Abstract
Recently, we have introduced [tris(1,10-phenanthroline)lanthanum(III)] trithiocyanate (KP772, FFC24) as a new lanthanum compound which has promising anticancer properties in vivo and in vitro. Aim of this study was to investigate the impact of ABC transporter-mediated multidrug resistance (MDR) on the anticancer activity of KP772. Here, we demonstrate that all MDR cell models investigated, overexpressing ABCB1 (P-glycoprotein), ABCC1 (multidrug resistance protein 1), or ABCG2 (breast cancer resistance protein) either due to drug selection or gene transfection, were significantly hypersensitive against KP772. Using ABCB1-overexpressing KBC-1 cells as MDR model, KP772 hypersensitivity was demonstrated to be based on stronger apoptosis induction and/or cell cycle arrest at unaltered cellular drug accumulation. KP772 did neither stimulate ABCB1 ATPase activity nor alter rhodamine 123 accumulation arguing against a direct interaction with ABCB1. Accordingly, several drug resistance modulators did not sensitize but rather protect MDR cells against KP772-induced cytotoxicity. Moreover, long-term KP772 treatment of KBC-1 cells at subtoxic concentrations led within 20 passages to a complete loss of drug resistance based on blocked MDR1 gene expression. When exposing parental KB-3-1 cells to subtoxic, stepwise increasing KP772 concentrations, we observed, in contrast to several other metallo-drugs, no acquisition of KP772 resistance. Summarizing, our data demonstrate that KP772 is hyperactive in MDR cells and might have chemosensitizing properties by blocking ABCB1 expression. Together with the disability of tumor cells to acquire KP772 resistance, our data suggest that KP772 should be especially active against notoriously drug-resistant tumor types and as second line treatment after standard chemotherapy failure.
Keywords: Multidrug resistance, P-glycoprotein, Collateral sensitivity, Phenanthroline, Lanthanum
1. Introduction
Drug resistance continues to be a major impediment to effective cancer chemotherapy [1,2]. Frequently, tumor cells exhibit resistance not only against a single class of drugs but against diverse chemotherapeutics, even with unrelated modes of action. This phenomenon, known as multidrug resistance (MDR), is often based on the overexpression of ATP-binding cassette (ABC) transporters. Under physiological conditions, this family of ATP-driven, membrane-spanning proteins is involved in the transport of molecules including toxins, peptides, sugars, lipids, but also chemotherapeutical drugs and diverse pharmaceutical compounds across biological membranes [2]. Most prominent in mediating MDR with respect to antineoplastic agents are members of three ABC transporter subfamilies: ABCB including the well-known drug-transporter ABCB1 (P-glycoprotein), ABCC comprising the nine multidrug resistance proteins, and ABCG harbouring the breast cancer resistance protein ABCG2 [1,2]. The substrate specificities of these proteins overlap and thus certain drugs are substrate of more than one ABC transporter [2]. Several tissues, especially those exposed to endogenous or environmental toxins, express per se high levels of some ABC transporters, representing an important protection mechanism of the healthy organisms [2]. Consequently, tumors derived from such tissues are often intrinsically chemoresistant. In contrast, acquired resistance develops frequently during chemotherapy in initially chemosensitive tumors [1]. Thus, it is essential for the successful clinical development of new anticancer drugs to clarify possible limitations by drug resistance mechanisms in order to select an appropriate patient collective and/or drug combination strategies.
Recently, we have introduced the new lanthanum compound [tris(1,10-phenanthroline)lanthanum(III)] trithiocyanate (KP772; FFC24; Fig. 1A) demonstrating promising anticancer activity in vitro as well as in vivo [3]. KP772 exposure potently arrested cancer cells in G0/G1 phase of the cell cycle and/or induced apoptosis via the mitochondrial pathway. These cytostatic/cytotoxic activities were widely independent of the cellular p53 status and not based on drug-mediated radical formation [3].
Fig. 1.
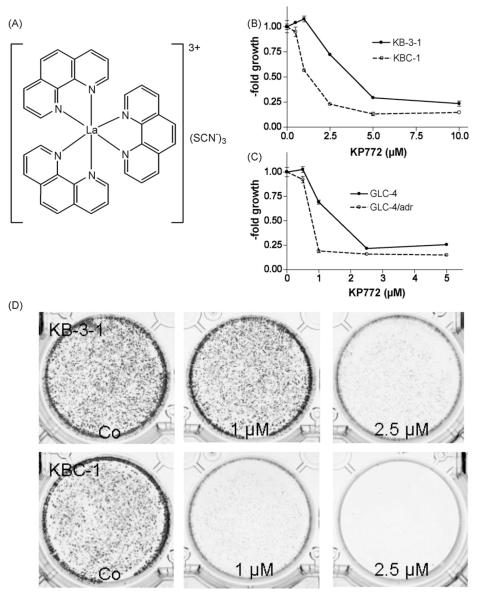
(A) [Tris(1,10-phenanthroline)lanthanum(III)] trithiocyanate (KP772; FFC24). KP772-induced cytotoxicity against (B) KB-3-1 or (C) GLC-4 and their chemoresistant sublines KBC-1 (P-gp-overexpressing) or GLC-4/adr (MRP1-overexpressing) was measured using MTT assay after 72 h drug exposure. (D) Clonogenic survival of KB-3-1 and KBC-1 cells was determined after exposure to the indicated concentrations of KP772 for 6 days. Cell colonies were visualised by crystal violet staining.
Here, we demonstrate that the potent anticancer activity of KP772 is not hampered by overexpression of the most important drug-transporter proteins ABCB1, ABCC1, and ABCG2. In contrast, all ABC transporter-overexpressing cell lines exhibited significant collateral sensitivity to this new drug. Additionally, selection against KP772 did not generate cells with acquired resistance. Moreover, long-term subtoxic KP772 treatmentled to loss of ABCB1 expression in a MDR cell model and consequently to restored sensitivity against ABCB1 substrate drugs. In summary, our data suggest that KP772 might be especially promising for treatment of patients suffering from chemotherapy-resistant tumors based on ABC transporter-mediated MDR.
2. Material and methods
2.1. Drugs
[Tris(1,10-phenanthroline)lanthanum(III)] trithiocyanate (KP772; FFC24) and acetatobis[1-(azepanyl)-4(2-pyridyl)-2, 3-diazapenta-1,3-dien-1-thiolato-N′,N3,S]bismuth(III) (KP1255) were prepared at the Institute of Inorganic Chemistry, University of Vienna (Vienna, Austria) [3]. For in vitro studies, KP772 was dissolved in water (1 mM stock) and diluted into culture media at the concentrations indicated. Verapamil (VP) was purchased from Abbott (Vienna, Austria), cyclosporin A (CSA) from Sandoz (Basel, Switzerland), dipyridamole from Aldrich (Milwaukee, USA), and TMAH from Merck (Darmstadt, Germany). All other substances were purchased from Sigma–Aldrich (St. Louis, USA). All solutions were freshly prepared before use.
