Abstract
Introduction
Pacemaker and defibrillator infections are an uncommon, but catastrophic complication of device implantation. The present study examined the prevalence of device-related infections, the patterns of antibiotic resistance, and the presence of methicillin resistant staphylococcus aureus (MRSA) nares colonization in device implant recipients.
Methods
Two protocols were employed using a retrospective and a prospective analysis. A retrospective chart review of 218 patients with suspected device infection from 1/2000 to 1/2011 was performed. Demographics, infection rates, and patterns of antibiotic resistance were compared. The prospective analysis enrolled one hundred eighty two patients undergoing device implantations or generator replacements. The nares were swabbed and analyzed for the presence of staphylococcus aureus, and tested for methicillin sensitivity.
Results
Over a period of ten years, 12,771 device implants/generator changes/system revisions were performed, with an infection rate of 1.2%. Methicillin resistance (MR) was identified in 98/218 (44.9%) of patients. Those with MR infection had more diabetes and cardiomyopathy. There was no significant increase in methicillin resistance over time (p=0.30). Our prospective analysis included 110 men. A total of 32 patients (17.6%) had positive cultures for SA: 6.6% with MRSA. Patients positive for MRSA nares colonization had a statistically significant greater length of hospital stay 8.5 days (mean) versus 4.4 days (P=0.049).
Conclusions
Methicillin resistant organisms appear to be emerging and persistent pathogens in device implants. The screening of MRSA colonization may identify new populations at risk. Further studies and analysis are needed to determine the cost effectiveness of a screening protocol.
Keywords: Pacemaker infections, MRSA infections, methicillin resistant infections, nares colonization, pacemaker generator changes, antibiotic prophylaxis
Introduction
It is estimated that 223,226 pacemakers and 48,127 implantable defibrillators are implanted in the United States annually [1]. These procedures typically have minimal morbidity, although complications do occur. The reported rate of infection related to the device implant ranges from 0.5 - 5.5% [2]. The spectrum of infection can be superficial, localized to the pulse generator pocket, or deeper infections that involve the intravenous portion of the lead, with or without involvement of the pocket. More severe infection may spread to the endocardium, causing endocarditis or vegetations to form on the pacing wire and/or valves. Even if the infection is localized, likelihood of sterilization of the device and surrounding tissue with antibiotic therapy alone is very low [3-5]. Therefore, pacemaker system removal is required to achieve an acceptable clinical outcome [6]. Complications due to the infection or to attempts at removal of chronic lead systems can lead to significant morbidity and mortality [7-9].
The most common pathogens implicated in device infections include staphylococcus aureus and coagulase-negative staphylococci [10,11]. Between 40-50 % of pacemaker patients who become infected have been shown to carry these organisms as skin contaminants [12,13]. Antibiotic prophylaxis has been shown to decrease the incidence of serious infective complications following permanent pacemaker implantation [12,14]. Most institutions currently use a prophylactic regimen prior to implantation directed against these common pathogens, such as cephalosporins, clindamycin, or vancomycin. Over the past two decades, methicillin-resistant (MR) staphylococcal organisms have been identified as frequent pathogens in nosocomial infections. Increasingly, these pathogens have been identified in patients with community-acquired infections [13,15]. In addition, asymptomatic colonization with methicillin-resistant staphylococcal aureas (MRSA) has been described as a risk factor for subsequent MRSA infection [16].
It was hypothesized that the prevalence of MR infections among patients undergoing device implants is relatively high. The study sought to determine the prevalence of MR organism device infection over the past decade, to prospectively define the prevalence of MR organism colonization in patients undergoing device implants, and to identify any comorbidities that may predispose to this colonization.
Methods
Retrospective assessment of prevalence of device infections with MR organisms
The medical records of all patients undergoing pacemaker or implantable cardioverter defibrillator implants at Beaumont Health System were reviewed for evidence of infection related to the device implant between the dates of January 2000 through January 2011. We used ICD code 996.61 (infection due to heart device), and search variables for pacemaker infections and extractions. Patients meeting clinical criteria for pacemaker infection were retrospectively identified. These criteria included any infection related to the device implant requiring removal of device, or systemic antibiotics. All device implants were included in the analysis, including single, dual, and biventricular systems, and new implants and device generator changes. The demographics, types of organisms, incidence of MR infections per year were recorded. The patients were then dichotomized into two groups, depending on methicillin resistance sensitivity, and demographics compared. Patients were also dichotomized into institutional infections versus outside referrals presenting for lead and generator extraction.
