Abstract
Voltage-gated ion channels are a diverse family of signaling proteins that mediate rapid electrical signaling events. Among these, voltage-gated potassium or Kv channels are the most diverse, in part due to the large number of principal (or α) subunits and auxiliary subunits that can assemble in different combinations to generate Kv channel complexes with distinct structures and functions. The diversity of Kv channels underlies much of the variability in the active properties between different mammalian central neurons, and the dynamic changes that lead to experience-dependent plasticity in intrinsic excitability. Recent studies have revealed that Kv channel α subunits and auxiliary subunits are extensively phosphorylated, contributing to additional structural and functional diversity. Here we highlight recent studies that show that auxiliary subunits exert some of their profound effects on dendritic Kv4 and axonal Kv1 channels through phosphorylation-dependent mechanisms, either due to phosphorylation on the auxiliary subunit itself, or by influencing the extent and/or impact of α subunit phosphorylation. The complex effects of auxiliary subunits and phosphorylation provide a potent mechanism to generate additional diversity in the structure and function of Kv4 and Kv1 channels, as well as allowing for dynamic reversible regulation of these important ion channels.
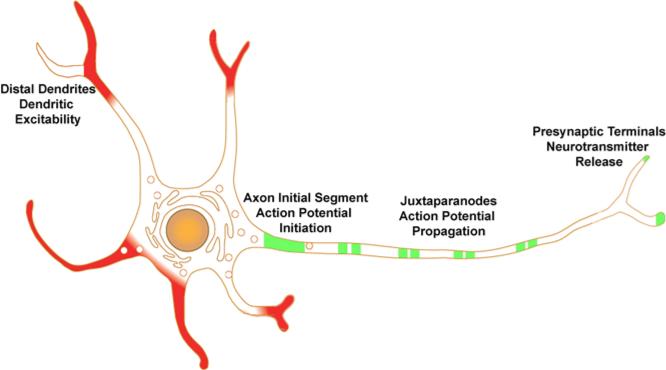
Voltage-gated potassium or Kv channels are diverse components of the ion channel repertoire that controls electrical activity of mammalian neurons, cardiac and skeletal muscle [1]. The diversity of mammalian Kv channel function is derived from genetic diversity, with over two dozen genes encoding the pore-forming and voltage-sensing α subunits, and that the α subunits can coassemble into functional tetramers in different combinations [2]. This genetic and combinatorial diversity is not observed in the highly related but pseudotetrameric voltage-gated sodium and calcium channel α subunit repertoire, and is crucial to generating the functional diversity that is a hallmark of Kv channels [3]. Kv channel functional diversity is further enhanced by coassembly with a wide array of auxiliary subunits, which in themselves cannot form functional channels but which can greatly impact channel function upon coassembly with α subunits [4]. In addition to the non-covalent interactions of α subunits with one another, and with auxiliary subunits, that define the structure and function of Kv channels, recent studies have revealed that Kv channel α and auxiliary subunits are extensively covalently modified with phosphate [5]. While a large body of work has defined how coassembly with auxiliary subunits and dynamic changes in phosphorylation state can separately impact Kv channel α subunits, and influence channel expression, localization and function, recent studies have provided compelling evidence for a more concerted coupling between these distinct molecular events. Here we review recent studies on how auxiliary subunit phosphorylation, and auxiliary subunit-dependent phosphorylation of the principal α subunits of dendritic Kv4 channels, and axonal Kv1 channels (Fig. 1), can impact diverse events that determine the fate of a Kv channel at crucial points in its lifetime.
Fig. 1.
Cartoon of Kv4 and Kv1 channel localization in an idealized neuron. Kv4 channels (red) are often found prominently expressed in distal dendrites, in some cases in a branch-specific manner, where they impact dendritic excitability. Kv1 channels (green) are found prominently on axons where they are clustered at high density at the axon initial segment, at or near nerve terminals, and, in myelinated axons, at juxtaparanodes of nodes of Ranvier.
Regulation of dendritic Kv4 channels by auxiliary subunits and phosphorylation
Principal and auxiliary subunits of native dendritic Kv4 channels
Kv4 channels are primarily localized to dendrites, and, to a lesser extent, somata, of diverse neurons throughout the mammalian brain [6]. Kv4 channels form low voltage-activated, 4-AP-sensitive, inactivating currents that underlie dendritic excitability [7]. In many cases Kv4 channels are not found uniformly expressed in dendritic membranes, specifically in distal dendrites (Fig. 1) but are localized in specific membrane subdomains where they can profoundly affect local electrical signaling events [8]. In mammalian brain, dendritic Kv4 channels primarily comprise heteromers or homomers of Kv4.2 and Kv4.3 α subunits, with the nature of the α subunit composition driven primarily by cellular patterns of gene expression. For example, glutamatergic pyramidal neurons in hippocampal CA1, and in layer 5 of neocortex, express Kv4.2 in the absence of detectable Kv4.3, such that the Kv4 channels in these cells are primarily homotetramers of Kv4.2 [9]. Inhibitory interneurons in hippocampus express only Kv4.3, yielding the corresponding homomeric Kv4.3 channels [9, 10]. Other neurons, for example hippocampal CA3 pyramidal neurons and dentate granule cells express high levels of both Kv4.2 and Kv4.3 [9], as do intralaminar thalamocortical relay neurons [11]. In some brain regions, more complex scenarios exist, for example the expression of Kv4.2 and Kv4.3 in cerebellar granule cells exhibits a striking anterior (Kv4.2) to posterior (Kv4.3) gradient [12].
In each of these cases, the diversity of Kv4 channel structure that is defined by distinct cellular patterns of α subunit expression is further enhanced by expression of a diverse set of auxiliary subunits. A yeast two hybrid screen using the N-terminus of Kv4.3 as bait identified a set of highly-related cytoplasmic Kv4 Channel Interacting Proteins or KChIPs [13]. KChIPs are members of a larger family of neuronal calcium sensor proteins, and contain EF hand motifs that confer Ca2+ binding to KChIPs [13]. This initial study revealed that KChIP coexpression has a profound impact on Kv4 channels, including increasing plasma membrane expression levels of Kv4.2 as exhibited by 10X increases in whole cell current amplitude, enhancing the voltage-dependent activation of Kv4.2 currents (evidenced by a 40 mV hyperpolarizing shift in the V1/2 of activation), slowing the kinetics of inactivation ≈ 5X, and accelerating the kinetics of recovery from inactivation ≈5X [13]. A number of subsequent studies (e.g., [14, 15]) revealed additional effects, as subsequently reviewed [7].
Subsequent to the identification of these cytoplasmic KChIPs, a second type of Kv4 channel auxiliary subunits was identified as a component of Kv4 channels purified from rat cerebellum [16]. In this case, the associated subunit was DPPX/DPP6, a single-pass transmembrane type II polypeptide with a large extracellular C-terminal domain exhibiting sequence similarity to the dipeptidyl peptidase (DPP) CD26, although lacking in enzyme activity [17]. The discovery of DPP6 as a Kv4 channel-associated protein led to further studies that revealed that other DPP-like proteins associate with and exert profound and diverse effects on coexpressed Kv4 channels [7], some of which are strikingly similar to those triggered by KChIPs, for example enhancing Kv4 channel surface expression, left-shifting the voltage-dependent activation of Kv4 currents, accelerating their recovery from inactivation, and enhancing Kv4.2 phosphorylation [16, 18]. However, coexpression with DPPs accelerates the kinetics of Kv4.2 inactivation [7, 16], an effect distinct from that of KChIPs. The binding of KChIPs and DPPs to Kv4 α subunits is not mutually exclusive, and a wealth of available evidence suggests that Kv4 channels can and do exist as ternary complexes of Kv4 α subunits, KChIPs and DPPs [19].
Immunohistochemical and biochemical studies reveal that different KChIP and DPP family members exhibit distinct patterns of expression, and of colocalization and association with Kv4.2 and Kv4.3 α subunits, in mammalian brain [6]. More specifically, there exist precise pairings of Kv4, KChIP and DPP isoforms in specific classes of neurons, for example hippocampal CA1 pyramidal cells express Kv4.2 α subunits together with KChIP2, KChIP3, KChIP4 [9], and DPP6 [20], while hippocampal interneurons express Kv4.3 α subunits together with KChIP1 [9, 10], and DPP10 [21]. These isoform-specific pairings of distinct Kv4: auxiliary subunit combinations suggests a cell-specific requirement for Kv4 channels with specific properties as defined by the ternary complexes formed by Kv4 α subunits, KChIPs and DPPs [19]. The precise patterns of regional, cellular and subcellular co-localization and co-association of these principal and auxiliary subunits also suggests a primary role for the auxiliary subunits as components of Kv4 channels [6].