2.2. Cell culture
The following human cell lines and their chemoresistant sublines were used in this study: the epidermal carcinoma-derived cell line KB-3-1 and its ABCB1-overexpressing subline KBC-1 (generously donated by Dr. Shen, Bethesda, USA) [4]; the promyelocytic leukaemia cell line HL60 and its ABCC1-over-expressing subline HL60/adr (by Dr. Center, Kansas State University, USA) [5], the small cell lung carcinoma cell line GLC-4 and its ABCC1- and lung resistance protein (LRP)-overexpressing subline GLC-4/adr (by Dr. deVries, Groningen, The Netherlands) [6]; the breast adenocarcinoma cell line MCF-7 with the respective ABCG2-transfected subclone MCF-7/bcrp by Dr. Ross, University of Maryland, Greenbaum Cancer Centre, USA) [7]. Additionally, the non-small cell lung cancer cell line A549 (from American Type Culture Collection, Manassas, VA) was used. All cell lines were grown in RMPI 1640 supplemented with 10% fetal calf serum, with the exception of MCF-7 cells, which were grown in MEME with 10% fetal calf serum.
2.3. Cytotoxicity assays
Cells were plated (2 × 104 cells/ml for KB, A549, 5 × 104 cells/ml for HL60 and MCF-7; 4 × 104 cells/ml for GLC-4) in 100 μl per well in 96-well plates and allowed to attach for 24 h. Drugs were added in another 100 μl growth medium and cells exposed for 72 h. The proportion of viable cells was determined by MTT assay following the manufacturer’s recommendations (EZ4U, Biomedica, Vienna, Austria). Cytotoxicity was expressed as IC75, IC50, and IC25 values calculated from full dose-response curves (drug concentrations inducing a 25, 50 and 75% reduction of cell survival in comparison to the control cultured in parallel without drugs).
2.4. Western blot analysis
Cell fractionation, protein separation, and Western blotting were performed as described [8] and densitometric evaluation done using the GelDoc 1000 system (Biorad, Hercules, CA). The following antibodies were used: anti-ABCB1 monoclonal mouse C219 (Signet, Dedham, USA), dilution 1:100; Apoptosis Sampler kit: anti-PARP, anti-caspase 3, anti-caspase 7, anti-cleaved caspase 7 (Cell Signalling Technology, Beverly, MA), all polyclonal rabbit, dilution 1:1000; anti-cyclin A (sc-751), anti-cyclin E (sc-481), anti-cyclin D1 (sc-246), anti-cdk2 (sc-163): all are polyclonal rabbit (Santa Cruz Biotechnology), dilution 1:200; anti-cyclin B1 monoclonal mouse sc-245 (Santa Cruz Biotechnology), dilution 1:1000; anti-cdk4 monoclonal mouse DSC156 (Cell Signaling Technology), dilution 1:200; anti-cdk1 monoclonal mouse AB-3 (Neomarkers, CA, USA), dilution 1:200; anti-β-actin monoclonal mouse AC-15 (Sigma), dilution 1:1000. All secondary, peroxidase-labelled antibodies from Pierce were used at working dilutions of 1:10,000.
2.5. Cell cycle analysis
KB-3-1 and KBC-1 cells (106 per well) seeded into 6-well plates and cultured for 24 h were treated for another 24 h with 0.5, 1, 2.5 and 5 μM KP772. Then cells were collected, washed with PBS, fixed in 70% ethanol and stored at −20 °C. To determine the cell cycle distribution, cells were transferred into PBS, incubated with RNAse (10 μg/ml) for 30 min at 37 °C, treated with 5 μg/ml propidium iodide for 30 min and then analysed by flow cytometry using FACS Calibur (Becton Dickinson, Palo Alto, CA). The resulting DNA histograms were quantified using the ModeFit software (Becton Dickinson and Company, New York, USA).
2.6. Mitochondrial membrane potential detection
Breakdown of mitochondrial membrane potential was determined by FACS analysis using JC-1 (5,5,6,6-tetrachloro-1,1,3,3-tetraethylbenzimidazol-carbocyanine iodide) [3]. For this purpose the Mitochondrial Membrane Potential Detection Kit (Stratagene, La Jolla, CA, USA) was used following the manufacturer’s instructions. In short, 106 adherent KB-3-1 and KBC-1 cells were treated for 24 h with 1, 2.5 and 5 μM KP772. After trypsinisation and PBS washing, cells were incubated for 10 min in freshly prepared JC-1 solution (10 μg/ml in medium) at 37 °C. Spare dye was removed by washing in PBS and cell-associated fluorescence measured via FACS.
2.7. Measuring ABCB1 ATPase activity
Preparation of plasma membrane vesicles from CCRF-ADR5000 cells (gift of Dr. V. Gekeler) and measurement of ABCB1 ATPase activity were performed exactly as described previously [9].
2.8. Drug accumulation assay
KB-3-1 cells (1 × 105 cells per well) were exposed to KP772 for 60 min at 37 °C. After three washes with PBS, cells were lysed by incubation in 400 μl TMAH at room temperature. Lysates were diluted in 0.6N HNO3 and lanthanum concentrations determined by inductively coupled plasma mass spectroscopy (ICP-MS) using an Elan 6100, Perkin-Elmer/Sciex Corporation. Values represent means of at least three independent experiments.
2.9. 3H-thymidine incorporation assay
KB-3-1 and KBC-1 cells (2 × 104 cells/ml) were seeded into 96-well plates and after 24 h recovery treated with KP772 for another 24 h. Medium was replaced by a 2 nM 3H-thymidine solution (diluted in complete culture medium; radioactivity: 25 Ci/mM). After 2 h incubation at 37 °C, cells were washed two times with PBS. Cell lysates were prepared and the radioactivity determined as described [10].
2.10. Clonogenic assay
KB-3-1 and KBC-1 cells (103 cells per well) were seeded into 6-well plates. Following 24 h recovery, cells were treated with 1 or 2.5 μM KP772. At day 6 of exposure, cells were washed twice with PBS, stained with crystal violet and analysed for colony formation.
2.11. RT-PCR
ABCB1 gene expression was determined by RT-PCR as described previously [11].
2.12. Rhodamine 123 (Rh123) accumulation studies
Rh123 accumulation assays were performed as described previously [11]. Briefly, 5 × 105 KB-3-1 or KBC-1 cells were incubated for 1 h at 37 °C with Rh123 (0.25 mg/ml) both in the presence and in the absence of VP or KP772 (5, 10 and 20 μM both) added 30 min before Rh123. After 60 min exposure, fluorescence of Rh123 was collected through a 530/30 nm bandpass filter on the FACS Calibur (Becton Dickinson, Palo Alto, CA).
3. Results
3.1. Activity of KP772 against drug-sensitive and - resistant cell sublines
Cytotoxic activity of KP772 was tested using a panel of chemosensitive tumor cell lines and their chemoresistant sublines expressing defined resistance mechanisms (Fig. 1B and C; Table 1). In general, the sensitivity of all tumor cell lines against KP772 was in the very low μM range. Unexpectedly, all ABC transporter-overexpressing MDR sublines demonstrated significant hypersensitivity against KP772 with IC50 values of about 1.5-fold and IC75 values up to 5.3-fold lower as compared to those of the parental cells (Table 1). To evaluate the effect of prolonged KP772 treatment, the capacity of single tumor cells to form colonies was analysed by clonogenic assays. Treatment with KP772 distinctly reduced colony formation in a dose-dependent manner (Fig. 1D). Corroborating the data from MTT assays, the ABCB1-overexpressing subline KBC-1 was again demonstrated to be KP772-hypersensitive with a profound inhibition of colony formation already at 1 μM, a concentration inactive in the parental KB-3-1 cells.