Prospective assessment of prevalence of colonization with MR organisms
One hundred eighty one patients undergoing pacemaker/defibrillator device implantations or generator replacements were prospectively enrolled in the study at William Beaumont Hospital in Royal Oak, Michigan during a six month period between October 2006 and March 2007. Subjects included both inpatients and outpatients. All patient’s undergoing a new pacemaker implant, a generator change of an existing device, or a revision, were eligible for inclusion in the study. After obtaining consent, the patient’s nares were swabbed bilaterally with a cotton-tipped culture swab (BD Diagnostics, Maryland) by an experienced nurse, and were placed in sterile containers. The samples were then used to inoculate sheep blood agar plates, and CNA plates, which contain antibiotics to inhibit gram-negative species. Following incubation for 18-24 hours the plates were spot tested for catalase and coagulase reactivity to confirm the presence of staphylococcus aureas. If both tests were found to be positive and growth of staphylococcus was observed, antibiotic sensitivity testing was performed using a microscan device over another period of 18-24 hours. Those samples resistant to oxacillin were defined as methicillin-resistant. Patient demographics, implant characteristics, and historical data were collected. A three-year follow up consisting of chart reviews was conducted to assess infection rates following device implantation.
Statistical analysis
Data were entered in a computerized database. Comparison of discrete variables was accomplished with Chi-square test where appropriate (expected frequency >5), otherwise a Fisher’s exact test was used. Continuous variables were examined using a Wilcoxon rank test. The latest SAS® version, Cary, NC was used for the prospective and retrospective analysis. Logistic regression was performed on the retrospective data where appropriate.
Results
Retrospective analysis
A total of 12,771 cases of pacemaker or ICD device implants/generator changes/system revisions were performed between January 2000 and January 2011. Two hundred eighteen cases of device infection were identified. Of those 64/218 (29%) were outside referrals for explant of device, yielding an institutional infection rate of 1.2%. Of these 218 cases, wound and/or blood cultures identified the putative infectious organism in 190 patients (87.1%). Of those with positive cultures, the organisms identified in order of prevalence were coagulase negative staphylococcus, MRSA, methicillin sensitive staphylococcus aureus, staphlococcus epidermidis, enterococcus faecalis, cornebacterium, and propionibacterium (Figure 1).
Figure 1.
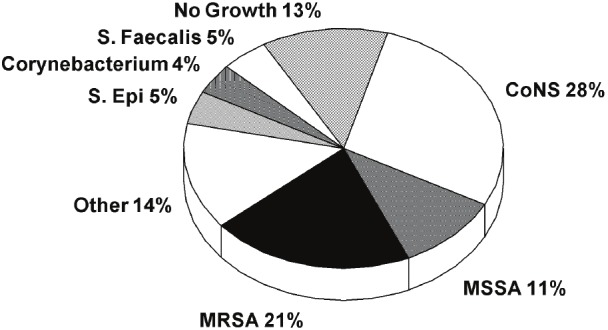
Culture results representing the distribution of pacemaker/ICD infection organism types. CoNS =coagulase negative staphylococci; E.Faecalis = enterococcus faecalis; MRSA = methicillin resistant staphylococcus aureus; MSSA=methicillin resistant staphylococcus aureus; S.Epi = staphylococcus epidermidis; Other = propionibacterium, candida albicans, pseudomonas, proteus, mycobacterium, escherichia.coli, enterobacter, fungemia, other staphylococcus and streptococcus species.
MR organisms, including staphylococcus aureus, coagulase negative staphylococcus (CoNS), and staphylococcus epidermidis, were identified in a total of 98/218 (45%) device infections over the last decade. Those with CoNS isolates without identified sensitivities were considered MR due to the high prevalence of methicillin resistance among this species, upwards of 80% in the literature [4]. Of the total MR organisms, CoNS without known sensitivities represented 34/98 (34.7%). Of the CoNS with known sensitivities, methicillin resistance represented 16/27 (59.2%). The other resistant organisms included MRSA in 45/98 (45.9%), and MR staphylococcus epidermidis in 3/98 (3.0%) of infections (see Figure 2).
