with essential hypertension [5]. Blood pressure is a function of cardiac output and peripheral resistance. Weight gain leads to increased cardiac output and blood volume, which might be related to activation of SNS and RAS, and to physical compression of the kidneys by fat accumulation within and around the kidneys and excessive visceral fat. SNS activation and physical compression of the kidneys both cause activation of the RAS, and pharmacological blockade of either the RAS or the SNS attenuates obesity induced hypertension by at least 50-60% [48]. Several mediators in the obesity-associated hypertension have been suggested, including hyperinsulinemia, angiotensin II, FFAs and leptin. Hyperinsulinemia could lead to a rise in blood pressure by enhancing sodium retention [49,50]. Adipocytes secrete angiotensinogen, which can be converted to angiotensin I and then angiotensin II, a potent vasoconstrictor and promoter of sodium and water absorption [12,14]. FFAs could promote hypertension by means of adrenergic stimulation, increase in oxidative stress, endothelial dysfunction, or stimulation of vascular cell growth [51,52]. Leptin activates SNS by stimulating opiomelanocortin neurons, with subsequent activation of central nervous system melanocortin 4 receptors [53]. The physiological importance of leptin in blood pressure regulation has been demonstrated in animal models [54]. Obese Ob/ob mice deficient in leptin have significantly decreased blood pressure [55]. Increased peripheral resistance due primarily to changes in vascular structure and function appear to be the fundamental abnormality in hypertension. Adipokines induce the functional and structural changes in the vessels by endothelial dysfunction, VSMC proliferation and migration and vascular inflammation, thereby regulating vascular responses to constrictor and dilator stimuli and contribute to the increased arterial pressure.
Conclusion and future perspectives
Adipocyte dysfunction and obesity contributes to hypertension by the endocrine and paracrine effects of adipose tissues-derived adipokines on vascular endothelial cells, VSMCs, and inflammatory cells including macrophages. Thus, investigations of adipokines and their local and systemic effects on vascular function may aid identification of novel molecular targets for treatment of obesity and obesity-associated hypertension.
Acknowledgement
Dr. Gangjian Qin is supported by the NIH grants R01 HL093439 and R01 HL113541, and American Heart Association Grant 0430135N. Dr. Junlan Zhou is supported by American Heart Association Grant 10POST4360009.
References
- 1.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WPT, Loria CM, Smith SC. Harmonizing the Metabolic Syndrome. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.James RS. Obesity as a cardiovascular risk factor. The American Journal of Medicine. 2003;115:37–41. doi: 10.1016/j.amjmed.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Reaven G, Abbasi F, McLaughlin T. Obesity, Insulin Resistance, and Cardiovascular Disease. Recent Prog Horm Res. 2004;59:207–223. doi: 10.1210/rp.59.1.207. [DOI] [PubMed] [Google Scholar]
- 4.Sowers JR. Obesity and cardiovascular disease. Clinical Chemistry. 1998;44:1821–1825. [PubMed] [Google Scholar]
- 5.Garrison RJ, Kannel WB, Stokes J, Castelli WP. Incidence and precursors of hypertension in young adults: The Framingham offspring study. Preventive Medicine. 1987;16:235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 6.Dyer A, Elliott P, Shipley M. Body mass index versus height and weight in relation to blood pressure. Finding for the 10,079 persons in the INTERSALT Study. Am J Epidemiol. 1990;131:589–596. doi: 10.1093/oxfordjournals.aje.a115543. [DOI] [PubMed] [Google Scholar]