Table 1. Cytotoxic activity of KP772 against various cell models at 72 h treatment.
| Cell line | Overexpressed protein | Fold resistancea | IC75 (μM) |
IC50 (μM) |
IC25 (μM) |
|||
|---|---|---|---|---|---|---|---|---|
| Meanb | ±S.D. | Meanb | ±S.D. | Meanb | ±S.D. | |||
| GLC-4 | Parental | - | 0.92 | 0.03 | 1.31 | 0.03 | 2.16 | 0.04 |
| GLC-4/adr | ABCC1, LRP | 0.61*** | 0.64 | 0.03 | 0.80 | 0.02 | 0.96 | 0.02 |
| HL60 | Parental | - | 1.33 | 0.58 | 1.77 | 0.04 | 2.20 | 0.03 |
| HL60/adr | ABCC1 | 0.44*** | 0.46 | 0.09 | 0.78 | 0.04 | 2.26 | 0.31 |
| KB-3-1 | Parental | - | 1.68 | 0.06 | 2.49 | 0.24 | 4.93 | 0.06 |
| KBC-1 | ABCB1 | 0.50* | 0.82 | 0.03 | 1.43 | 0.17 | 3.51 | 0.44 |
| MCF-7 | Parental | - | 1.6 | 0.24 | 4.29 | 0.43 | >10.0 | - |
| MCF-7/bcrp | ABCG2 | 0.54* | 0.31 | 0.03 | 2.33* | 0.21 | >10.0 | - |
| A459 | ABCC1, ABCC2, ABCG2, LRP | - | 0.9 | 0.1 | 1.5 | 0.10 | 2.6 | 0.2 |
Differences in KP772 sensitivity calculated by dividing IC50 values of the MDR subline by those of the parental cell lines.
IC75, IC50, and IC25 were calculated from whole dose–response curves. Values given are means ± S.D. of 1 representative experiment out of three, performed in triplicates.
Significantly different from parental cell line ( p < 0.05).
Significantly different from parental cell line ( p < 0.001).
3.2. Influence of ABCB1 expression on KP772-induced cell cycle arrest
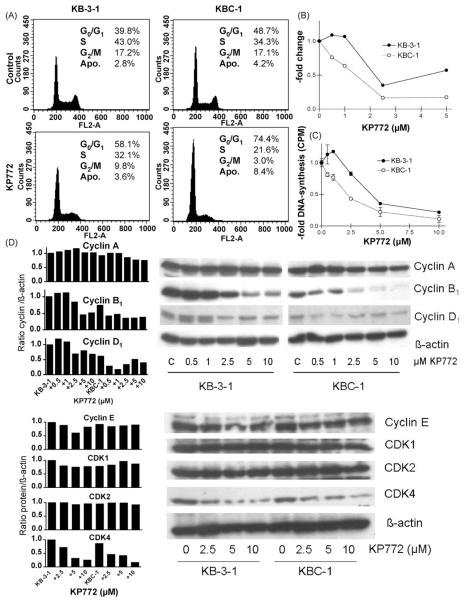
Recently, we have shown that KP772 reduces DNA synthesis and induces a profound accumulation of cells in G0/G1 phase of the cell cycle accompanied by a potent downregulation of cyclin B1 [3]. In order to determine whether the increase of KP772 sensitivity of MDR cell models is based on increased cell cycle arrest, we analysed cell cycle distribution of KP772-treated KB-3-1 in comparison to KBC-1 cells. After 18 h treatment with 5 μM KP772 (Fig. 2A), the percentage of both G2/M and S phase cells were significantly (two-way ANOVA, p < 0.05) stronger reduced and, accordingly, those of G0/G1 cells increased in KBC-1 as compared to KB-3-1 cells. This effect was especially noticeable in the G2/M phase cell population (Fig. 2B). Corresponding to cell cycle changes, KP772 treatment had a distinctly stronger inhibiting effect (two-way ANOVA, p < 0.0001) on DNA synthesis of KBC-1 as compared to KB-3-1 cells (Fig. 2C). Additionally, a profound reduction of cyclin B1 expression in KBC-1 cells was detectable already after 24 h treatment with 0.5 μM KP772 (Fig. 2D), while in KB-3-1 cells similar levels of reduction were not observed up to 2.5 μM KP772. Also the reduction of cyclin D1 was more pronounced in the ABCB1-overexpressing subline while CDK4 expression was lost at comparable levels. All other investigated cell cycle proteins (cyclin A and E, CDK1 and 2) remained widely unchanged during KP772 treatment.
Fig. 2.
Impact of KP772 on cell cycle progression and DNA synthesis of KB-3-1 and ABCB1-overexpressing KBC-1 cells. (A) Changes in the cell cycle distribution of the indicated cell lines treated with 5 μM KP772 for 24 h were analysed by PI staining and FACS. Percentages of cells in G0/G1, S and G2/M phases of the cell cycle as well as apoptotic cells (Apo) are indicated. (B) Changes in the proportion of cells in G2/M phase of the cell cycle at increasing doses of KP772 are shown. The amount of G2/M cells in the untreated control group was set as 1. One of three experiments delivering comparable results is shown. (C) DNA synthesis was determined by 3H-thymidine incorporation after 24 h treatment with KP772 at the indicated concentrations. Values given are means ± S.D. from at least two independent experiments performed in triplicates. (D) The impact of the indicated drug concentrations on the expression pattern of cyclin A, B1, D1, E and CDK1, 2, 4 after a 24 h treatment was analysed by Western blot (right) followed by densiometric evaluation (left). β-Actin was used as loading control. Antibodies are described under Section 2.