Figure 2.
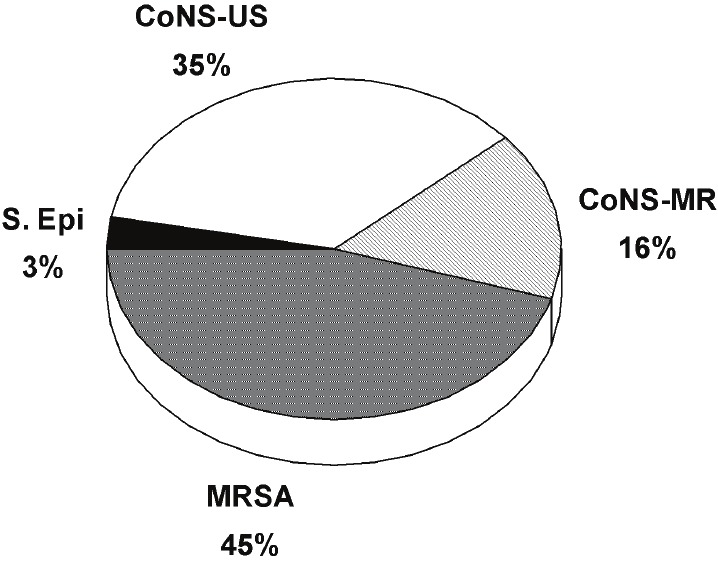
Distribution of methicillin resistant organismtypes in pacemaker/ICD infections. CoNS-MR=coagulase negative staphylococci-methicillinresistant; CoNS-US = coagulase negative staphylococci-unknown sensitivities; MRSE = methicillinresistance staphylococcus aureus; MRSA = methicillin resistance staphylococcus aureus; S.epi = Staphylococcus epidermidis.
Patient demographics with MR infections were compared to those without resistant organisms (see Table 1). Among the clinical differences observed, diabetes (42% vs 28%, p=0.037) and cardiomyopathy (64% vs 47%, p=.00093) were statistically seen more frequently among the methicillin resistance cohort. There was a trend seen among the MR cohort with respect to percent of ICDs (67% vs 55%, p=0.064). No other clinical variables were found to be different. Institutional patients were compared to outside referrals presenting for device explantation. No clinical differences were observed other than a greater amount of ICDs seen in the institutional cohort 66% vs 47% (p=0.008). The overall median time to infection from day of implantation or last device procedure (generator changes, lead revisions, upgrades) was 226 days (interquartile range 43-653 days) with the earliest infection occurring within 4 days, and the latest 2287 days post implant. Logistical regression did not reveal an increase in institutional methicillin resistance infections over time (P=0.304) using the number of implants performed per year. The annual prevalence of MR infections ranged between 33-78% of the device infections seen.
Table 1.
Comparable demographics of methicillin resistant infections to those patients without resistant organisms
| MR (+) | MR (-) | P value | |
|---|---|---|---|
| N | 98 | 120 | |
| Age (mean) | 69.9+/-14.5 | 70.4+/-14.2 | 0.91 |
| Males | 68 (69%) | 81 (68%) | 0.77 |
| Diabetes | 41(42%) | 34 (28%) | 0.037 |
| Coronary artery disease | 60 (61%) | 72 (60%) | 0.52 |
| Hypertension | 63 (64%) | 80 (67%) | 0.71 |
| Cardiomyopathy* | 63 (64%) | 56 (47%) | 0.0093 |
| Chronic kidney disease | 25 (26%) | 22 (18%) | 0.20 |
| Valvular Disease | 13 (13%) | 13 (11%) | 0.72 |
| Congenital Disease | 1 (1%) | 4 (3.3%) | 0.23 |
| ICD | 66 (67%) | 66 (55%) | 0.064 |
| PPM | 32 (33%) | 54 (45%) | |
| Days to infection | N=89 | N = 108 | 0.48 |
| Median (25th,75th) | 177 (39,503) | 242 (44,670) |
ICD = implantable cardioverter defibrillator; PPM = permanent pacemaker.