To further reinforce this concept, Kv4.2 knockout mice display a dramatic downregulation of certain KChIPs, but not others, in specific patterns that precisely reflect their extent of interaction with Kv4.2 in wild-type mice [22]. The interdependent roles of individual KChIP subunits are underscored by recent studies that used selective genetic ablation of individual KChIP isoforms. KChIP3 knockout mice have partially reduced A-type currents in hippocampal dentate granule cells, supporting an in vivo role for KChIPs in promoting functional expression of Kv4 channels [23]. The lack of complete elimination of expression is likely due to expression of other auxiliary subunits in these cells, in this case KChIP2, KChIP4 [9, 12], and DPP6 [16]. Consistent with proposed roles of Kv4 channels in dendrites, these mice also exhibit enhanced LTP in perforant path-dentate granule cell synapses [23]. In neocortex, KChIP2 knockout mice have increased expression of KChIP3 and KChIP4, and KChIP3 knockout mice have increased expression of KChIP2 and KChIP4 [24]. Together these compensatory changes in KChIP expression presumably support relatively normal expression of A-type Kv currents. However, acute elimination of expression of these same KChIPs resulted in marked decreases in A-type currents [24], suggesting that the relatively subtle phenotype of the constitutive KChIP knockouts resulted from compensatory upregulation of other auxiliary subunits. Acute (albeit partial) knockdown of DPP6 in CA1 pyramidal neurons resulted in altered gating of Kv4.2-based A-type currents, and dramatic effects on neuronal excitability [25]. A recent study showed that acute knockdown of DPP6 in cerebellar granule cells also led to a dramatic loss in Kv4-based A-type currents [26]. A similar approach to acutely knockdown KChIP1 in CA1 interneurons, where it is found in the absence of other KChIPs, yielded only partial inhibition of Kv4.3 expression [27]. This could be due to the presence of DPP10 in these cells [21], or to the fact that the extent of KChIP1 knockdown was modest. These results together suggest that while KChIPs and DPPs are indistinguishable in their ability to support efficient expression of functional Kv4 channels in heterologous cells [18], each auxiliary subunit has a distinct and crucial role in neurons in vivo.
Phosphorylation-dependent modulation of Kv4 currents in dendrites
Individual branches of CA1 pyramidal cell dendrites have distinct active electrical properties that affect the coupling of local dendritic spikes to the soma [28]. The distinctions between such branches are due to differences in the levels of A-type Kv currents, which, in CA1 pyramidal cells, are mediated by complexes of Kv4.2, KChIP and DPP subunits [29]. The coupling of dendritic spikes to the soma can be dynamically enhanced though NMDA receptor-dependent downregulation of the amplitude of the A-type Kv currents [30], such that local changes in the excitability of individual dendritic branches can serve as an additional mechanism of information storage (Fig. 1). The compartmentalized plasticity of dendritic branches can be dynamically modified in vivo by experience, for example by housing rats in an enriched environment for a few days [31]. The role of Kv4.2-based channels in neuronal function is underscored by studies in Kv4.2 knockout mice, which have decreased neuronal A-type currents [32-34], an increase in dendritic back-propagating action potentials [33, 34], increased theta burst-induced LTP [34], and a loss of the distance dependent scaling of mEPSCs attributed to the lack of the gradient of Kv4.2 channel expression seen in wild-type neurons, although the increased inhibitory drive due to enhanced tonic GABA receptor-mediated currents may obscure the full impact of the knockout phenotype [33]. Kv4.2 knockout mice also exhibit enhanced susceptibility to kainate-induced seizures [32], consistent with a proposed role for Kv4.2 downregulation in seizure susceptibility associated with epileptogenesis [35-37].
One of the key aspects in regulation of dendritic excitability by the Kv4.2-based A-type current is that it is highly sensitive to activity-dependent modulation, for example in response to induction of LTP [38]. Kv4-based neuronal A-type currents have long been recognized as substrates for phosphorylation-dependent modulation [29]. These currents are downregulated in CA1 pyramidal neurons upon activation of PKA, PKC [39] and ERK [40], and upregulated by CamKII [41]. Kv4.2 is a direct substrate for phosphorylation by PKA [42], PKC [43, 44], ERK [45, 46], and CamKII [41]. Phosphorylation of Kv4.2 in heterologous cells by PKA [47] and ERK [44] yields a right-shifted activation curves and an overall reduction of Kv4.2 current, while activation of PKC yielded reduced currents without effects on gating [43, 44]. Phosphorylation of Kv4.2 by CamKII yields increased peak currents without affecting voltage-dependent activation or inactivation [41].
Phosphorylation-dependent modulation of Kv4 channels requires KChIPs
In addition to modulating expression levels as described above, PKA activity in hippocampal CA1 pyramidal neurons also leads to changes in the gating of Kv4-based A-type [39]. Kv4.2 was identified as being a direct substrate for PKA phosphorylation by studies in which recombinant bacterially expressed fragments of Kv4.2 were subjected to in vitro phosphorylation reactions using purified PKA [42]. Phosphopeptide mapping and amino acid sequencing revealed that T38 in the cytoplasmic N-terminus, and S552 in the cytoplasmic C-terminus, were sites phosphorylated directly by PKA [42]. Phosphospecific antibodies generated against these sites revealed PKA-stimulated phosphorylation at these sites in hippocampal slices [42]. Studies in the Xenopus oocyte heterologous cell expression system revealed that unlike the Kv4.2-based A-type current in CA1 pyramidal neurons, recombinant Kv4.2 currents were not modulated by PKA [47]. However, coexpression of Kv4.2 with KChIP3 led to PKA-mediated changes in the gating properties of the expressed currents typical of those observed upon PKA modulation of neuronal A-type currents [47]. Modulation of Kv4.2 by arachadonic acid, another potent regulator of native Kv4.2-based A current in neurons [48], also requires KChIP co-expression [49], showing that KChIP auxiliary subunits mediate diverse modes of Kv4.2 regulation. Note that Kv4.2 channels are also regulated by ERK/MAPK phosphorylation [45], and at least some modes of regulation of Kv4.2 channels by this pathway are also dependent on association with KChIP auxiliary subunits [46]
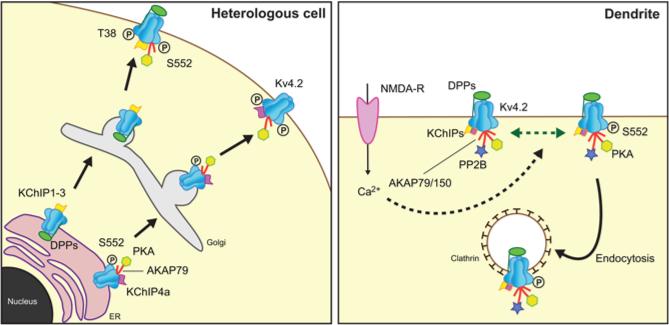
Expression of Kv4.2 in heterologous mammalian COS-1 cells yields a form of Kv4.2 that is distinct from that seen in brain in that it has an increased relative electrophoretic mobility (“lower molecular weight”) on SDS gels that corresponds to the mobility of brain Kv4.2 that has been subjected to extensive dephosphorylation with exogenous alkaline phosphatase (AP) treatment [14]. Moreover, Kv4.2 expressed in these cells is retained intracellularly, in either the ER [14] or post-ER compartment [15], with no detectable staining of intact cells with externally-directed anti-Kv4.2 antibodies [14, 18]. Coexpression with KChIPs yielded a form of Kv4.2 with electrophoretic mobility more similar to the native brain form, and which underwent similar mobility shifts upon AP-dependent dephosphorylation [14]. Moreover, while no detectable immunoreactivity against the S552 phosphorylation site could be detected in cells expressing Kv4.2 alone, by either immunoblot or immunofluorescence staining, both assays revealed robust immunoreactivity on Kv4.2 coexpressed with KChIPs [14]. The immunofluorescence staining was intriguing in that it was specific for plasma membrane-associated Kv4.2, and did not stain intracellular Kv4.2 [14]. One interpretation of these staining results is that phosphorylation at S552 may be involved in biogenic trafficking of Kv4.2 to the plasma membrane, such that unphosphorylated Kv4.2 was retained intracellularly while phosphorylation at S552 was a permissive or instructive switch for trafficking to the plasma membrane (Fig. 2). However, changing the chemistry of this phosphorylation site in the S552A and S552D mutants did not alter trafficking in the presence or absence of KChIPs, suggesting that S552 phosphorylation is associated with but not a primary determinant of the biogenic plasma membrane expression of Kv4.2 [14]. While a precise role in biogenic trafficking of Kv4 channels remains unclear, phosphorylation at S552 serves as a useful marker to distinguish newly synthesized and intracellular Kv4 channels from those that have been trafficked the plasma membrane (Fig. 2).
Fig. 2.
Modulation of Kv4 channels. Left panel. Studies in heterologous cells have revealed diverse roles for auxiliary subunit-dependent phosphorylation of Kv4.2, including forward trafficking from ER though Golgi to the plasma membrane. T38 and S552 refer to Kv4.2 phosphorylation sites. Right panel. Activity-dependent changes in Kv4.2 phosphorylation, mediated by enzymes associated with AKAP79/150, lead to changes internalization of Kv4.2 channels via endocytosis.
KChIP subunits are also required for the acute modulation of dendritic Kv4.2 trafficking mediated through PKA phosphorylation [50]. PKA-mediated phosphorylation of Kv4.2 has diverse effects on endocytosis and recycling. Acute activation of PKA (e.g., 10 min stimulation with forskolin) leads to downregulation of Kv4.2 expression in neurons through enhanced internalization, a process that requires phosphorylation at S552 [51]. However, the effects of PKA activation are complex, such that prolonged (e.g., 24 hour) exposure to PKA activators leads to increased expression of Kv4.2, an effect that requires KChIP coexpression and Kv4.2 phosphorylation at S552 [50]. Together, these results suggest that while KChIP coexpression in unstimulated cells increases Kv4.2 expression and S552 phosphorylation, further activation of PKA can enhance surface expression via a separate mechanism that again requires both KChIP coexpression and Kv4.2 S552 phosphorylation, perhaps through translation and biogenic trafficking of new channels (Fig. 2).