3.3. Influence of ABCB1 expression on KP772-induced apoptosis
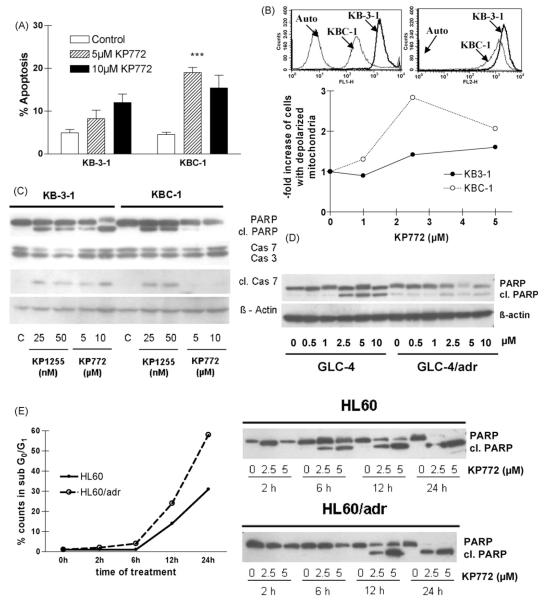
As described previously [3], KP772 treatment led to typical signs of apoptosis, i.e. condensed chromatin and fragmentation of nuclei into apoptotic bodies. To characterise whether the collateral sensitivity of ABC transporter-overexpressing cells is also reflected by enhanced apoptosis, the impact of KP772 on nuclear morphology was assessed in KB-3-1 and KBC-1 cells by DAPI staining. After 48 h treatment with 5 μM KP772, 8.2 and 19.0% of KB-3-1 and KBC-1 cells, respectively, exhibited apoptotic features, while 10 μM induced almost comparable apoptosis rates in both cell lines (12.0% versus 14.4%) (Fig. 3A). Additionally, the fluorescent cationic dye JC-1 was used to detect the mitochondrial permeability transition, an early step in the induction of apoptosis by the intrinsic pathway [12]. In healthy, non-apoptotic cells the dye accumulates and aggregates within the mitochondria, resulting in bright red staining. In apoptotic cells, JC-1 cannot accumulate within the mitochondria due to the collapse of the membrane potential and remains in the cytoplasm in its green-fluorescent monomeric form. JC-1 is a substrate for ABCB1 [13] and consequently accumulation of the cytoplasmic, green-fluorescent form is strongly reduced in KBC-1 as compared to KB-3-1 cells (Fig. 3B upper panel, left). However, the red-fluorescent, mitochondrial JC-1 signal does not strongly differ between the cell lines and thus can be used for mitochondrial membrane potential detection (Fig. 3B upper panel, right). Fig. 3B (lower panel) shows the increase of cells with depolarised mitochondria measured via FACS analyses of JC-1-stained KB-3-1 or KBC-1 cells after 24 h KP772 treatment. KP772 led to a significantly (two-way ANOVA, p < 0.05) enhanced fraction of pre-apoptotic cells in KBC-1 as compared to equally treated KB-3-1 cells. The maximum difference between parental cells and the ABCB1-overexpressing subline was found at 2.5 μM. In order to further characterise KP772-induced cell death in these cell lines, the caspase-mediated cleavage of PARP (poly(ADP-ribosyl) polymerase), caspase 7, and caspase 3 was analysed by Western blotting (Fig. 3C). After 24 h KP772 treatment, a dose-dependent increase of cleaved PARP and caspase 7 as well as loss of full-length caspase 3 was detectable in KB-3-1 but not in KBC-1 cells. As positive control for apoptosis induction, KP1255, a bismuth compound, was used. Diminished PARP cleavage was also detected in ABCC1- and LRP-overexpressing GLC-4/adr cells (Fig. 3D), while in the drug-resistant HL60/adr subline PARP cleavage under KP772 treatment was significantly delayed (Fig. 3E, right panel). In parallel, apoptosis induction in the HL60 model was analysed by determination of the subG0/G1 population in cell cycle analyses. Comparable to the KB cell model, a significantly enhanced number of apoptotic cells were induced by KP772 in the drug-resistant HL60/adr as compared to the parental cell line (Fig. 3E, left panel).
Fig. 3.
Induction of apoptosis in KB-3-1 and ABCB1-overexpressing KBC-1 cells after treatment with KP772 for 24 h. (A) Percentages of apoptotic nuclei in untreated controls and cells treated with 5 and 10 μM KP772 were determined microscopically after DAPI staining. Three hundred to 500 nuclei of at least two cytospin slides for each concentration and cell line were counted. (B) Accumulation of green-fluorescent cytoplasmic (FL-1, left) and red-fluorescent mitochondrial (FL-2, right) JC-1 in the indicated cell lines is shown in the upper panel. The lower panel indicates loss of mitochondrial membrane potential after treatment with KP772. The proportions of apoptotic KB-3-1 and KBC-1 cells after treatment with the indicated drug concentrations are shown normalised to the untreated control. (C) Caspase-induced cleavage of PARP, caspase 7 and caspase 3 in KB-3-1 and KBC-1 cells after treatment with KP772 at the indicated concentrations was determined via Western blotting. The bismuth compound KP1255 was used as positive control. Antibodies used are described under Section 2. (D) Caspase-induced cleavage of PARP in GLC-4 and ABCC1- and LRP-overexpressing GLC-4/adr cells after treatment with KP772 at the indicated concentrations was determined via Western blotting. (E) After treatment with KP772 for the indicated time periods, HL60 and ABCC1-overexpressing HL60/adr cells were analysed for apoptosis induction by both Western blotting and FACS. Immunoblots (right panel) show the time-dependent cleavage of PARP by treatment with 2.5 and 5 μM KP772. For FACS analysis cells treated with 2.5 μM KP772 were fixed and stained with ethanol and PI, respectively. Percentages of the apoptotic subG0/G1 compartment were calculated by Cell Quest Software.
3.4. Influence of ABCB1 and ABCC1 modulation on KP772 cytotoxicity
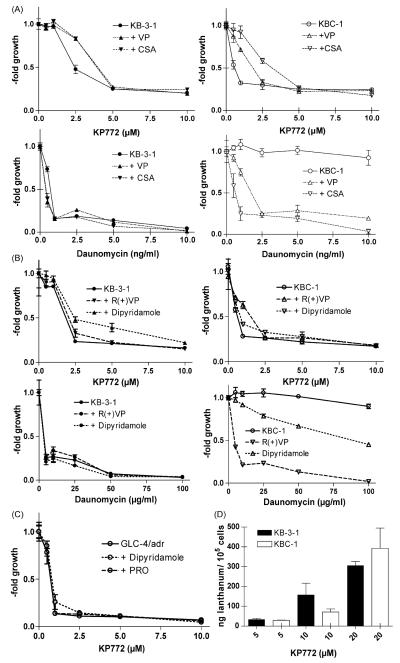
Several substances, e.g. VP and CSA are known to inhibit ABCB1- and/or ABCC1-mediated drug efflux [14]. These MDR modulators are highly effective at restoring sensitivity against the known ABCB1 and ABCC1 substrate daunomycin in ABCB1-overexpressing KBC-1 cells (Fig. 4A and B). However, neither VP, CSA, R(+)VP (dex-verapamil), nor dipyridamole enhanced the sensitivity of KBC-1 cells against KP772. In contrast, these ABCB1 modulators were found to reduce KP772 sensitivity not only in ABC transporter-overexpressing but also in parental cell lines (two-way ANOVA, p < 0.0001 for both). The ABCC1 modulator probenecid (PRO) did not induce this effect, neither in KB (data not shown) nor in GLC-4/adr (Fig. 4C). With regard to VP and CSA, comparable results were also observed in all other MDR cell models included in Table 1 (data not shown).
Fig. 4.
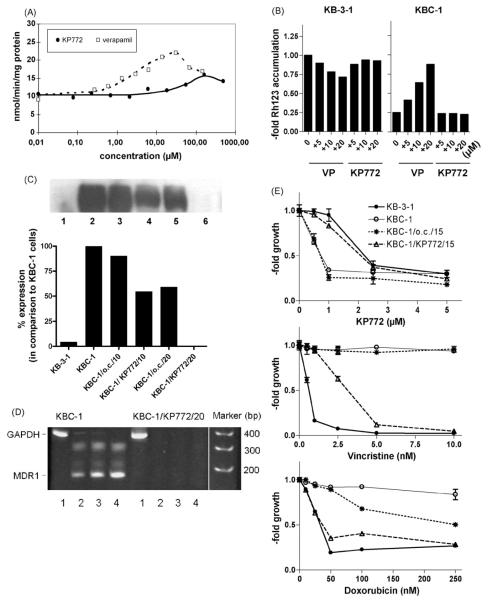
Impact of MDR modulators on KP772 anticancer activity. KB-3-1 cells and the ABCB1-overexpressing subline KBC-1 were incubated for 72 h with increasing concentrations of KP772 in combination with the ABCB1/ABCC modulators (A) VP (10 μM) and CSA (1 μM) and (B) R(+)VP (10 μM) and dipyridamole (10 μM) as indicated. Values given are means ± S.D. of one representative experiment performed in triplicate. At least three experiments were done delivering comparable results. (C) Impact of dipyridamole (10 μM) and PRO (1 mM) on KP772-treated GLC-4/adr cells. (D) Lanthanum levels of KB-3-1 (full bars) and KBC-1 cells (open bars) were measured by ICP-MS after 1 h incubation with the indicated KP772 concentrations. Means ± S.D. of at least three experiments are given.