- 7.Dyer A, Elliott P. The INTERSALT study: relations of body mass index to blood pressure. INTERSALT Co-operative Research Group. J Hum Hypertens. 1989;3:299–308. [PubMed] [Google Scholar]
- 8.Aucott L, Poobalan A, Smith WCS, Avenell A, Jung R, Broom J. Effects of Weight Loss in Overweight/Obese Individuals and Long-Term Hypertension Outcomes. Hypertension. 2005;45:1035–1041. doi: 10.1161/01.HYP.0000165680.59733.d4. [DOI] [PubMed] [Google Scholar]
- 9.Whelton SP, Chin A, Xin X, He J. Effect of Aerobic Exercise on Blood Pressure. Annals of Internal Medicine. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 10.Kokkinos PF, Narayan P, Colleran JA, Pittaras A, Notargiacomo A, Reda D, Papademetriou V. Effects of Regular Exercise on Blood Pressure and Left Ventricular Hypertrophy in African-American Men with Severe Hypertension. New England Journal of Medicine. 1995;333:1462–1467. doi: 10.1056/NEJM199511303332204. [DOI] [PubMed] [Google Scholar]
- 11.Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010;33:386–393. doi: 10.1038/hr.2010.9. [DOI] [PubMed] [Google Scholar]
- 12.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic Activation in Obese Normotensive Subjects. Hypertension. 1995;25:560–563. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- 13.Landsberg L, Krieger DR. Obesity, metabolism, and the sympathetic nervous system. Am J Hypertens. 1989;2:125–132. doi: 10.1093/ajh/2.3.125s. [DOI] [PubMed] [Google Scholar]
- 14.Campbell D. Circulating and tissue angiotensin systems. J Clin Invest. 1987;79:1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyton A. The surprising kidney-fluid mechanism for pressure control--its infinite gain! Hypertension. 1990;16:725–730. doi: 10.1161/01.hyp.16.6.725. [DOI] [PubMed] [Google Scholar]
- 16.Hall J. Mechanisms of Abnormal Renal Sodium Handling in Obesity Hypertension. American Journal of Hypertension. 1997;10:49S–55S. [PubMed] [Google Scholar]
- 17.Cano P, Cardinali DP, Rios-Lugo MJ, Fernandez-Mateos MP, Reyes Toso CF, Esquifino AI. Effect of a High-fat Diet on 24-Hour Pattern of Circulating Adipocytokines in Rats. Obesity. 2009;17:1866–1871. doi: 10.1038/oby.2009.200. [DOI] [PubMed] [Google Scholar]
- 18.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 19.Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, Merval R, Esposito B, Clément K, Holvoet P, Tedgui A, Mallat Z. Defective Leptin/Leptin Receptor Signaling Improves Regulatory T Cell Immune Response and Protects Mice From Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:2691–2698. doi: 10.1161/ATVBAHA.107.149567. [DOI] [PubMed] [Google Scholar]
- 20.Leslie PK. Brown Fat and the Myth of Diet-Induced Thermogenesis. Cell Metabolism. 2010;11:263–267. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katugampola SD, Kuc RE, Maguire JJ, Davenport AP. G-protein-coupled receptors in human atherosclerosis: comparison of vasoconstrictors (endothelin and thromboxane) with recently de-orphanized (urotensin-II, apelin and ghrelin) receptors. Clin Sci. 2002;103(Suppl 48):171S–175S. doi: 10.1042/CS103S171S. [DOI] [PubMed] [Google Scholar]
- 22.Linscheid P, Seboek D, Zulewski H, Keller U, Müller B. Autocrine/Paracrine Role of Inflammation-Mediated Calcitonin Gene-Related Peptide and Adrenomedullin Expression in Human Adipose Tissue. Endocrinology. 2005;146:2699–2708. doi: 10.1210/en.2004-1424. [DOI] [PubMed] [Google Scholar]
- 23.Eringa EC, Bakker W, Smulders YM, Serné EH, Yudkin JS, Stehouwer CDA. Regulation of Vascular Function and Insulin Sensitivity by Adipose Tissue: Focus on Perivascular Adipose Tissue. Microcirculation. 2007;14:389–402. doi: 10.1080/10739680701303584. [DOI] [PubMed] [Google Scholar]