Includes ischemic cardiomyopathy, non-ischemic cardiomyopathy, hypertrophic cardiomyopathy
Includes single chamber PPM/ICD, dual chamber PPM/ICD
Prospective analysis
Of the 181 patients tested, 110 were men, and the average age was 72 years. Other demographic data are shown in Table 2. Procedures performed included new implants in 73%, generator changes in 13% and system modifications/upgrades in 14% of subjects. Eighty patients (43%) were outpatients, hospitalized for 1 day or less, and 102 patients (56%) were inpatients with a median length of stay of 7 days. Comorbidities included diabetes (32%), chronic kidney disease (23%), cancer history (6%) and steroid use (3%). Seventy-eight percent of the nasal cultures were negative for any staphylococcal growth after 48 hours. Of the 22% of patients with positive cultures, 32 pts (17.6%) had positive cultures for SA of which 6.6% were MRSA, and 11% were methicillin sensitive SA. Cultures from 5 patients grew CoNS, and one culture grew S. epidermidis (Figure 3). There were no demographic differences of significance noted among the MRSA positive group versus the MRSA negative group (Table 2). However, patients positive for MRSA nares colonization had a statistically significant greater mean length of hospital stay (8.5 ± 9.6 days versus 4.4 ± 5.2 days, P=0.049) suggesting that many MRSA colonizations were hospital acquired. No other clinical predictors of MR colonization were identified.
Table 2.
Demographics of patients comparing results of nares cultures
| MRSA (-) | MRSA (+) | P value | |
|---|---|---|---|
| N | 169 | 12 | 0.38 |
| Age (mean) | 71 | 74 | 0.77 |
| Males | 102 (60%) | 8 (67%) | 0.53 |
| DM | 54 (32%) | 5 (42%) | 0.62 |
| CAD | 116 (69%) | 7 (58%) | 0.53 |
| HTN | 140 (83%) | 10 (83%) | 1.00 |
| CMP* | 83 (49%) | 5 (42%) | 0.62 |
| Steroids | 5 (3.0%) | 0 (0%) | 1.00 |
| Cancer | 11 (6.5%) | 0 (0%) | 1.00 |
| CKD | 39 (23%) | 4 (33%) | 0.48 |
| Previous Device** | 47 (28%) | 2 (17%) | 0.52 |
| Inpatient length of stay(Mean days +/- SD) | 4.4 +/- 5.2 (2.0) | 8.5 +/- 9.6 (6.0) | 0.049 |
ICD = implantable cardioverter defibrillator; PPM = permanent pacemaker.
Includes ischemic cardiomyopathy, non-ischemic cardiomyopathy, hypertrophic cardiomyopathy
Includes single chamber PPM/ICD, dual chamber PPM/ICD
Figure 3.
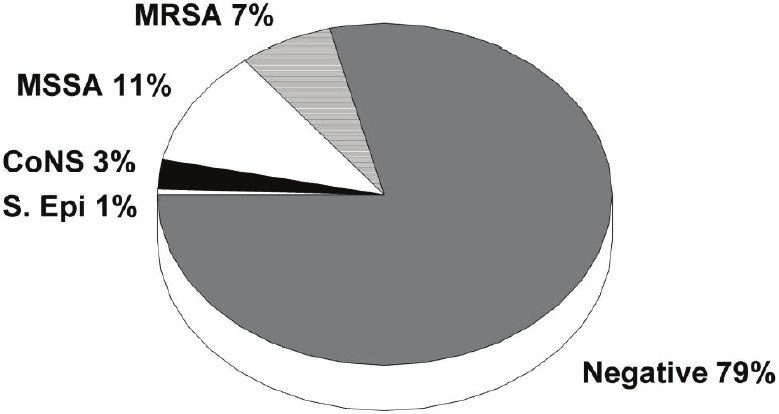
Prevalence of nares colonization with staphylococcus species. CoNS = coagulase negative staphylococci; MRSA = methicillin resistant staphylococcus aureus; MSSA = methicillin sensitive staphylococcus aureus; S.epi = staphylococcus epidermidis.
All patients enrolled in the prospective study arm were followed up to three years after device implant. There was one device-related infection that occurred in this cohort (infection rate 0.55%). That patient was one of 12 with a positive MRSA nares culture prior to device implantation, and presented with a MRSA infection one month after implant. The organism had similar sensitivities to that cultured earlier.