3.5. Impact of drug-transporter overexpression on intracellular accumulation of KP772
To examine whether the hypersensitivity of ABCB1-over-expressing cells is based on increased drug uptake, lanthanum levels in KP772-treated KB-3-1 and KBC-1 cells were determined. As shown in Fig. 4D, the amount of lanthanum was equal in both tested cell lines. Additionally, coadministration of VP had no detectable effects on KP772 accumulation (data not shown).
3.6. Impact of KP772 on ABCB1 ATPase activity
In order to analyse whether KP772 directly interacts with ABCB1, the impact on the ABCB1 ATPase activity was measured as described previously [9]. ABCB1 ATPase activity is stimulated by several substrate drugs in a concentration-dependent manner with biphasic characteristic of the curve and thus a definite maximal value for each compound. The activation constant is calculated by fitting a hyperbolic concentration response curve to the ascending part of the curve using the method of non-linear least squares. While KP772 lacked any influence at those concentrations required for growth inhibition and apoptosis induction, very high concentrations (up to 100-fold above the respective IC50 values) led to a detectable stimulation of ABCB1 ATPase activity (Fig. 5A). A maximal effect was observed at a concentration of approximately 200 μM KP772 and the half maximal stimulation was only reached at a concentration exceeding 100 μM. In contrast, the ABCB1 substrate/inhibitor VP showed an activation constant of below 5 μM.
Fig. 5.
Impacts of KP772 on ABCB1 expression and function. (A) The impact on ABCB1 ATPase activity was determined by analysing the rate of ATP hydrolysis in ABCB1-containing plasma membrane vesicles [9] at increasing concentrations of KP772 (straight line) or VP (dashed line). The concentration–response curves were fitted to the data points by non-linear regression analysis. (B) Rh123 accumulation in KB-3-1 and KBC-1 cells with and without coadministration of KP772 and VP at the indicated concentrations was measured after 1 h drug exposure by FACS analysis. Data were normalised to Rh123 accumulation of untreated KB-3-1 cells. One of three experiments delivering comparable results is shown. (C) ABCB1 expression levels of KB-3-1 (lane 1), KBC-1 (lane 2), and KBC-1 cells cultured without colchicine selection for 10 passages (KBC-1/o.c./10; lane 3), and 20 passages (KBC-1/o.c./20; lane 5) or under exposure to 0.7 μM KP772 for the identical time periods (KBC-1/KP772/10; lane 4 and KBC-1/KP772/20; lane 6, respectively) were measured in membrane-enriched fractions by Western blot and quantified by Molecular Analyst software (Biorad). (D) ABCB1 mRNA expression in KBC-1 and KBC-1/772/20 cells was analysed by RT-PCR. Amplification products obtained using GAPDH-specific (358 bp product; 25 cycles, lane 1) and ABCB1-specific (167 bp product; 25, 30, 35 PCR cycles, lanes 2–4) oligonucleotide primers, respectively, were separated by acrylamide gel electrophoresis and stained by ethidium bromide. (E) Concentration–response curves were established for the indicated drugs in KB-3-1, KBC-1, KBC-1/o.c./15 and KBC-1/KP772/15 cells. Following 72 h drug exposure, cell viability was determined by MTT assays.
3.7. Impact of KP772 on ABCB1 transport function
To gain more insight in the interaction between KP772 and ABCB1, the effect of KP772 on cellular Rh123 accumulation was studied [11]. Drug-sensitive and -resistant KB cells were exposed to Rh123 in the absence or presence of increasing concentrations of KP772 and the MDR modulator VP as positive control. Up to the highest concentration tested, KP772 had no effect on Rh123 levels in both cell lines. As expected VP restored decreased Rh123 levels of KBC-1 cells while it did not enhance Rh123 retention in parental KB-3-1 cells (Fig. 5B).
3.8. Impact of KP772 on ABCB1 expression
To test whether long-term KP772 treatment has an impact on ABCB1 expression of MDR cells, KBC-1 cells were cultured under continuous exposure to a subtoxic concentration (0.7 μM) of the lanthanum drug. After 10 passages under KP772 exposure, Western blots revealed a significant reduction of ABCB1 expression to 55% of the control (Fig. 5C). After 20 passages, KP772-treated KBC-1 cells were devoid of ABCB1 expression (Fig. 5C) accompanied by complete lack of MDR1 mRNA as detected by RT-PCR (Fig. 5D). Accordingly, the reduction in ABCB1 expression attenuated resistance against the known ABCB1 substrate drug vincristine and doxorubicin and reduced collateral sensitivity against KP772 (Fig. 5E). In all experiments, KBC-1 cells grown for indicated passages without colchicine (KBC-1/o.c.) were used to indicate whether the observed loss of ABCB1 expression was based on the missing selection pressure. Up to 20 passages analysed, strong ABCB1 expression was detectable in KBC-1/o.c. cells (~60% of original KBC-1 cells, Fig. 5C, lane 5) in contrast to complete loss under KP772 treatment (Fig. 5C, lane 6).
3.9. Selection of KP772-resistant cell lines
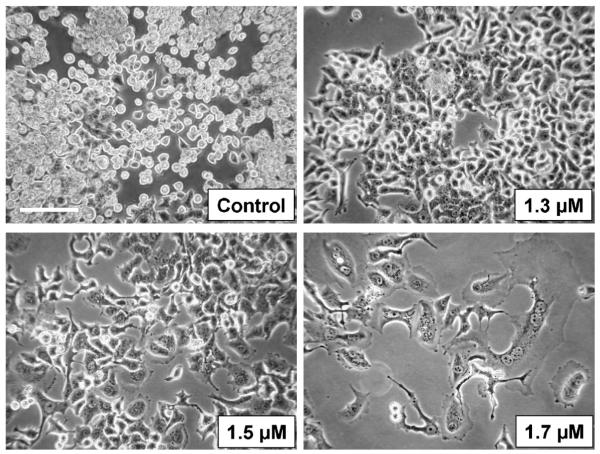
In order to establish cell models for acquired KP772 resistance, KB-3-1 and HL60 cells were selected against increasing drug concentrations. The selection process started at 0.5 μM KP772, a concentration distinctly below the cytotoxic range. In case of unaltered cell proliferation for more than six passages, drug concentration was increased by 0.2 μM. For KB-3-1 cells, the maximum concentration allowing cell proliferation was 1.5 μM KP772, and any attempt to escalate beyond this concentration led to cell cycle arrest and cell death. Additionally, distinct morphological changes with cell flattening and enhanced stress fibre formation was observable at 1.5 and 1.7 μM KP772 (Fig. 6). Even prolongation of the selection process by recovery periods up to 2 weeks without drug exposure did not lead to the development of KP772-resistant subclones. HL60 cells were even more sensitive as compared to KB-3-1. In repeated selection experiments, 1 μM was the highest concentration of KP772 compatible with cell proliferation.
Fig. 6.
Morphologic changes in KB-3-1 cells after 8-month selection against KP772. Drug concentrations were stepwise, slowly increased (compare Section 2) until the indicated levels were reached. Photomicrographs shown were taken by using a 10× objective and phase contrast settings using Nikon Eclipse TE300 (Nikon Instruments, Japan). Bar: 50 μm.