- 24.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of Monocyte Chemoattractant Protein-1 in Adipose Tissues Causes Macrophage Recruitment and Insulin Resistance. Journal of Biological Chemistry. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 25.Singhal A, Farooqi IS, Cole TJ, O’Rahilly S, Fewtrell M, Kattenhorn M, Lucas A, Deanfield J. Influence of Leptin on Arterial Distensibility. Circulation. 2002;106:1919–1924. doi: 10.1161/01.cir.0000033219.24717.52. [DOI] [PubMed] [Google Scholar]
- 26.Hui X, Lam KSL, Vanhoutte PM, Xu A. Adiponectin and Cardiovascular Health: an Update. British Journal of Pharmacology. 2011;165:574–590. doi: 10.1111/j.1476-5381.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1[agr] and mitochondria by Ca2+ and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 28.Gao YJ, Lu C, Su LY, Sharma AM, Lee RMKW. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. British Journal of Pharmacology. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinozaki K, Kashiwagi A, Masada M, Okamura T. Molecular Mechanisms of Impaired Endothelial Function Associated with Insulin Resistance. Current Drug Targets - Cardiovascular & Hematological Disorders. 2004;4:1–11. doi: 10.2174/1568006043481248. [DOI] [PubMed] [Google Scholar]
- 30.Ohashi K, Ouchi N, Sato K, Higuchi A, Ishikawa T-o, Herschman HR, Kihara S, Walsh K. Adiponectin Promotes Revascularization of Ischemic Muscle through a Cyclooxygenase 2-Dependent Mechanism. Mol Cell Biol. 2009;29:3487–3499. doi: 10.1128/MCB.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, Shin HK, Moskowitz MA, Ouchi N. Adiponectin Prevents Cerebral Ischemic Injury Through Endothelial Nitric Oxide Synthase–Dependent Mechanisms. Circulation. 2008;117:216–223. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- 32.Wong Wing T, Tian Xiao Y, Xu A, Yu J, Lau Chi W, Hoo Ruby LC, Wang Y, Lee Vivian WY, Lam Karen SL, Vanhoutte Paul M, Huang Y. Adiponectin Is Required for PPARγ-Mediated Improvement of Endothelial Function in Diabetic Mice. Cell Metabolism. 2011;14:104–115. doi: 10.1016/j.cmet.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Verma S, Li S-H, Wang C-H, Fedak PWM, Li R-K, Weisel RD, Mickle DAG. Resistin Promotes Endothelial Cell Activation. Circulation. 2003;108:736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 34.Zemse SM, Chiao CW, Hilgers RHP, Webb RC. Interleukin-10 inhibits the in vivo and in vitro adverse effects of TNF-α on the endothelium of murine aorta. American Journal of Physiology - Heart and Circulatory Physiology. 2010;299:H1160–H1167. doi: 10.1152/ajpheart.00763.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, Saito Y, Nagai R, Sata M. Periadventitial Adipose Tissue Plays a Critical Role in Vascular Remodeling. Circulation Research. 2009;105:906–911. doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- 36.Souza DSR, Johansson B, Bojö L, Karlsson R, Geijer H, Filbey D, Bodin L, Arbeus M, Dashwood MR. Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: Results of a randomized longitudinal trial. The Journal of Thoracic and Cardiovascular Surgery. 2006;132:373–378. doi: 10.1016/j.jtcvs.2006.04.002. e375. [DOI] [PubMed] [Google Scholar]
- 37.Lo IC, Shih J-M, Jiang MJ. Reactive oxygen species and ERK 1/2 mediate monocyte chemotactic protein-1-stimulated smooth muscle cell migration. Journal of Biomedical Science. 2005;12:377–388. doi: 10.1007/s11373-005-1703-2. [DOI] [PubMed] [Google Scholar]
- 38.Madamanchi NR, Moon S-K, Hakim ZS, Clark S, Mehrizi A, Patterson C, Runge MS. Differential Activation of Mitogenic Signaling Pathways in Aortic Smooth Muscle Cells Deficient in Superoxide Dismutase Isoforms. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:950–956. doi: 10.1161/01.ATV.0000161050.77646.68. [DOI] [PubMed] [Google Scholar]