Discussion
Over the past decade, methicillin-resistant organisms have emerged as frequent pathogens within the hospital and in the community. These resistant organisms cause more serious disease, lengthen hospital stay, and increase costs compared to other pathogens. Identifying patients at risk of developing resistant organism infections may lead to strategies to decrease this risk.
The retrospective data collected at Beaumont Health System showed a high prevalence of MR pacemaker infections, almost 45% among the total cohort. Patients with diabetes and cardiomyopathy were more frequently found to have MR infection. These patients are part of a sicker patient population, and are more likely to be predisposed to colonization with MR organisms and have increased risk for developing infection. In the prospective study, up to 18% of patients had colonization with SA, and nearly 7% of patients were colonized with MRSA. One patient with MRSA colonization did go on to develop a pacemaker infection one month later with the same organism after three year follow up.
Mechanisms of device infection with MR
Not all patients assume the same risk of infection. Those with diabetes mellitus, malignancy, elderly, on corticosteroids, or recent surgery involving a pacemaker device, have a greater inherent risk of developing infections with implantation of foreign bodies [2,17-19]. Another method of identyfing patients at risk of MR infection has been through identifying positive nares colonization with the organism. Davis et al reported an increase risk for MRSA infection in those patients identified with MRSA colonization. They suggested that as many as 25% patients colonized with MRSA later went on to develop MRSA infection [16,20-22].
Current guidelines for antibiotic prophylaxis prior to device implants, employed by the investigators, consist of pre-procedure treatment with cephalexin or clindamycin. These antibiotics are appropriate for the common skin pathogens, but may not be sufficient to treat emergent resistant bacterial strains. Although there are no randomized control trials that have showed a benefit with antibiotic prophylaxis, observational data has identified the use of antibiotic prophylaxis as a negative risk factor for infection after device implantation [20-22].
Although there are current treatment strategies in treating cardiac device infections, the ultimate goal would be to prevent these deadly infections [23-29]. Identifying those patients who may be at increased risk of developing methicillin resistant infection is a clinical challenge. The present study confirmed that patients with a longer hospital stay were more likely to be colonized with MRSA. Other risk factors for MRSA colonization described in the literature include previous antibiotic use, hospitalization during the past year, and HIV infection [22,29-32]. Patients with positive MRSA colonization also seem to be at greater risk of developing infection overall [16,19]. In the present study, patients with a history of diabetes and cardiomyopathy were more likely to be infected with a MR organism. Developing a strategy for identifying these high risk patients may lead to more appropriate prophylactic antibiotic selection, and possibly decrease morbidity and mortality.
One such strategy for identifying patients receiving a device implant who are at risk for MR infections may be to perform nares cultures on inpatients, diabetics, those patients with cardiomyopathies, and those who have had lengthy hospital stays. If the culture is positive, one could consider prophylaxis with an antibiotic appropriate against MR organisms. It is not known rather this intervention will indeed lead to any benefit in reduction of MR infections or impact mortality.
Limitations
In our retrospective chart review, culture data regarding sensitivities of CoNS organisms was incomplete. Given that over 80% of CoNS is methicillin resistant, and is treated as such on a clinical basis, we chose to label these organisms as methicillin resistant which may have overestimated the true prevalence of MR. The sample size for the prospective study is small, and the number of patients found with methicillin resistance was considerably less compared to those without resistance. Our follow up of nares culture patients for development of infection consisted of institutional chart review. We did not account for patients that may have presented at other institutions with device infections.
Conclusions
Methicillin resistance infections continue to represent almost half of PPM or ICD implantation infections. Clinical risk factors alone may be not be sufficient enough to identify at-risk patients. Nares culture data may help to risk stratify patients undergoing device implants and drive choice of prophylactic antibiotics. Such strategies may also help minimize overuse of antibiotics directed toward resistant organisms. Future studies should prospectively test strategies to identify patients at increased risk for the development of MR infections, and determine whether prophylactic antibiotics against these organisms are effective at lowering the subsequent infection rate.