4. Discussion
Up to now, the escape of cancer cells from chemotherapy through activation of MDR mechanisms is a major reason for systemic cancer treatment failure. Recently, we have demonstrated that the new lanthanum drug KP772 has promising anticancer activity in vitro and in vivo [3]. In this study, we demonstrate that ABC transporter-overexpressing MDR cells are preferential targets for the anticancer activity of the lanthanum drug KP772. Moreover, in contrast to several other metallo-drugs [15-17] and despite intense efforts, selection of a cell line resistant to this compound failed. In contrast, continuous exposure of ABCB1-overexpressing, highly drug-resistant KBC-1 cells to subtoxic KP772 concentrations led to a progressive loss of ABC transporter expression both at the mRNA and protein levels and accordingly to re-sensitization of the cells against diverse ABCB1 substrate drugs. In summary, our data suggest that neither intrinsic expression of ABC transporters nor acquired resistance should represent major impediments for treatment of cancer patients with KP772. In contrast, KP772 is expected to be especially active as single agent against multidrug-resistant tumors. Moreover, in combination therapies KP772 might select against acquired drug resistance and/or have chemosensitizing activities at least against ABCB1 substrate drugs.
So far, hypersensitivity of MDR cells, a phenomenon termed “collateral sensitivity” [18], has been described for a limited number of drugs including antimetabolic agents such as 5-fluorouracil (5-FU) [19], gemcitabine [20], and 2-deoxy-d-glucose [21], the phosphatidylinositol-3-kinase inhibitor LY294002 [22], as well as ABCB1 substrates/modulators like the calcium channel antagonist VP [23]. Recently, Szakacs et al. correlated the ABC transporter expression profiles of the 60 cancer cell lines used by the National Cancer Institute with the growth inhibitory profiles of 1429 anticancer drugs. This approach led to the discovery of a small number of new compounds displaying enhanced sensitivity against ABCB1-overexpressing cells with the thiosemicarbazone NSC73306 as the most promising candidate drug [24].
Previous studies suggested that hypersensitivity of MDR cells might be caused by direct or indirect interactions of the respective drugs with the ABC transporter mechanism or with other alterations obtained during drug selection. In case of NSC73306, a strict dependence of hypersensitivity on ABCB1 expression was suggested based on the fact that not only drug-selected but also ABCB1-transfected cells exhibited collateral sensitivity [24]. Accordingly, suppression of ABCB1 expression by siRNA reversed this hypersensitivity [25]. Corroboratingly, in our study ABCG2-transfected cell clones, in addition to multiple drug-selected cell models with both ABCB1 and ABCC1 overexpression, displayed enhanced sensitivity against KP772. This is interesting as the vast majority of previous reports [19,21,25-27] on collateral sensitivity concerned ABCB1-overexpressing cell models, only. Additionally, in the screening approach [24] positive correlations between ABC transporter expression and drug activity were predominantly detected with regard to the overexpression of ABCB1. Also hypersensitivity to NSC73306 was ABCB1-specific [25]. Thus our data on collateral sensitivity against KP772 also of ABCC1- and ABCG2-expressing cells suggest for the first time that a general ABC transporter-mediated mechanism can underlie hypersensitivity of MDR cells against a cytotoxic drug.
Several agents with hyperactivity against MDR cells, as for example VP [28], are ABCB1 transport substrates and/or modulators. As these substances activate the ABCB1 ATPase function, they were suggested to cause apoptosis due to enhanced oxidative stress as a consequence of reactively stimulated ATP production [29]. Several observations make a comparable mechanism in case of KP772 unlikely: (1) In MDR cells, KP772 neither enhanced accumulation of the ABCB1 substrate Rh123 nor sensitivity to vincristine and adriamycin (data not shown). (2) Accordingly, KP772 did not stimulate ATPase activity of ABCB1 at concentrations already exhibiting profound cytotoxicity. (3) ATP pools of both KB-3-1 and KBC-1 cells remained unchanged during KP772 treatment for several days (data not shown). (4) ROS scavenging agents do not protect cells against the cytostatic/toxic activity of KP772 [3]. Thus it is unlikely that hypersensitivity of MDR cells against KP772 is based on a direct interaction with the transporter molecule and suggests that other alterations cause by the ABC transporter function might make MDR cells more vulnerable to KP772. Interestingly, the so far reported hyperactive substance panel includes several antimetabolites [24,19-21]. Although, the precise cellular targets of KP772 remain to be elucidated [3], recent experiments revealed that at least part of the anticancer activity of KP772 is based on the inhibition of ribonucleotide reductase (manuscript in preparation). Additionally, the only substances showing (weakly) correlating activity with KP772 in the NCI profile are anti-metabolic drugs [3]. This suggests that the ATP-transport activity might cause differences in the cellular metabolic state which sensitize MDR cells to certain antimetabolites including KP772.
In previous studies it has been reported that ABCB1-overexpressing cells in certain situations might be generally resistant against apoptosis induction [30-32]. These effects were independent of ATP hydrolysis [31] but reversible by VP or anti-ABCB1 antibodies [32] suggesting a transport-unrelated impact of ABCB1 on apoptosis execution. In our study PARP and caspase 7-cleavage did not correlate with the enhanced apoptosis rate induced by KP772 in three different MDR cell models (KBC-1, GLC-4/adr, HL60/adr) but rather was reduced in comparison to the parental cells. In contrast, for several other hyperactive substances stronger PARP cleavage in ABCB1-positive cells was reported [19,22,27,33]. This suggests that altered apoptosis execution might play a role in KP772 hypersensitivity of MDR cells.
Additionally, we found significant differences with regard to cell cycle arrest of KB-3-1 and KBC-1 cells. Whether ABCB1 expression has also a modifying impact on the cell cycle regulation and whether this is the reason why MDR cells also display enhanced cell cycle arrest in response to KP772 treatment remains speculative. The enhanced loss of G2/M cells in case of KBC-1 is well reflected by a stronger down-regulation of the M phase cyclin B1. However, additionally also the G1 phase cyclin D1 was preferentially decreased in the MDR cell model while the downregulation of CDK4 was almost similar in both cell lines. The loss of cyclin D1 would indicate that KP772 treatment might not only arrest cells in G1-phase of the cell cycle but also induce a G0-like state. This, however, is unlikely due to the unchanged expression of cyclin A in KP772-treated cells. The concomitant downregulation of cyclin D1 and CDK4 has already been reported for several other substance inducing G0/G1 arrest and apoptosis including sphingosine [34] as well as staurosporine [35]. This suggests that KP772 might interfere with the CDK4/cyclin D signalling complex thus inducing the profound accumulation of cells in G0/G1 [3]. It has, however, to be taken into account that KB cells as Hela derivatives have no functional Rb due to degradation by the HPV-16 E7 protein [36] and thus might be characterised by unusual cell cycle regulation mechanisms. Currently, experiments are underway to clarify the exact pathway of KP772-induced cell cycle arrest and to elucidate whether ABCC1- and ABCG2-overexpressing cells show similar patterns of cyclin/CDK deregulation.