- 39.Motobayashi Y, Izawa-Ishizawa Y, Ishizawa K, Orino S, Yamaguchi K, Kawazoe K, Hamano S, Tsuchiya K, Tomita S, Tamaki T. Adiponectin inhibits insulin-like growth factor-1-induced cell migration by the suppression of extracellular signal-regulated kinase 1/2 activation, but not Akt in vascular smooth muscle cells. Hypertens Res. 2009;32:188–193. doi: 10.1038/hr.2008.19. [DOI] [PubMed] [Google Scholar]
- 40.Calabro P, Samudio I, Willerson JT, Yeh ETH. Resistin Promotes Smooth Muscle Cell Proliferation Through Activation of Extracellular Signal- Regulated Kinase 1/2 and Phosphatidylinositol 3-Kinase Pathways. Circulation. 2004;110:3335–3340. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- 41.Begum N, Sandu OA, Duddy N. Negative Regulation of Rho Signaling by Insulin and Its Impact on Actin Cytoskeleton Organization in Vascular Smooth Muscle Cells. Diabetes. 2002;51:2256–2263. doi: 10.2337/diabetes.51.7.2256. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Nakayama T. Inflammation, a Link between Obesity and Cardiovascular Disease. Mediator Inflamm. 2010;2010:535918. doi: 10.1155/2010/535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor Necrosis Factor-α Induces Endothelial Dysfunction in the Prediabetic Metabolic Syndrome. Circulation Research. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 44.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-γ, a Th1 Cytokine, Regulates Fat Inflammation. Circulation Research. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, Manabe I, Utsunomiya K, Nagai R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine–endothelial cell interactions. Biochemical and Biophysical Research Communications. 2004;314:415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 46.Hui X, Li H, Zhou Z, Lam KSL, Xiao Y, Wu D, Ding K, Wang Y, Vanhoutte PM, Xu A. Adipocyte Fatty Acid-binding Protein Modulates Inflammatory Responses in Macrophages through a Positive Feedback Loop Involving c-Jun NH2-terminal Kinases and Activator Protein-1. Journal of Biological Chemistry. 2010;285:10273–10280. doi: 10.1074/jbc.M109.097907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K. Sfrp5 Is an Anti-Inflammatory Adipokine That Modulates Metabolic Dysfunction in Obesity. Science. 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol. 2004;286:R803–R813. doi: 10.1152/ajpregu.00707.2003. [DOI] [PubMed] [Google Scholar]
- 49.Creager MA, Liang CS, Coffman JD. Beta adrenergic-mediated vasodilator response to insulin in the human forearm. Journal of Pharmacology and Experimental Therapeutics. 1985;235:709–714. [PubMed] [Google Scholar]
- 50.Hall J. Hyperinsulinemia: a link between obesity and hypertension? Kidney Int. 1993;43:1402–1417. doi: 10.1038/ki.1993.197. [DOI] [PubMed] [Google Scholar]
- 51.Stepniakowski KT, Goodfriend TL, Egan BM. Fatty Acids Enhance Vascular α-Adrenergic Sensitivity. Hypertension. 1995;25:774–778. doi: 10.1161/01.hyp.25.4.774. [DOI] [PubMed] [Google Scholar]
- 52.Oishi K, Zheng B, Kuo JF. Inhibition of Na,K-ATPase and sodium pump by protein kinase C regulators sphingosine, lysophosphatidylcholine, and oleic acid. Journal of Biological Chemistry. 1990;265:70–75. [PubMed] [Google Scholar]
- 53.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced Hypertension: Role of Sympathetic Nervous System, Leptin, and Melanocortins. Journal of Biological Chemistry. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic Cardiovascular and Renal Actions of Leptin. Hypertension. 2002;39:496–501. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- 55.Silvani A, Bastianini S, Berteotti C, Franzini C, Lenzi P, Lo Martire V, Zoccoli G. Sleep Modulates Hypertension in Leptin-Deficient Obese Mice. Hypertension. 2009;53:251–255. doi: 10.1161/HYPERTENSIONAHA.108.125542. [DOI] [PubMed] [Google Scholar]