References
- 1.Mond H, Irwin M, Morillo C, Ector H. The world survey of cardiac pacing and cardioverter defibrillators:calendar year 2001. Pacing Clin Electrophysiol. 2004;27:955–964. doi: 10.1111/j.1540-8159.2004.00565.x. [DOI] [PubMed] [Google Scholar]
- 2.Eggimann P, Waldvogel F. Infections associated with indwelling medical devices. Washington DC: Am Soc Microbiol Press; 2000. Pacemaker and defibrillator infections; p. 247. [Google Scholar]
- 3.Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med. 2000;133:604–608. doi: 10.7326/0003-4819-133-8-200010170-00011. [DOI] [PubMed] [Google Scholar]
- 4.Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, Beach M SENTRY Partcipants Group. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis. 2001;32(Suppl 2):S114. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 5.Fowler VG Jr, Kong LK, Corey GR, Gottlieb GS, McClelland RS, Sexton DJ, Gesty-Palmer D, Harrell LJ. Recurrent Staphyloccus aureus bacteremia:pulsed-field gel electrophoresis findings in 29 patients. J Infect Dis. 1999;179:1157–1161. doi: 10.1086/314712. [DOI] [PubMed] [Google Scholar]
- 6.Bracke FA, Meijer A, Van Gelder LM. Pacemaker lead complications: when is extraction appropriate and what can we learn from published data? Heart. 2001;85:254–259. doi: 10.1136/heart.85.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karchmer AW, Longworth DL. Infections of intracardiac devices. Infect Dis Clin North Am. 2002;16:477–505. doi: 10.1016/s0891-5520(01)00005-8. [DOI] [PubMed] [Google Scholar]
- 8.Hauser RG, Katsiyiannis CC, Gornick CC, Almquist AK, Kallinen LM. Deaths and cardiovascular injuries due to device-assisted implantable cardioverter-defibrillator and pacemaker lead extraction. Europace. 2010;12:395–401. doi: 10.1093/europace/eup375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banman TS, Gupta SK, Valle JA, Yamada E. Risk factors for mortality in patients with cardiac device-related infection. Circ Arrhythm Electrophysiol. 2009;2:129–134. doi: 10.1161/CIRCEP.108.816868. [DOI] [PubMed] [Google Scholar]
- 10.Baddour LM, Wilson WR. Infections of prosthetic valves and intravascular devices. In: Mandell GL, Bennett JE, Dolin R, editors. principles and practice of infectious diseases. 6th ed. Philadelphia PA: Churchill Livingstone; 2005. p. 1022. [Google Scholar]
- 11.Baddour LM, Bettmann MA, Bolger AF, Epstein AE, Ferrieri P, Gerber MA, Gewitz MH, Jacobs AK, Levison ME, Newburger JW, Pallasch TJ, Wilson WR, Baltimore RS, Falace DA, Shulman ST, Tani LY, Taubert KA AHA. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108:2015–2031. doi: 10.1161/01.CIR.0000093201.57771.47. [DOI] [PubMed] [Google Scholar]
- 12.Da Costa A, Kirkorian G, Cucherat M, Delahaye F, Chevalier P, Cerisier A, Isaaz K, Touboul P. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation. 1998;97:1796–1801. doi: 10.1161/01.cir.97.18.1796. [DOI] [PubMed] [Google Scholar]
- 13.Da Costa A, Lelievre H, Kirkorian G, Célard M, Chevalier P, Vandenesch F, Etienne J, Touboul P. Role of the preaxillary flora in pacemaker infections: a prospective study. Circulation. 1998;97:1791–1795. doi: 10.1161/01.cir.97.18.1791. [DOI] [PubMed] [Google Scholar]
- 14.de Oliveira JC, Martinelli M, D’Orio Nishioka SA, Varejão T, Uipe D, Pedrosa AA, Costa R, D'Avila A, Danik SB. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo controlled trial. Circ Arrhythm Electrophysiol. 2009;2:29–34. doi: 10.1161/CIRCEP.108.795906. [DOI] [PubMed] [Google Scholar]
- 15.Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26:166–174. doi: 10.1086/502522. [DOI] [PubMed] [Google Scholar]
- 16.Davis KA, Stewart JJ, Crouch HK, Florez CE, Hospenthal DR. Methicillin-resistant staphylococcus aureus (MRSA) nares colinization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis. 2004;39:776–782. doi: 10.1086/422997. [DOI] [PubMed] [Google Scholar]