With respect to KP772, long-term treatment of highly ABCB1-overexpressing cells with subtoxic concentrations led to a progressive loss of the ABC transporter mRNA and protein expression to undetectable levels. Correspondingly, it reversed the high resistance of these cells against ABCB1 substrate drugs. This is surprising and suggests a strong selection pressure of even low KP772 concentrations against MDR cells. Comparable observations as for KP772 have been made after long-term exposure to subtoxic concentrations of the thiosemicarbazone NSC73306 [25]. In both cases it is unclear whether loss of ABCB1 expression is a consequence of selection of ABCB1-negative subclones or adaptation of ABCB1-overexpressing cells. In our study, exposure to KP772 was done at low, subtoxic concentrations avoiding substantial cell death. Nevertheless, selection cannot be excluded as besides apoptosis also the KP772-induced cell cycle arrest [3] was significantly stronger in ABCB1-overexpressing cells. Currently, studies are performed in our lab to clarify the molecular mechanisms underlying MDR1 gene suppression by KP772. From the therapeutic view, application of low KP772 doses might represent a new strategy for MDR reversal at least in ABCB1-overexpressing tumors as also previously suggested for NSC73306 [25].
Surprisingly and in contrast to several other metallo-drugs investigated in parallel ([17] and data not shown), generation of KP772-resistant cell models by selection against stepwise increasing drug concentrations failed repeatedly. This suggests that tumor cells generally lack an effective amatory to escape the cytotoxic insult induced by this drug. Lack of acquired resistance development to KP772 seems not to be a general consequence of collateral sensitivity as there exist several reports on cell models resistant against other hyperactive drugs like 5-FU, gemcitabine, and VP [26,37,38]. In these cases the acquired resistance was not caused by ABC transporters but based on other drug-specific molecular alterations.
In summary, we have demonstrated that both acquired and intrinsic drug resistance should not represent major impediments to a successful clinical development of the new anticancer lanthanum compound KP772. In contrast, simultaneous or alternate application of KP772 with ABCB1 substrate drugs might prevent induction of MDR and/or even chemosensitize intrinsically ABCB1-positive tumors. Corroboratingly, KP772 was highly effective against the DLD-1 colon cancer xenograft in vivo, a tumor model distinctly resistant to several chemotherapeutic agents due of oncogene mutations [39] and the intrinsic overexpression of ABCB1 [40]. All these features suggest the further (pre)clinical development of the lanthanum drug KP772 as anticancer agent.
Acknowledgments
We are indebted to Marlies Spannberger and Vera Bachinger for the skilful handling of cell culture, Elisabeth Rabensteiner, Rosa-Maria Weiss, and Christian Balcarek for competent technical assistance, Paul Breit for preparing photomicrographs and Irene Herbacek for FACS analysis.
This work was supported by the Austrian Science Fond (FWF) grant L212-B11 and the Medizinischer-Wissenschaftlicher Fonds des Bürgermeisters der Bundeshauptstadt Wien, grant 2460, as well as by the Faustus Forschung Austria Translational Drug Development AG.
Abbreviations
- 5-FU
5-fluorouracil
- ABC
ATP-binding cassette
- ABCB1
P-glycoprotein
- ABCC
multidrug resistance-related protein
- ABCG2
breast cancer resistance protein
- BSA
bovine serum albumin
- CSA
cyclosporin A
- DMSO
dimethyl sulfoxide
- ICP-MS
inductively coupled plasma mass spectroscopy
- FACS
fluorescence-activated cell sorting
- KP1255
acetatobis[1-(azepanyl)-4(2-pyridyl)-2,3-diaza-penta-1,3-dien-1-thiolato-N′,N3,S]bismuth(III)
- KP772
[tris(1,10-phenanthroline)lanthanum(III)] trithiocyanate
- LRP
lung resistance protein
- MDR
multidrug resistance
- PBS
phosphate-buffered saline
- Rh123
rhodamine 123
- RT-PCR
reverse transcriptase polymerase chain reaction
- PRO
probenecid
- TMAH
tetramethylammonium hydroxide
- VP
verapamil
REFERENCES
- [1].Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- [2].Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- [3].Heffeter P, Jakupec MA, Korner W, Wild S, von Keyserlingk NG, Elbling L, et al. Anticancer activity of the lanthanum compound [tris(1,10-phenanthroline)lanthanum(III)]trithiocyanate (KP772; FFC24) Biochem Pharmacol. 2006;71:426–40. doi: 10.1016/j.bcp.2005.11.009. [DOI] [PubMed] [Google Scholar]
- [4].Shen DW, Cardarelli C, Hwang J, Cornwell M, Richert N, Ishii S, et al. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986;261:7762–70. [PubMed] [Google Scholar]
- [5].McGrath T, Center MS. Mechanisms of multidrug resistance in HL60 cells: evidence that a surface membrane protein distinct from P-glycoprotein contributes to reduced cellular accumulation of drug. Cancer Res. 1988;48:3959–63. [PubMed] [Google Scholar]
- [6].Zijlstra JG, de Vries EG, Mulder NH. Multifactorial drug resistance in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1987;47:1780–4. [PubMed] [Google Scholar]
- [7].Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–70. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Berger W, Elbling L, Micksche M. Expression of the major vault protein LRP in human non-small-cell lung cancer cells: activation by short-term exposure to antineoplastic drugs. Int J Cancer. 2000;88:293–300. [PubMed] [Google Scholar]
- [9].Schmid D, Ecker G, Kopp S, Hitzler M, Chiba P. Structure–activity relationship studies of propafenone analogs based on P-glycoprotein ATPase activity measurements. Biochem Pharmacol. 1999;58:1447–56. doi: 10.1016/s0006-2952(99)00229-4. [DOI] [PubMed] [Google Scholar]
- [10].Berger W, Elbling L, Minai-Pour M, Vetterlein M, Pirker R, Kokoschka EM, et al. Intrinsic MDR-1 gene and P-glycoprotein expression in human melanoma cell lines. Int J Cancer. 1994;59:717–23. doi: 10.1002/ijc.2910590522. [DOI] [PubMed] [Google Scholar]
- [11].Elbling L, Berger W, Weiss RM, Printz D, Fritsch G, Micksche M. A novel bioassay for P-glycoprotein functionality using cytochalasin D. Cytometry. 1998;31:187–98. doi: 10.1002/(sici)1097-0320(19980301)31:3<187::aid-cyto6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- [12].Kim R, Emi M, Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol. 2006;57:545–53. doi: 10.1007/s00280-005-0111-7. [DOI] [PubMed] [Google Scholar]
- [13].Chaoui D, Faussat AM, Majdak P, Tang R, Perrot JY, Pasco S, et al. JC-1, a sensitive probe for a simultaneous detection of P-glycoprotein activity and apoptosis in leukemic cells. Cytom B Clin Cytom. 2006;70:189–96. doi: 10.1002/cyto.b.20100. [DOI] [PubMed] [Google Scholar]
- [14].Fojo T, Bates S. Strategies for reversing drug resistance. Oncogene. 2003;22:7512–23. doi: 10.1038/sj.onc.1206951. [DOI] [PubMed] [Google Scholar]