Recent studies have suggested a role for other proteins associated with the Kv4: KChIP complex in dynamic regulation of Kv4 channels via PKA (Fig. 2). Of particular note is a recently defined role for AKAP79/150, a protein scaffold that can profoundly influence phosphorylation-dependent signaling of diverse protein targets through its interaction with both PKA and the Ca2+/calmodulin-dependent protein phosphatase calcineurin [52]. Phosphorylation-dependent mechanisms link NMDA-receptor signaling to acute changes in the activity of Kv4.2 channels that underlie A-type currents in CA1 pyramidal neurons (Fig. 2). Kv4.2 channels on CA1 pyramidal cell dendrites are subjected to clathrin-mediated endocytosis in response to NMDA-receptor activation [30]. Internalization is triggered by PKA-mediated phosphorylation of Kv4.2 at a specific residue (S552) on the Kv4.2 C-terminus [51]. Efficacy of the coupling between NMDA receptor activation and Kv4.2 phosphorylation is achieved through the physical coupling of PKA to Kv4.2 channels via AKAP79/150 scaffolding protein [50]. Disrupting the PKA anchoring function of this AKAP leads to enhanced steady-state plasma membrane expression of Kv4.2 in both cultured hippocampal neurons and heterologous cells, while overexpressing the AKAP led to decreased surface expression [53]. The AKAP-dependent changes in Kv4.2 expression were dependent on PKA-dependent phosphorylation at the S552 site on Kv4.2 [53]. Interestingly, the calcineurin binding activity of AKAP79/150 is also involved in regulation of Kv4.2 [53]. As such, the role of AKAP79/150 is complex, and is strongly influenced by the identity of the accessory KChIP cytoplasmic auxiliary subunit associated with the Kv4.2 channel complex [50]. Kv4.2 channels with associated KChIP4a subunits have enhanced expression in the presence of AKAP79/150, while those associated with other KChIP subunits do not [50] (Fig. 2). It should be noted while discussing a potential role of the Ca2+/calmodulin-dependent phosphatase calcineurin in Kv4 channel regulation that KChIPs themselves are Ca2+binding proteins [13], and mutation of the EF hand motifs in KChIPs leads to loss of their ability to modulate Kv4 channel gating in heterologous cells, even though Kv4: KChIP binding was retained [13, 54]. This suggests that KChIPs may act in concert with calcineurin, and other Ca2+-sensing Kv4 channel interacting proteins, in regulating Kv4 channel function under the diverse conditions that impact local or global intracellular [Ca2+].
That manipulating AKAP79/150 impacts excitability of hippocampal neurons underscores the important role of AKAP: Kv4.2 interaction in neuronal function. That AKAP79/150 can tether both PKA and calcineurin to Kv4.2 channels suggests that different modes of regulation of Kv4.2 phosphorylation state can be achieved based on the modality of signaling occurring at particular sites of Kv4 channel expression, analogous to regulation of glutamate receptor function underlying synaptic plasticity [55]. While the role of KChIPs in the AKAP-dependent regulation of Kv4.2 was not directly addressed, it is likely, given the previously described requirement for KChIP association in regulating both the extent and impact of PKA-mediated phosphorylation of Kv4.2 at S552 [14, 47, 50], that KChIPs are playing a prominent role in mediating the dynamic regulation of Kv4.2 via AKAP-associated PKA and calcineurin. It is important to highlight the distinct yet crucial roles of the direct association of Kv4.2 with these diverse interacting proteins in regulating Kv4.2 through phosphorylation at the critical S552 phosphorylation site. KChIPs co-assemble with Kv4 α subunits early in biogenesis, and all available evidence suggests that they remain as permanent component subunits of Kv4 channel complexes for the lifetime of the channel, being trafficked along with the channel to its site of function, and remaining associated as the Kv4 channels are dynamically internalized and reinserted during activity- and phosphorylation-dependent modulation (Fig. 2). Presumably, the association of Kv4 channels with AKAP79/150 occurs at a later stage in the channel's lifetime, perhaps after it has been inserted into the plasma membrane at its ultimate site of function, and one can imagine that this association could be dynamically regulated, such that Kv4: AKAP association may be limited to plasma membrane Kv4 channels, or even a subset of these channels should the association be conditional and dependent on certain physiological conditions. This example highlights the distinction between an “auxiliary subunit” such as a KChIP, and an “interacting protein” such as an AKAP, although clearly there are instances where the distinction (and nomenclature) blurs.
Regulation of axonal Kv1 channels by auxiliary subunits and phosphorylation
Principal and auxiliary subunits of native axonal Kv1 channels
The most prominent Kv1 channels in the axons of mammalian neurons are heteromers of Kv1.1, Kv1.2, and/or Kv1.4 α subunits [6]. These form Kv channels that underlie low voltage-activated, dendrotoxin-sensitive, non-inactivating or inactivating currents. These Kv1 channels are present in high densities in specific axonal sites (Fig. 1), including the axon initial segment or AIS, the juxtaparanodal domain of the nodes of Ranvier of myelinated axons, or JPN, and the preterminal segment of the axon immediately adjacent to the nerve terminal [56]. At these locations, Kv1 channels have many important roles as regulators of cell excitability, controlling spike threshold and shape, and repetitive firing [57]. Disruption of Kv1 channel function at each site by disease or injury, or experimentally by pharmacological blockade or genetic ablation has been linked to specific effects on neuronal function, including conduction block and altered coupling of cell firing to neurotransmitter release from presynaptic terminals [58]. The properties of these Kv1 channels are profoundly affected by their subunit composition, with heteromers of distinct α subunit composition and stoichiometry exhibiting distinct properties [2]. Early biochemical studies on native Kv1 channels revealed the presence of stoichiometric amounts of a copurifying low molecular weight components that were proposed to be β subunits of these channels [59]. These Kvβ subunits were eventually purified in amounts that allowed for amino acid sequencing [60], leading to the design of probes resulting in the cloning of a set of these auxiliary subunits [60, 61]. Biochemical and immunohistochemical studies provided support that the bulk of Kv1 channels in mammalian brain are associated with Kvβ subunits, and vice-versa [62-65].
In the mammalian genome, three Kvβ subunit genes exist (Kvβ1, Kvβ2 and Kvβ3), and from these, alternative splicing can generate a number of functionally distinct isoforms [66]. Protein sequence alignment of Kvβ family members showed that these polypeptides generally display subtype-specific N-terminal sequences of 30-70 amino acids, which are followed by a highly conserved protein core sequence (~330 amino acids) exhibiting >80% amino acid sequence identity [4]. Certain Kvβ N-termini, for example of Kvβ1.1 and Kvβ3.1, contain a domain that is necessary and sufficient to confer rapid “N-type” inactivation to otherwise noninactivating Kv channels [61]. The N-terminus of these cytoplasmic auxiliary subunits behaves similarly to the inactivation particle or ball domain present on the N-terminus of Kv1.4, which rapidly occludes the pore of activated Kv1 channels [61]. It is now recognized that Kvβ subunits are members of the extended oxidoreductase protein superfamily [67]. Studies of Kvβ1 and Kvβ2 have shown that the conserved core domains are functional aldo-keto reductases (AKR) that use NADPH as a cofactor [68, 69]. The AKR activity of the Kvβ1 core and the ability of the Kvβ1 N terminus to induce channel inactivation seem to be linked [70]. Conversion of the bound NADPH to NADP+, whether enzymatically by a substrate, nonenzymatically by an oxidant such as H2O2, or by exchange with NADP+, leads to reduced inactivation and an increase in Kv1 current [68, 69]. Enzymatic activity of Kvβ subunits against artificial substrates has recently been demonstrated [68], but it is however still unclear whether Kvβ subunits act in vivo as genuine oxidoreductase enzymes.