- 17.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Stoner SM, Baddour LM. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis. 2007;45:166–173. doi: 10.1086/518889. [DOI] [PubMed] [Google Scholar]
- 18.Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, Masoudi FA, Okum EJ, Wilson WR, Beerman LB, Bolger AF, Estes NA 3rd, Gewitz M, Newburger JW, Schron EB, Taubert KA American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management. Circulation. 2010;121:458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 19.Huang S, Platt R. Risk of methicillin-resistant Staphyloccus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36:281–285. doi: 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- 20.Klug D, Balde M, Pavin D, Hidden-Lucet F, Clementy J, Sadoul N, Rey JL, Lande G, Lazarus A, Victor J, Barnay C, Grandbastien B, Kacet S PEOPLE Study Group. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116:1349–1355. doi: 10.1161/CIRCULATIONAHA.106.678664. [DOI] [PubMed] [Google Scholar]
- 21.Ott M, Shen J, Sherwood S. Evidenced-based practice for control of methicillin-resistant Staphylococcus aureus. AORN J. 2005;81:361–364, 367-372. doi: 10.1016/s0001-2092(06)60418-3. [DOI] [PubMed] [Google Scholar]
- 22.Da Costa A, Kirkorian G, Cucherat M, Delahaye F, Chevalier P, Cerisier A, Isaaz K, Touboul P. Antibiotic prophylaxis for permanent implantation:a meta analysis. Circulation. 1998;97:1796–1801. doi: 10.1161/01.cir.97.18.1796. [DOI] [PubMed] [Google Scholar]
- 23.Gemmell CG, Edwards D, Fraise AP, Gould K, Ridgway GL, Warren RE. Guidelines for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J Antimicrob Chemother. 2006;57:589–608. doi: 10.1093/jac/dkl017. [DOI] [PubMed] [Google Scholar]
- 24.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Stoner S, Baddour LM. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49:1851–1859. doi: 10.1016/j.jacc.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 25.Sampedro MF, Patel R. Infections associated with long term prosthetic devices. Infect Dis Clin North Am. 2007;21:785–819. doi: 10.1016/j.idc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 27.Korantzopoulos P, Kostantinos PL, Grekas G, Goudevenos JA. Pacemaker dependency after implantation of electrophysiological devices. Europace. 2009;11:1151–1155. doi: 10.1093/europace/eup195. [DOI] [PubMed] [Google Scholar]
- 28.Uslan DZ, Dowsley TF, Sohail MR, Hayes DL, Friedman PA, Wilson WR, Steckelberg JM, Baddour LM. Cardiovascular implantable electronic device infection in patients with Staphylococcus aureus bacteremia. Pacing Clin Eletrophysiol. 2010;33:407. doi: 10.1111/j.1540-8159.2009.02565.x. [DOI] [PubMed] [Google Scholar]
- 29.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Jenkins SM, Baddour LM. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clinic Proc. 2008;83:46–53. doi: 10.4065/83.1.46. [DOI] [PubMed] [Google Scholar]
- 30.Hidron AI, Kourbatova E, Halvosa S, Terrell BJ, McDougal LK, Tenover FC, Blumberg HM, King MD. Risk factors for colonization with methicillin-resistant Staphyloccus aureus (MRSA) in patients admitted to an urban hospital: emergence of community-associated MRSA nasal carriage. Clin Infect Dis. 2005;41:159–166. doi: 10.1086/430910. [DOI] [PubMed] [Google Scholar]
- 31.Coello R, Jiminez J, Garcia M, Arroyo P, Minguez D, Fernández C, Cruzet F, Gaspar C. Prospective study of infection, colonization and carriage of methicillin-resistant Staphyloccus aureus in an outbreak affecting 990 patients. Eur J Clin Microbio Infect Dis. 1994;13:74–81. doi: 10.1007/BF02026130. [DOI] [PubMed] [Google Scholar]
- 32.Sanford MD, Widmer AF, Bale MJ, Jones RN, Wenzel RP. Efficient detection and long-term persistant of the carriage of methicillin-resistant Staphyloccus aureus. Clin Infect Dis. 1994;19:1123–1128. doi: 10.1093/clinids/19.6.1123. [DOI] [PubMed] [Google Scholar]