- [15].McKeage MJ. New-generation platinum drugs in the treatment of cisplatin-resistant cancers. Expert Opin Invest Drug. 2005;14:1033–46. doi: 10.1517/13543784.14.8.1033. [DOI] [PubMed] [Google Scholar]
- [16].Davies NP, Rahmanto YS, Chitambar CR, Richardson DR. Resistance to the antineoplastic agent gallium nitrate results in marked alterations in intracellular iron and gallium trafficking: identification of novel intermediates. J Pharmacol Exp Ther. 2006;317:153–62. doi: 10.1124/jpet.105.099044. [DOI] [PubMed] [Google Scholar]
- [17].Heffeter P, Pongratz M, Steiner E, Chiba P, Jakupec MA, Elbling L, et al. Intrinsic and acquired forms of resistance against the anticancer ruthenium compound KP1019 [indazolium trans-[tetrachlorobis(1H-indazole)ruthenate (III)] (FFC14A)] J Pharmacol Exp Ther. 2005;312:281–9. doi: 10.1124/jpet.104.073395. [DOI] [PubMed] [Google Scholar]
- [18].Fattman CL, Allan WP, Hasinoff BB, Yalowich JC. Collateral sensitivity to the bisdioxopiperazine dexrazoxane (ICRF-187) in etoposide (VP-16)-resistant human leukemia K562 cells. Biochem Pharmacol. 1996;52:635–42. doi: 10.1016/0006-2952(96)00338-3. [DOI] [PubMed] [Google Scholar]
- [19].Warr JR, Bamford A, Quinn DM. The preferential induction of apoptosis in multidrug-resistant KB cells by 5-fluorouracil. Cancer Lett. 2002;175:39–44. doi: 10.1016/s0304-3835(01)00721-2. [DOI] [PubMed] [Google Scholar]
- [20].Bergman AM, Pinedo HM, Talianidis I, Veerman G, Loves WJ, van der Wilt CL, et al. Increased sensitivity to gemcitabine of P-glycoprotein and multidrug resistance-associated protein-overexpressing human cancer cell lines. Br J Cancer. 2003;88:1963–70. doi: 10.1038/sj.bjc.6601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bentley J, Quinn DM, Pitman RS, Warr JR, Kellett GL. The human KB multidrug-resistant cell line KB-C1 is hypersensitive to inhibitors of glycosylation. Cancer Lett. 1997;115:221–7. doi: 10.1016/s0304-3835(97)04739-3. [DOI] [PubMed] [Google Scholar]
- [22].Nicholson KM, Quinn DM, Kellett GL, Warr JR. LY294002, an inhibitor of phosphatidylinositol-3-kinase, causes preferential induction of apoptosis in human multidrug resistant cells. Cancer Lett. 2003;190:31–6. doi: 10.1016/s0304-3835(02)00615-8. [DOI] [PubMed] [Google Scholar]
- [23].Cano-Gauci DF, Riordan JR. Action of calcium antagonists on multidrug resistant cells. Specific cytotoxicity independent of increased cancer drug accumulation. Biochem Pharmacol. 1987;36:2115–23. doi: 10.1016/0006-2952(87)90139-0. [DOI] [PubMed] [Google Scholar]
- [24].Szakacs G, Annereau JP, Lababidi S, Shankavaram U, Arciello A, Bussey KJ, et al. Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell. 2004;6:129–37. doi: 10.1016/j.ccr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- [25].Ludwig JA, Szakacs G, Martin SE, Chu BF, Cardarelli C, Sauna ZE, et al. Selective toxicity of NSC73306 in MDR1-positive cells as a new strategy to circumvent multidrug resistance in cancer. Cancer Res. 2006;66:4808–15. doi: 10.1158/0008-5472.CAN-05-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sosinski J, Thakar JH, Germain GS, Dias P, Harwood FC, Kuttesch JF, et al. Cross-resistance to antitumor diarylsulfonylureas and collateral sensitivity to mitochondrial toxins in a human cell line selected for resistance to the antitumor agent N-(5-indanylsulfonyl)-N′-(4-chlorophenyl)urea. Mol Pharmacol. 1994;45:962–70. [PubMed] [Google Scholar]
- [27].Nicholson KM, Quinn DM, Kellett GL, Warr JR. Preferential killing of multidrug-resistant KB cells by inhibitors of glucosylceramide synthase. Br J Cancer. 1999;81:423–30. doi: 10.1038/sj.bjc.6690711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Litman T, Zeuthen T, Skovsgaard T, Stein WD. Competitive, non-competitive and cooperative interactions between substrates of P-glycoprotein as measured by its ATPase activity. Biochim Biophys Acta. 1997;1361:169–76. doi: 10.1016/s0925-4439(97)00027-6. [DOI] [PubMed] [Google Scholar]
- [29].Karwatsky J, Lincoln MC, Georges E. A mechanism for P-glycoprotein-mediated apoptosis as revealed by verapamil hypersensitivity. Biochemistry. 2003;42:12163–7. doi: 10.1021/bi034149+. [DOI] [PubMed] [Google Scholar]
- [30].Ruefli AA, Tainton KM, Darcy PK, Smyth MJ, Johnstone RW. P-glycoprotein inhibits caspase-8 activation but not formation of the death inducing signal complex (disc) following Fas ligation. Cell Death Differ. 2002;9:1266–72. doi: 10.1038/sj.cdd.4401081. [DOI] [PubMed] [Google Scholar]
- [31].Tainton KM, Smyth MJ, Jackson JT, Tanner JE, Cerruti L, Jane SM, et al. Mutational analysis of P-glycoprotein: suppression of caspase activation in the absence of ATP-dependent drug efflux. Cell Death Differ. 2004;11:1028–37. doi: 10.1038/sj.cdd.4401440. [DOI] [PubMed] [Google Scholar]
- [32].Robinson LJ, Roberts WK, Ling TT, Lamming D, Sternberg SS, Roepe PD. Human MDR 1 protein overexpression delays the apoptotic cascade in Chinese hamster ovary fibroblasts. Biochemistry. 1997;36:11169–78. doi: 10.1021/bi9627830. [DOI] [PubMed] [Google Scholar]
- [33].Bell SE, Quinn DM, Kellett GL, Warr JR. 2-Deoxy-d-glucose preferentially kills multidrug-resistant human KB carcinoma cell lines by apoptosis. Br J Cancer. 1998;78:1464–70. doi: 10.1038/bjc.1998.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–23. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McGahren-Murray M, Terry NH, Keyomarsi K. The differential staurosporine-mediated G1 arrest in normal versus tumor cells is dependent on the retinoblastoma protein. Cancer Res. 2006;66:9744–53. doi: 10.1158/0008-5472.CAN-06-1809. [DOI] [PubMed] [Google Scholar]
- [36].Huh KW, DeMasi J, Ogawa H, Nakatani Y, Howley PM, Munger K. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc Natl Acad Sci USA. 2005;102:11492–7. doi: 10.1073/pnas.0505337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- [38].Davidson JD, Ma L, Flagella M, Geeganage S, Gelbert LM, Slapak CA. An increase in the expression of ribonucleotide reductase large subunit 1 is associated with gemcitabine resistance in non-small cell lung cancer cell lines. Cancer Res. 2004;64:3761–6. doi: 10.1158/0008-5472.CAN-03-3363. [DOI] [PubMed] [Google Scholar]
- [39].Kavgaci H, Ozdemir F, Ovali E, Yavuz A, Yavuz M, Aydin F. Effect of the farnesyl transferase inhibitor L-744,832 on the colon cancer cell line DLD-1 and its combined use with radiation and 5-FU. Chemotherapy. 2005;51:319–23. doi: 10.1159/000088954. [DOI] [PubMed] [Google Scholar]
- [40].Eliason JF, Ramuz H, Yoshikubo T, Ishikawa T, Yamamoto T, Tsuruo T. Novel dithiane analogues of tiapamil with high activity to overcome multidrug resistance in vitro. Biochem Pharmacol. 1995;50:187–96. doi: 10.1016/0006-2952(95)00115-g. [DOI] [PubMed] [Google Scholar]