In contrast to Kvβ1 and Kvβ3 subunits, Kvβ2 subunits lack an N-terminal ball domain and as such do not dramatically affect the inactivation of Kv1 channels, but do control Kv1 axonal targeting, as detailed below. All Kvβ subunit isoforms are able to promote biosynthetic endoplasmic reticulum (ER) export of associated Kv1 channel complexes [71, 72]. Kvβ1 knockout mice have deficiencies in learning and memory [73, 74]. Kvβ2 knockout and Kvβ1/Kvβ2 double-knockout mice are characterized by a reduced life span, an increased neuronal excitability, occasional seizures, and cold swim-induced tremors [75, 76]. Elimination of Kvβ2 did not induce marked changes in the gross characteristics of Kv1.2 glycosylation, indicative of a lack of a dramatic impact on intracellular trafficking of Kv1.2, and Kv1.2 expression in cerebellar basket cell terminals and juxtaparanodes of nodes of Ranvier was not detectably affected [75]. Kvβ1/Kvβ2 double-knockout mice did exhibit variably reduced levels of Kv1.2 in basket cell terminals, suggesting altered expression and/or trafficking of Kv1.2 from cerebellar basket cell sonata to terminals [76]. The impact of genetic ablation of Kvβ2 was markedly strain-dependent, with Kvβ2 knockouts on the C57BL/6 strain background showing a more pronounced phenotype and an increased mortality compared to Kvβ2 knockouts on the 129/SvEv background [76]. A recent study revealed a pronounced behavioral phenotype in Kvβ2 knockout mice maintained on the 129/SvEv background, which exhibit deficits in associative learning and memory, and a reduction in the slow afterhyperpolarization, and increased excitability, in neurons in the lateral nucleus of the amygdala [77]. Some aspects of the phenotype resembled the cognitive impairment observed in patients with 1p36 deletion syndrome [78], who lack portions of the distal end of the short arm of chromosome 1 that in some patients includes the region containing the Kvβ2 gene. Clinical studies have suggested a strong association between the severity of the seizures that are seen in many but not all of these patients, and the loss of the Kvβ2 gene [79]. Thus while initial studies of Kvβ subunit knockout mice suggested a subtle phenotype relative to mice lacking the Kv1.1 [80] and Kv1.2 [81] α subunits, which exhibit severe epileptic phenotypes, these more recent studies suggest that the phenotype of these mice may in fact support a fundamental role for these auxiliary subunits in regulating neuronal excitability through effects on Kv1 channel expression, localization and function. Acute knockdown of individual Kvβ subunits in vivo will be revealing, as it will avoid many of the potential mechanisms (e.g., upregulation of other Kvβ subunits) that may act to compensate for the loss of individual Kvβ subunits, and obscure their role generating and maintaining the normal expression, localization and function of Kv1 channels in brain neurons.
Structural determination by X-ray crystallography of a truncated mammalian Kvβ2 subunit, both alone [82] and in complex with Kv1.2 α subunits [83, 84], confirmed the stoichiometry of the complex previously found in earlier biochemical and molecular biology studies. Kv1 channels function as supramolecular protein complexes, composed of four pore-forming and voltage-sensing principal, or α subunits, with four Kvβ subunits. Biosynthesis studies of Kv1 channels show that Kv1 α and Kvβ subunits coassemble in the ER and remain together as a permanent complex [71]. Kvβ-subunits primarily attach to the tetramerization domain (or T1 domain) present on the cytoplasmic N-termini of Kv1 α subunits [83-85]. The amino acid sequence of the contact loop is highly conserved among members of the Kv1 α subunit family, providing a molecular explanation for the specificity of Kv1 α- and Kvβ-subunit interactions. The interaction of Kv1 channels with Kvβ subunits extends the cytoplasmic domains of the channel complex an additional ~100 Å into the cytoplasm [84]., providing a large surface for interaction with cellular proteins impacting channel function through diverse signaling pathways (e.g., protein kinases, protein phosphatases, and other signaling molecules).
Interplay between Kv1 and Kvβ subunit phosphorylation regulates ER export and cell surface trafficking
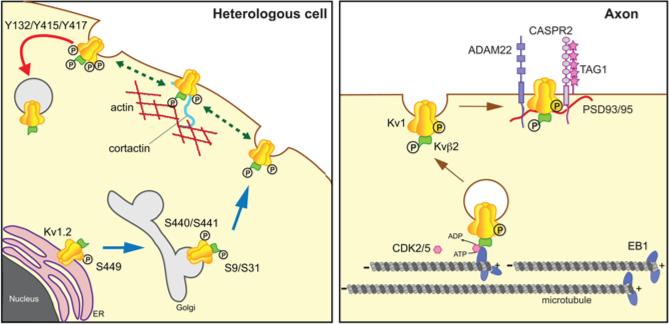
Biosynthesis studies showed that Kvβ auxiliary subunits and Kv1 subunits co-assemble before the resultant α4β4 channel complexes exit the ER [71, 86] and that Kvβ2 association is crucial for efficient cell surface trafficking of Kv1.2 [71, 72]. Recent in vivo and in vitro LC-MS/MS analyses of Kv1.2 [87] and Kvβ2 [88] identified several phosphorylation sites crucial for delivery of Kv1.2-containing channel complexes to the plasma membrane. These studies revealed that phosphorylation of Kv1.2 at S440 and S441 was specifically associated with cell surface localized Kv1.2, while phosphorylation at S449 was associated with newly synthesized and ER-localized Kv1.2 [87] (Fig. 3). Site-directed mutagenesis analysis showed that Ser-to-Ala mutations at either S440 or S441 diminished Kv1.2 surface expression levels, and increased the levels of intracellular Kv1.2, resulting in decreased ionic current. Furthermore, mutation of S449 led to a decrease in phosphorylation at both S440 and S441, and effects on trafficking similar to those seen in the S440A and S441A mutants [87]. Co-expression of Kvβ2 impacted Kv1.2 phosphorylation at S440 and S441, either as a correlate or determinant of enhanced Kvβ2-mediated Kv1.2 surface expression [87]. Importantly, incorporation of Kv1.2 subunits into heteromeric Kv1 channel complexes confers upon them phosphorylation-dependent trafficking regulation [87], suggesting that Kv1.2 could play a role in contributing conditional, phosphorylation-dependent regulation to the diverse forms of Kv1.2-containing Kv1 channels found in mammalian brain [62, 64, 65, 89, 90]. Similarly, mutation of Kvβ2 phosphorylation sites S9 and S31 led to decreased Kv1.2 surface expression levels resulting in reduced ionic current. Together, these observations strongly suggest interplay between these phosphosites, in which a specific phosphorylation event serves as a prerequisite for triggering the subsequent forward trafficking step (Fig. 3). It is also important to highlight the role of phosphorylation as a key mechanism for acute suppression of Kv1 channels. Tyrosine phosphorylation of Kv1.2 at Y132, Y466 and Y482 leads to enhanced endocytosis of Kv1.2 channels [91, 92]. Phosphorylation of Kv1.2 at Y466 and Y482 partially released Kv1.2 from its interaction with cortactin [92], triggering endocytosis, which was then dependent on phosphorylation at Y132 [91]. As such, phosphorylation of Kv1.2 and Kvβ2 may play a general role in providing phosphorylation-dependent regulation of the expression levels of the diverse repertoire of Kv1.2-containing heteromeric Kv1 channel complexes present in mammalian brain.
Fig. 3.
Modulation of Kv1 channels. Left panel. Studies in heterologous cells have revealed diverse roles for phosphorylation of both Kv1.2 and Kvβ2, including ER export, forward trafficking to the plasma membrane, association with the actin-based cortical cytoskeleton, and endocytosis. S9 and S31 refer to phosphorylation sites on Kvβ2, and Y132, Y415, Y417, S440/S441, and S449 on Kv1.2. Right panel. Altering phosphorylation of Kvβ2 leads to changes in binding to EB1 and dissocation of Kv1 channel complexes from MTs, allowing for local insertion into the plasma membrane and direct or indirect association with interacting proteins ADAM22, CASPR2, PSD93/95, and TAG1.
To date, few candidate signaling pathways responsible for dynamically regulating these phosphosites have been identified. Kv1.2 S440 and S449 are substrates for PKA [93, 94], and Kvβ2 S31 is a substrate for CDK2 and CDK5, and inhibition of CDKs in heterologous cells leads to a decrease in Kv1 surface expression [88]. This suggests that signaling pathways leading to PKA and CDK activation could modulate expression of Kv1.2 through effects on cell surface trafficking. Interestingly, Kvβ2 subunits, which are also a substrate for PKC [95], bind specifically to the PKC-zeta interacting protein or ZIP [96, 97]. ZIP proteins act as a physical link between PKCζ and Kvβ2, providing a mechanism for specificity and efficacy of PKCζ–mediated Kvβ2 phosphorylation. However, the effects of PKCζ activation on Kv1 channels, and whether PKCζ phosphorylates specific sites on Kvβ2, or on associated Kv1 α subunits, remain unclear.
Kvβ2 phosphorylation modulates Kv1 axonal targeting
In neurons, Kvβ2 orchestrates axonal targeting of Kv1 channels [98], via its interaction with the microtubule plus-end tracking protein (+TIP) EB1 [99] and the microtubule-based motors KIF3A [100]. KIF5B has also been shown to be required for efficient targeting of Kv1 channels to axons [101], but any potential Kvβ2/KIF5B interaction has not yet been characterized. A recent study [88] revealed a new regulatory mechanism for the targeting of Kv1 complexes to the axonal membrane through the reversible phosphorylation-dependent binding of Kvβ2 auxiliary subunits to EB1 (Fig. 3). In this study, in vivo phosphosites (S9, S20, S31 and S112) on Kvβ2 purified from mammalian brain were identified using a phosphoproteomic approach [88]. Site-directed mutagenesis analyses revealed that mutations at two of the identified phosphosites, S9 and S31 in the unique Kvβ2 N-terminus, impact Kvβ2-EB1 interaction. Moreover, CDK-mediated phosphorylation of Kvβ2 negatively regulates its interaction with EB1 [88]. CDK2 and CDK5 directly phosphorylated Kvβ2 in vitro, and pharmacological inhibition of CDKs in heterologous cells led to an increase of Kvβ2 recruitment to MTs and a decrease of Kv1 surface expression [88]. Moreover, acute inhibition of CDKs in cultured hippocampal neurons led to an increase in the levels of intracellular populations of axonal Kvβ2, EB1 and Kv1 channels without affecting the levels of either the surface population of Kv1 channels, or the Kv1 channel anchoring protein PSD-93 [88]. This suggests a model whereby CDK-mediated phosphorylation of Kvβ2 on its unique N-terminal domain disrupts its binding to EB1, which consequently releases the Kv1/Kvβ2-containing vesicles from their association with EB1 and axonal MTs (Fig. 3). Thus, it is likely that CDK activity at or near sites of high densities of axonal Kv1 channels (e.g., axon initial segments, juxtaparanodes) spatially restricts where the phosphorylation events that regulate Kvβ2: EB1 interaction, and Kv1 unloading from MTs, will occur. This ensures that Kv1 channels are localized at the correct subcellular locations, and prevents their ectopic expression at sites that could result in deranged neuronal excitability. Similarly, tyrosine phosphorylation may play a central role in clustering of axonal Kv1 channels at their proper locations. A recent study showed Kv1 channel clustering in myelinated axons was mediated by phosphorylation of Y458 on the Kv1.2 C-terminus [102], near the previously identified cluster of Ser sites S440/S441 and S449 [87]. Mutating Y458 altered TAG-1-induced clustering of Kv1.2 in axons in cultured neurons, and a general anti-phosphotyrosine antibody showed staining that colocalized with TAG-1 and Kv1.2 clusters [102], although the molecular identity of the tyrosine phosphorylated proteins that were stained here was not determined. Long-term (48 h) inhibition of tyrosine kinase activity decreased the coclustering of Kv1.2 and TAG-1 while increasing overall levels of axonal Kv1.2. The latter result is consistent with previous studies suggesting a role for tyrosine phosphorylation in Kv1.2 internalization [103]. However, the model presented for tyrosine phosphorylation-dependent Kv1.2 clustering, namely that TAG-1 aggregation recruits Kv1 channels containing Kv1.2 subunits phosphorylated at Y458 and anchored to the actin cytoskeleton [102], is counter to a previous report that showed that tyrosine phosphorylation triggered dissociation of Kv1.2 from the actin cytoskeleton [92]. Future studies will help to solve the mechanism of phosphorylation-dependent clustering of Kv1.2-containing Kv1 channels at precise sites in axons.
Modulation of auxiliary subunit-mediated inactivation of Kv1 channels by phosphorylation
Inactivation of voltage-gated Kv1 channels can be altered by certain Kvβ subunits, which contain an N-terminal ball domain that blocks the ion-conducting pore to induce a rapid N-type inactivation [61]. Phosphorylation of the Kvβ subunits themselves can influence the extent of these Kvβ subunit-mediated effects on Kv1 inactivation gating. Studies in Xenopus oocytes and mammalian heterologous cell expression systems revealed that phosphorylation of Kvβ1.3 by PKA causes marked slowing of Kvβ1.3-mediated fast inactivation, with a resulting increase in total Kv1.5 current [104]. These effects were attributed to phosphorylation of a specific PKA consensus site (S24) in the unique N- terminus of Kvβ1.3 subunit by PKA [104]. Interestingly, Kvβ1.2 was not modulated by PKA activation even though it possesses an analogous PKA consensus site and has similar effects on Kv1.5 currents [104]. Conversely, activation of PKC led to a specific decrease of Kv1.5 current only when Kv1.5 was coexpressed with Kvβ1.2, but not with Kvβ1.3, even though Kvβ1.3 also contains PKC consensus sites [105]. It is intriguing that phosphorylation of the N-terminus of Kv1.4, which also contains a ball inactivation domain, can modulate inactivation of Kv1.4-containing Kv1 channels [106]. Together these observations underscore that modulation of distinct Kvβ subunits by specific protein kinases can yield diverse functional responses. The diverse nature of the effects of phosphorylation-dependent modulation of specific sites on α and/or auxiliary subunits represents a potent mechanism for regulating the expression, localization and functional diversity of Kv1 channels in vivo.
Conclusions
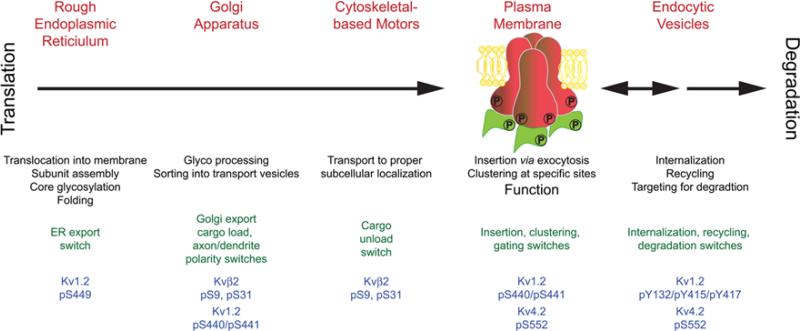
A number of crucial events occur through the lifetime of a Kv channel (Fig. 4). At each step in this timeline the channel's fate is determined by cellular mechanisms that operate via molecular switches affecting protein-protein interactions, many of these are impacted by auxiliary subunits. Reversible phosphorylation is a common mechanism to directly or indirectly, dynamically, and reversibly flip molecular switches, and not surprisingly many of the events in the lifetime of Kv channels are dynamically regulated by phosphorylation. For example, Kv1.2 phosphorylation at S449 is associated with the newly synthesized population of Kv1.2 present in the ER. Kvβ2 phosphorylation at S9 and S31, and Kv1.2 phosphorylation at S440/S441 direct ER export and forward trafficking of Kv1.2: Kvβ2-containing channel complexes. Phosphorylation of Kvβ2 also seems to be involved in unloading of channel complexes from MT-based motors at sites of insertion. Phosphorylation of Kv1.2 at S440/S441, and of Kv4.2 at S552, are associated with plasma membrane channels, although the role of this phosphorylation is complex, as Kv4.2 S552 phosphorylation also directs Kv4.2 endocytosis, as does Kv1.2 phosphorylation at Y132. Much recent data, as described in detail in the sections above, suggest that Kv channel auxiliary subunits and phosphorylation exhibit complex interplay as fundamental modes of Kv channel regulation. The underlying basis for auxiliary subunit-dependent changes in α subunit phosphorylation is as yet unknown. On possibility is that association with auxiliary subunits could generate conformational changes in the Kv1 and Kv4 α subunits that expose domains containing phosphorylation sites, thereby making them accessible to protein kinase-mediated phosphorylation. Alternatively, auxiliary subunit-dependent changes in the intracellular trafficking of Kv1 and Kv4 channel complexes could result in their targeting at specific cellular sites containing protein kinases with a restricted compartmentalized subcellular localization, for example those associated with a specific intracellular organelle, with the plasma membrane, or in the case of neurons with a specific neuronal compartment (e.g., the AIS). Another possibility is that auxiliary subunits are involved in the association of Kv1 and Kv4 channel complexes with the modifying enzymes themselves, for example by mediating associations with AKAPs or other protein kinase and protein phosphatase scaffolds. Whatever the mechanism, the studies detailed above suggest that phosphorylation of Kv channel auxiliary subunits, and auxiliary subunit-dependent phosphorylation of principal subunits, are important determinants of the molecular events that act to shape the expression, localization and function of neuronal Kv channels and critical aspects of neuronal function. Future research will likely lead to new insights as to these mechanistic details, as well as to how phosphorylation of Kv channel auxiliary subunits, and auxiliary subunit-dependent phosphorylation of principal subunits, is dynamically regulated to impact the crucial contributions made by Kv1 and Kv4 channels to neuronal function. Moreover, as many of the studies described above have been undertaken in heterologous cell systems, translating these findings into native systems remains an important future objective. Mass spectrometry-based proteomic analyses of phosphorylation of Kv1 and Kv4 channels [107], and of proteins associated with native Kv1 [108] and Kv4 [109] channel complexes purified from brain, provide a strong foundation for future in vivo studies of the complex and dynamic interplay between phosphorylation and protein: protein interactions, including that with auxiliary subunits, in regulating these important neuronal ion channels. Finally, it will be of great interest to determine how the roles of Kv channel phosphorylation and auxiliary subunit interaction are altered under conditions of disease and injury. Kv4.2 exhibits enhanced phosphorylation by ERK in animal subjects during status epilepticus [110], suggesting that acute modulation of Kv4 channels by phosphorylation could impact the function of brain neurons under pathological conditions. While no disease causing human mutations have been found in Kv1 or Kv4 phosphorylation sites, a recent large-scale comparison of ion channel gene sequences in sporadic idiopathic epilepsy patients versus unaffected controls suggests that even relatively severe ion channel mutations (e.g., large deletions or truncations) confer uncertain risk to an individual [111]. This may be related to the fact that while a large number of human diseases linked to ion channel mutations (channelopathies) have been identified, many of these are episodic or paroxysmal [112], suggesting a conditional expression of the disease phenotype in the affected individual, perhaps related to alterations in the dynamic regulation of ion channels by phosphorylation and auxiliary subunit interactions.
Fig. 4.
Key events in the lifetime of a Kv channel. After translation, a series of events (black text) occur in different subcellular compartments (red text) that define the expression, localization and function of Kv channels. Recent studies have defined a series of phosphorylation events on Kv1 and Kv4 channel principal and auxiliary subunits (blue text) that act as switches (green text) to determine the outcome of these events. In many cases, the phosphorylation events on the Kv1 and Kv4 α subunits can be influenced by auxiliary subunits.
Acknowledgments
Work from our laboratories reviewed here was supported by the Institut National de la Santé et de la Recherche Médicale and Marie Curie 7th framework program grant IRG-2008-239499 (to H. Vacher), and National Institutes of Health grants NS34383 and NS42225 (to J. S. Trimmer).
References
- 1.Hille B. Ionic channels of excitable membranes. 3 edn. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- 2.Papazian DM. Potassium channels: some assembly required. Neuron. 1999;23:7–10. doi: 10.1016/s0896-6273(00)80746-1. [DOI] [PubMed] [Google Scholar]
- 3.Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004:re15. doi: 10.1126/stke.2532004re15. 2004. [DOI] [PubMed] [Google Scholar]
- 4.Trimmer JS. Regulation of ion channel expression by cytoplasmic subunits. Curr Opin Neurobiol. 1998;8:370–4. doi: 10.1016/s0959-4388(98)80063-9. [DOI] [PubMed] [Google Scholar]
- 5.Cerda O, Trimmer JS. Analysis and functional implications of phosphorylation of neuronal voltage-gated potassium channels. Neurosci Lett. 2010;486:60–7. doi: 10.1016/j.neulet.2010.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–47. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–69. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Shah MM, Hammond RS, Hoffman DA. Dendritic ion channel trafficking and plasticity. Trends Neurosci. 2010;33:307–16. doi: 10.1016/j.tins.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, Strassle BW, Buchwalder L, Menegola M, Cao J, An WF, Trimmer JS. KChIPs and Kv4 alpha subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci. 2004;24:7903–15. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menegola M, Misonou H, Vacher H, Trimmer JS. Dendritic A-type potassium channel subunit expression in CA1 hippocampal interneurons. Neuroscience. 2008;154:953–64. doi: 10.1016/j.neuroscience.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanyshkova T, Broicher T, Meuth SG, Pape HC, Budde T. A-type K+ currents in intralaminar thalamocortical relay neurons. Pflugers Arch. 2011;461:545–56. doi: 10.1007/s00424-011-0953-2. [DOI] [PubMed] [Google Scholar]
- 12.Strassle BW, Menegola M, Rhodes KJ, Trimmer JS. Light and electron microscopic analysis of KChIP and Kv4 localization in rat cerebellar granule cells. J Comp Neurol. 2005;484:144–55. doi: 10.1002/cne.20443. [DOI] [PubMed] [Google Scholar]
- 13.An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–6. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 14.Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, Carroll KI, Sweatt JD, Rhodes KJ, Trimmer JS. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem. 2003;278:36445–54. doi: 10.1074/jbc.M306142200. [DOI] [PubMed] [Google Scholar]
- 15.Hasdemir B, Fitzgerald DJ, Prior IA, Tepikin AV, Burgoyne RD. Traffic of Kv4 K+ channels mediated by KChIP1 is via a novel post-ER vesicular pathway. J Cell Biol. 2005;171:459–69. doi: 10.1083/jcb.200506005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de Miera E, Ma Y, Mo W, Goldberg EM, Misumi Y, Ikehara Y, Neubert TA, Rudy B. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003;37:449–61. doi: 10.1016/s0896-6273(02)01185-6. [DOI] [PubMed] [Google Scholar]
- 17.Kin Y, Misumi Y, Ikehara Y. Biosynthesis and characterization of the brain-specific membrane protein DPPX, a dipeptidyl peptidase IV-related protein. J Biochem (Tokyo) 2001;129:289–95. doi: 10.1093/oxfordjournals.jbchem.a002856. [DOI] [PubMed] [Google Scholar]
- 18.Seikel E, Trimmer JS. Convergent modulation of Kv4.2 channel alpha subunits by structurally distinct DPPX and KChIP auxiliary subunits. Biochemistry. 2009;48:5721–30. doi: 10.1021/bi802316m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maffie J, Rudy B. Weighing the evidence for a ternary protein complex mediating A-type K+ currents in neurons. J Physiol. 2008;586:5609–23. doi: 10.1113/jphysiol.2008.161620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark BD, Kwon E, Maffie J, Jeong HY, Nadal M, Strop P, Rudy B. DPP6 Localization in Brain Supports Function as a Kv4 Channel Associated Protein. Front Mol Neurosci. 2008;1:8. doi: 10.3389/neuro.02.008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zagha E, Ozaita A, Chang SY, Nadal MS, Lin U, Saganich MJ, McCormack T, Akinsanya KO, Qi SY, Rudy B. DPP10 modulates Kv4-mediated A-type potassium channels. J Biol Chem. 2005;280:18853–61. doi: 10.1074/jbc.M410613200. [DOI] [PubMed] [Google Scholar]
- 22.Menegola M, Trimmer JS. Unanticipated region- and cell-specific downregulation of individual KChIP auxiliary subunit isotypes in Kv4.2 knock-out mouse brain. J Neurosci. 2006;26:12137–42. doi: 10.1523/JNEUROSCI.2783-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lilliehook C, Bozdagi O, Yao J, Gomez-Ramirez M, Zaidi NF, Wasco W, Gandy S, Santucci AC, Haroutunian V, Huntley GW, Buxbaum JD. Altered Abeta formation and long-term potentiation in a calsenilin knock-out. J Neurosci. 2003;23:9097–106. doi: 10.1523/JNEUROSCI.23-27-09097.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norris AJ, Foeger NC, Nerbonne JM. Interdependent roles for accessory KChIP2, KChIP3, and KChIP4 subunits in the generation of Kv4-encoded IA channels in cortical pyramidal neurons. J Neurosci. 2010;30:13644–55. doi: 10.1523/JNEUROSCI.2487-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Nadal MS, Clemens AM, Baron M, Jung SC, Misumi Y, Rudy B, Hoffman DA. Kv4 accessory protein DPPX (DPP6) is a critical regulator of membrane excitability in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2008;100:1835–47. doi: 10.1152/jn.90261.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadin BM, Pfaffinger PJ. Dipeptidyl peptidase-like protein 6 is required for normal electrophysiological properties of cerebellar granule cells. J Neurosci. 2010;30:8551–65. doi: 10.1523/JNEUROSCI.5489-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourdeau ML, Laplante I, Laurent CE, Lacaille JC. KChIP1 modulation of Kv4.3-mediated A-type K(+) currents and repetitive firing in hippocampal interneurons. Neuroscience. 2011;176:173–87. doi: 10.1016/j.neuroscience.2010.11.051. [DOI] [PubMed] [Google Scholar]
- 28.Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–41. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- 29.Johnston J, Forsythe ID, Kopp-Scheinpflug C. Going native: voltage-gated potassium channels controlling neuronal excitability. J Physiol. 2010;588:3187–200. doi: 10.1113/jphysiol.2010.191973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007;54:933–47. doi: 10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makara JK, Losonczy A, Wen Q, Magee JC. Experience-dependent compartmentalized dendritic plasticity in rat hippocampal CA1 pyramidal neurons. Nat Neurosci. 2009;12:1485–7. doi: 10.1038/nn.2428. [DOI] [PubMed] [Google Scholar]
- 32.Barnwell LF, Lugo JN, Lee WL, Willis SE, Gertz SJ, Hrachovy RA, Anderson AE. Kv4.2 knockout mice demonstrate increased susceptibility to convulsant stimulation. Epilepsia. 2009;50:1741–51. doi: 10.1111/j.1528-1167.2009.02086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrasfalvy BK, Makara JK, Johnston D, Magee JC. Altered synaptic and non-synaptic properties of CA1 pyramidal neurons in Kv4.2 knockout mice. J Physiol. 2008;586:3881–92. doi: 10.1113/jphysiol.2008.154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–51. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monaghan MM, Menegola M, Vacher H, Rhodes KJ, Trimmer JS. Altered expression and localization of hippocampal A-type potassium channel subunits in the pilocarpine-induced model of temporal lobe epilepsy. Neuroscience. 2008;156:550–62. doi: 10.1016/j.neuroscience.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–5. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- 37.Singh B, Ogiwara I, Kaneda M, Tokonami N, Mazaki E, Baba K, Matsuda K, Inoue Y, Yamakawa K. A Kv4.2 truncation mutation in a patient with temporal lobe epilepsy. Neurobiol Dis. 2006;24:245–53. doi: 10.1016/j.nbd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Rosenkranz JA, Frick A, Johnston D. Kinase-dependent modification of dendritic excitability after long-term potentiation. J Physiol. 2009;587:115–25. doi: 10.1113/jphysiol.2008.158816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman DA, Johnston D. Downregulation of transient K+channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J Neurosci. 1998;18:3521–8. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–8. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varga AW, Yuan LL, Anderson AE, Schrader LA, Wu GY, Gatchel JR, Johnston D, Sweatt JD. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci. 2004;24:3643–54. doi: 10.1523/JNEUROSCI.0154-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson AE, Adams JP, Qian Y, Cook RG, Pfaffinger PJ, Sweatt JD. Kv4.2 phosphorylation by cyclic AMP-dependent protein kinase. J Biol Chem. 2000;275:5337–46. doi: 10.1074/jbc.275.8.5337. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura TY, Coetzee WA, Vega-Saenz De Miera E, Artman M, Rudy B. Modulation of Kv4 channels, key components of rat ventricular transient outward K+ current, by PKC. Am J Physiol. 1997;273:H1775–86. doi: 10.1152/ajpheart.1997.273.4.H1775. [DOI] [PubMed] [Google Scholar]
- 44.Schrader LA, Ren Y, Cheng F, Bui D, Sweatt JD, Anderson AE. Kv4.2 is a locus for PKC and ERK/MAPK cross-talk. Biochem J. 2009;417:705–15. doi: 10.1042/BJ20081213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–87. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- 46.Schrader LA, Birnbaum SG, Nadin BM, Ren Y, Bui D, Anderson AE, Sweatt JD. ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of the pore-forming subunit. Am J Physiol Cell Physiol. 2006;290:C852–61. doi: 10.1152/ajpcell.00358.2005. [DOI] [PubMed] [Google Scholar]
- 47.Schrader LA, Anderson AE, Mayne A, Pfaffinger PJ, Sweatt JD. PKA modulation of Kv4.2-encoded A-type potassium channels requires formation of a supramolecular complex. J Neurosci. 2002;22:10123–33. doi: 10.1523/JNEUROSCI.22-23-10123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colbert CM, Pan E. Arachidonic acid reciprocally alters the availability of transient and sustained dendritic K(+) channels in hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:8163–71. doi: 10.1523/JNEUROSCI.19-19-08163.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmqvist MH, Cao J, Knoppers MH, Jurman ME, Distefano PS, Rhodes KJ, Xie Y, An WF. Kinetic modulation of Kv4-mediated A-current by arachidonic acid is dependent on potassium channel interacting proteins. J Neurosci. 2001;21:4154–61. doi: 10.1523/JNEUROSCI.21-12-04154.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin L, Sun W, Wikenheiser AM, Kung F, Hoffman DA. KChIP4a regulates Kv4.2 channel trafficking through PKA phosphorylation. Mol Cell Neurosci. 2010;43:315–25. doi: 10.1016/j.mcn.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammond RS, Lin L, Sidorov MS, Wikenheiser AM, Hoffman DA. Protein kinase a mediates activity-dependent Kv4.2 channel trafficking. J Neurosci. 2008;28:7513–9. doi: 10.1523/JNEUROSCI.1951-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dell'Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur J Cell Biol. 2006;85:627–33. doi: 10.1016/j.ejcb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Lin L, Sun W, Kung F, Dell'Acqua ML, Hoffman DA. AKAP79/150 impacts intrinsic excitability of hippocampal neurons through phospho-regulation of A-type K+ channel trafficking. J Neurosci. 2011;31:1323–32. doi: 10.1523/JNEUROSCI.5383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee L, Chen KC, Chang LS. Functional roles of EF-hands in human potassium channel-interacting protein 2.2. Protein Pept Lett. 2009;16:1081–7. doi: 10.2174/092986609789055368. [DOI] [PubMed] [Google Scholar]
- 55.Sanderson JL, Dell'Acqua ML. AKAP signaling complexes in regulation of excitatory synaptic plasticity. Neuroscientist. 2011;17:321–36. doi: 10.1177/1073858410384740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rasband MN, Trimmer JS. Ion Channel Localization in Axons. In: Squire LR, editor. Encyclopedia of Neuroscience. Academic Press, London; Oxford: 2009. pp. 229–235. [Google Scholar]
- 57.Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G. Axon physiology. Physiol Rev. 2011;91:555–602. doi: 10.1152/physrev.00048.2009. [DOI] [PubMed] [Google Scholar]
- 58.Judge SI, Bever CT., Jr Potassium channel blockers in multiple sclerosis: neuronal Kv channels and effects of symptomatic treatment. Pharmacol Ther. 2006;111:224–59. doi: 10.1016/j.pharmthera.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 59.Parcej DN, Dolly JO. Elegance persists in the purification of K+ channels. Biochem J. 1989;264:623–4. doi: 10.1042/bj2640623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scott VE, Rettig J, Parcej DN, Keen JN, Findlay JB, Pongs O, Dolly JO. Primary structure of a beta subunit of alpha-dendrotoxin-sensitive K+ channels from bovine brain. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:1637–1641. doi: 10.1073/pnas.91.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- 62.Rhodes KJ, Keilbaugh SA, Barrezueta NX, Lopez KL, Trimmer JS. Association and colocalization of K+ channel alpha- and beta-subunit polypeptides in rat brain. J Neurosci. 1995;15:5360–71. doi: 10.1523/JNEUROSCI.15-07-05360.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhodes KJ, Monaghan MM, Barrezueta NX, Nawoschik S, Bekele-Arcuri Z, Matos MF, Nakahira K, Schechter LE, Trimmer JS. Voltage-gated K+ channel beta subunits: expression and distribution of Kv beta 1 and Kv beta 2 in adult rat brain. J Neurosci. 1996;16:4846–60. doi: 10.1523/JNEUROSCI.16-16-04846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. J Neurosci. 1997;17:8246–58. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coleman SK, Newcombe J, Pryke J, Dolly JO. Subunit composition of Kv1 channels in human CNS. J Neurochem. 1999;73:849–58. doi: 10.1046/j.1471-4159.1999.0730849.x. [DOI] [PubMed] [Google Scholar]
- 66.Pongs O, Leicher T, Berger M, Roeper J, Bahring R, Wray D, Giese KP, Silva AJ, Storm JF. Functional and molecular aspects of voltage-gated K+ channel beta subunits. Ann NY Acad Sci. 1999;868:344–355. doi: 10.1111/j.1749-6632.1999.tb11296.x. [DOI] [PubMed] [Google Scholar]
- 67.McCormack T, McCormack K. Shaker K+ channel beta subunits belong to an NAD(P)H-dependent oxidoreductase superfamily. Cell. 1994;79:1133–5. doi: 10.1016/0092-8674(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 68.Weng J, Cao Y, Moss N, Zhou M. Modulation of voltage-dependent Shaker family potassium channels by an aldo-keto reductase. J Biol Chem. 2006;281:15194–200. doi: 10.1074/jbc.M513809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tipparaju SM, Liu SQ, Barski OA, Bhatnagar A. NADPH binding to beta-subunit regulates inactivation of voltage-gated K(+) channels. Biochem Biophys Res Commun. 2007;359:269–76. doi: 10.1016/j.bbrc.2007.05.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan Y, Weng J, Levin EJ, Zhou M. Oxidation of NADPH on Kvbeta1 inhibits ball-and-chain type inactivation by restraining the chain. Proc Natl Acad Sci U S A. 2011;108:5885–90. doi: 10.1073/pnas.1100316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi G, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS. Beta subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron. 1996;16:843–52. doi: 10.1016/s0896-6273(00)80104-x. [DOI] [PubMed] [Google Scholar]
- 72.Campomanes CR, Carroll KI, Manganas LN, Hershberger ME, Gong B, Antonucci DE, Rhodes KJ, Trimmer JS. Kv beta subunit oxidoreductase activity and Kv1 potassium channel trafficking. J Biol Chem. 2002;277:8298–305. doi: 10.1074/jbc.M110276200. [DOI] [PubMed] [Google Scholar]
- 73.Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvbeta1.1-deficient mice with impaired learning. Learn Mem. 1998;5:257–73. [PMC free article] [PubMed] [Google Scholar]
- 74.Need AC, Irvine EE, Giese KP. Learning and memory impairments in Kv beta 1.1-null mutants are rescued by environmental enrichment or ageing. Eur J Neurosci. 2003;18:1640–4. doi: 10.1046/j.1460-9568.2003.02889.x. [DOI] [PubMed] [Google Scholar]
- 75.McCormack K, Connor JX, Zhou L, Ho LL, Ganetzky B, Chiu SY, Messing A. Genetic analysis of the mammalian K+ channel beta subunit Kvbeta 2 (Kcnab2). J Biol Chem. 2002;277:13219–28. doi: 10.1074/jbc.M111465200. [DOI] [PubMed] [Google Scholar]
- 76.Connor JX, McCormack K, Pletsch A, Gaeta S, Ganetzky B, Chiu SY, Messing A. Genetic modifiers of the Kv beta2-null phenotype in mice. Genes Brain Behav. 2005;4:77–88. doi: 10.1111/j.1601-183X.2004.00094.x. [DOI] [PubMed] [Google Scholar]
- 77.Perkowski JJ, Murphy GG. Deletion of the mouse homolog of KCNAB2, a gene linked to monosomy 1p36, results in associative memory impairments and amygdala hyperexcitability. J Neurosci. 2011;31:46–54. doi: 10.1523/JNEUROSCI.2634-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shapira SK, McCaskill C, Northrup H, Spikes AS, Elder FF, Sutton VR, Korenberg JR, Greenberg F, Shaffer LG. Chromosome 1p36 deletions: the clinical phenotype and molecular characterization of a common newly delineated syndrome. Am J Hum Genet. 1997;61:642–50. doi: 10.1086/515520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heilstedt HA, Burgess DL, Anderson AE, Chedrawi A, Tharp B, Lee O, Kashork CD, Starkey DE, Wu YQ, Noebels JL, Shaffer LG, Shapira SK. Loss of the potassium channel beta-subunit gene, KCNAB2, is associated with epilepsy in patients with 1p36 deletion syndrome. Epilepsia. 2001;42:1103–11. doi: 10.1046/j.1528-1157.2001.08801.x. [DOI] [PubMed] [Google Scholar]
- 80.Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, Chiu SY, Schwartzkroin PA, Messing A, Tempel BL. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–19. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- 81.Brew HM, Gittelman JX, Silverstein RS, Hanks TD, Demas VP, Robinson LC, Robbins CA, McKee-Johnson J, Chiu SY, Messing A, Tempel BL. Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. J Neurophysiol. 2007;98:1501–25. doi: 10.1152/jn.00640.2006. [DOI] [PubMed] [Google Scholar]
- 82.Gulbis JM, Mann S, MacKinnon R. Structure of a voltage-dependent K+ channel beta subunit. Cell. 1999;97:943–52. doi: 10.1016/s0092-8674(00)80805-3. [DOI] [PubMed] [Google Scholar]
- 83.Gulbis JM, Zhou M, Mann S, MacKinnon R. Structure of the cytoplasmic beta subunit-T1 assembly of voltage- dependent K+ channels. Science. 2000;289:123–7. doi: 10.1126/science.289.5476.123. [DOI] [PubMed] [Google Scholar]
- 84.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 85.Sewing S, Roeper J, Pongs O. Kv beta 1 subunit binding specific for shaker-related potassium channel alpha subunits. Neuron. 1996;16:455–463. doi: 10.1016/s0896-6273(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 86.Nagaya N, Papazian DM. Potassium channel alpha and beta subunits assemble in the endoplasmic reticulum. J. Biol. Chem. 1997;272:3022–3027. doi: 10.1074/jbc.272.5.3022. [DOI] [PubMed] [Google Scholar]
- 87.Yang JW, Vacher H, Park KS, Clark E, Trimmer JS. Trafficking-dependent phosphorylation of Kv1.2 regulates voltage-gated potassium channel cell surface expression. Proc Natl Acad Sci U S A. 2007;104:20055–60. doi: 10.1073/pnas.0708574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vacher H, Yang JW, Cerda O, Autillo-Touati A, Dargent B, Trimmer JS. Cdk-mediated phosphorylation of the Kv{beta}2 auxiliary subunit regulates Kv1 channel axonal targeting. J Cell Biol. 2011;192:813–24. doi: 10.1083/jcb.201007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O. Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co- localizations in rat brain. Eur J Neurosci. 1995;7:2189–2205. doi: 10.1111/j.1460-9568.1995.tb00641.x. [DOI] [PubMed] [Google Scholar]
- 90.Monaghan MM, Trimmer JS, Rhodes KJ. Experimental localization of Kv1 family voltage-gated K+ channel alpha and beta subunits in rat hippocampal formation. J Neurosci. 2001;21:5973–83. doi: 10.1523/JNEUROSCI.21-16-05973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nesti E, Everill B, Morielli AD. Endocytosis as a mechanism for tyrosine kinase-dependent suppression of a voltage-gated potassium channel. Mol Biol Cell. 2004;15:4073–88. doi: 10.1091/mbc.E03-11-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williams MR, Markey JC, Doczi MA, Morielli AD. An essential role for cortactin in the modulation of the potassium channel Kv1.2. Proc Natl Acad Sci U S A. 2007;104:17412–7. doi: 10.1073/pnas.0703865104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Connors EC, Ballif BA, Morielli AD. Homeostatic regulation of Kv1.2 potassium channel trafficking by cyclic AMP. J Biol Chem. 2008;283:3445–53. doi: 10.1074/jbc.M708875200. [DOI] [PubMed] [Google Scholar]
- 94.Johnson RP, El-Yazbi AF, Hughes MF, Schriemer DC, Walsh EJ, Walsh MP, Cole WC. Identification and functional characterization of protein kinase A-catalyzed phosphorylation of potassium channel Kv1.2 at serine 449. J Biol Chem. 2009;284:16562–74. doi: 10.1074/jbc.M109.010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X, Zhang J, Berkowski SM, Knowleg H, Chandramouly AB, Downens M, Prystowsky MB. Protein kinase C-mediated phosphorylation of Kv beta 2 in adult rat brain. Neurochem Res. 2004;29:1879–86. doi: 10.1023/b:nere.0000042215.92952.3d. [DOI] [PubMed] [Google Scholar]
- 96.Croci C, Brandstatter JH, Enz R. ZIP3, a new splice variant of the PKC-zeta-interacting protein family, binds to GABAC receptors, PKC-zeta, and Kv beta 2. J Biol Chem. 2003;278:6128–35. doi: 10.1074/jbc.M205162200. [DOI] [PubMed] [Google Scholar]
- 97.Gong J, Xu J, Bezanilla M, van Huizen R, Derin R, Li M. Differential stimulation of PKC phosphorylation of potassium channels by ZIP1 and ZIP2. Science. 1999;285:1565–9. doi: 10.1126/science.285.5433.1565. [DOI] [PubMed] [Google Scholar]
- 98.Gu C, Jan YN, Jan LY. A conserved domain in axonal targeting of Kv1 (Shaker) voltage-gated potassium channels. Science. 2003;301:646–9. doi: 10.1126/science.1086998. [DOI] [PubMed] [Google Scholar]
- 99.Gu C, Zhou W, Puthenveedu MA, Xu M, Jan YN, Jan LY. The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron. 2006;52:803–16. doi: 10.1016/j.neuron.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 100.Gu Y, Gu C. Dynamics of Kv1 channel transport in axons. PLoS One. 2010;5:e11931. doi: 10.1371/journal.pone.0011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rivera J, Chu PJ, Lewis TL, Jr., Arnold DB. The role of Kif5B in axonal localization of Kv1 K(+) channels. Eur J Neurosci. 2007;25:136–46. doi: 10.1111/j.1460-9568.2006.05277.x. [DOI] [PubMed] [Google Scholar]
- 102.Gu C, Gu Y. Clustering and activity tuning of Kv1 channels in myelinated hippocampal axons. J Biol Chem. 2011 doi: 10.1074/jbc.M111.219113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cachero TG, Morielli AD, Peralta EG. The small GTP-binding protein RhoA regulates a delayed rectifier potassium channel. Cell. 1998;93:1077–85. doi: 10.1016/s0092-8674(00)81212-x. [DOI] [PubMed] [Google Scholar]
- 104.Kwak YG, Hu N, Wei J, George AL, Jr., Grobaski TD, Tamkun MM, Murray KT. Protein kinase A phosphorylation alters Kvbeta1.3 subunit-mediated inactivation of the Kv1.5 potassium channel. J Biol Chem. 1999;274:13928–32. doi: 10.1074/jbc.274.20.13928. [DOI] [PubMed] [Google Scholar]
- 105.Williams CP, Hu N, Shen W, Mashburn AB, Murray KT. Modulation of the human Kv1.5 channel by protein kinase C activation: role of the Kvbeta1.2 subunit. J Pharmacol Exp Ther. 2002;302:545–50. doi: 10.1124/jpet.102.033357. [DOI] [PubMed] [Google Scholar]
- 106.Roeper J, Lorra C, Pongs O. Frequency-dependent inactivation of mammalian A-type K+ channel KV1.4 regulated by Ca2+/calmodulin-dependent protein kinase. J Neurosci. 1997;17:3379–91. doi: 10.1523/JNEUROSCI.17-10-03379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baek JH, Cerda O, Trimmer JS. Mass spectrometry-based phosphoproteomics reveals multisite phosphorylation on mammalian brain voltage-gated sodium and potassium channels. Semin Cell Dev Biol. 2011;22:153–9. doi: 10.1016/j.semcdb.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schulte U, Muller CS, Fakler B. Ion channels and their molecular environments--glimpses and insights from functional proteomics. Semin Cell Dev Biol. 2011;22:132–44. doi: 10.1016/j.semcdb.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 109.Marionneau C, Townsend RR, Nerbonne JM. Proteomic analysis highlights the molecular complexities of native Kv4 channel macromolecular complexes. Semin Cell Dev Biol. 2011;22:145–52. doi: 10.1016/j.semcdb.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lugo JN, Barnwell LF, Ren Y, Lee WL, Johnston LD, Kim R, Hrachovy RA, Sweatt JD, Anderson AE. Altered phosphorylation and localization of the A-type channel, Kv4.2 in status epilepticus. J Neurochem. 2008;106:1929–40. doi: 10.1111/j.1471-4159.2008.05508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klassen T, Davis C, Goldman A, Burgess D, Chen T, Wheeler D, McPherson J, Bourquin T, Lewis L, Villasana D, Morgan M, Muzny D, Gibbs R, Noebels J. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell. 2011;145:1036–48. doi: 10.1016/j.cell.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ryan DP, Ptacek LJ. Episodic neurological channelopathies. Neuron. 2010;68:282–92. doi: 10.1016/j.neuron.2010.10.008. [DOI] [PubMed] [Google Scholar]