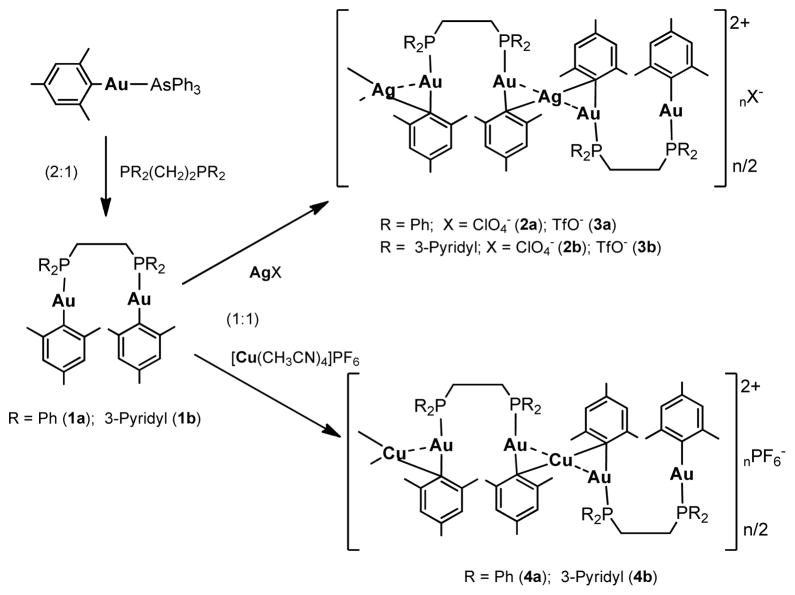
Abstract
The reaction of new dinuclear gold(I) organometallic complexes containing mesityl ligands and bridging bidentate phosphanes [Au2(mes)2(μ-LL)] (LL = dppe: 1,2-Bis(di-phenylphosphano)ethane 1a, and water-soluble dppy: 1,2-Bis(di-3-pyridylphosphano)ethane 1b) with Ag+ and Cu+ lead to the formation of a family of heterometallic clusters with mesityl bridging ligands of the general formula [Au2M(μ-mes)2(μ-LL)]A (M = Ag, A = ClO4−, L-L = dppe 2a, dppy 2b; M = Ag, A = SO3CF3−, L-L = dppe 3a, dppy 3b; M = Cu, A = PF6−, L-L = dppe 4a, dppy 4b). The new compounds were characterized by different spectroscopic techniques and mass spectrometry The crystal structures of [Au2(mes)2(μ-dppy)] 1b and [Au2Ag(μ-mes)2(μ-dppe)]SO3CF3 3a were determined by a single-crystal X-ray diffraction study. 3a in solid state is not a cyclic trinuclear Au2Ag derivative but it gives an open polymeric structure instead, with the {Au2(μ-dppe)} fragments “linked” by Ag(μ-mes)2 units. The very short distances of 2.7559(6) Å (Au-Ag) and 2.9229(8) Å (Au-Au) are indicative of gold-silver (metallophillic) and aurophilic interactions. A systematic study of their luminescence properties revealed that all compounds are brightly luminescent in solid state, at room temperature (RT) and at 77 K, or in frozen DMSO solutions with lifetimes in the microsecond range and probably due to the self-aggregation of [Au2M(μ-mes)2(μ-LL)]+ units (M= Ag or Cu; LL= dppe or dppy) into an extended chain structure, through Au-Au and/or Au-M metallophylic interactions, as that observed for 3a. In solid state the heterometallic Au2M complexes with dppe (2a–4a) show a shift of emission maxima (from ca. 430 to the range of 520–540 nm) as compared to the parent dinuclear organometallic product 1a while the complexes with dppy (2b–4b) display a more moderate shift (505 for 1b to a max of 563 nm for 4b).
More importantly, compound [Au2Ag(μ-mes)2(μ-dppy)]ClO4 2b resulted luminescent in diluted DMSO solution at room temperature. Previously reported compound [Au2Cl2(μ-LL)] (L-L dppy 5b) was also studied for comparative purposes. The antimicrobial activity of 1–5 and AgA (A= ClO4−, OSO2CF3−) against Gram-positive and Gram-negative bacteria and yeast was evaluated. Most tested compounds displayed moderate to high antibacterial activity while heteronuclear Au2M derivatives with dppe (2a–4a) were the more active (MIC 10 to 1 μg/mL). Compounds containing silver were ten times more active to Gram-negative bacteria than the parent dinuclear compound 1a or silver salts. Au2Ag compounds with dppy (2b, 3b) were also potent against fungi.
Keywords: luminescent, gold-silver, gold-copper, antimicrobial, synergism
Introduction
The photophysical and luminescent properties of closed-shell d10 gold(I) compounds have been widely investigated over the last decades.[1] The studies have focused on finding a correlation between the emission properties of polynuclear gold(I) derivatives and aurophilic interactions. Aurophilicity[2] or the weak Au(I)-Au(I) interaction displayed in most polynuclear gold(I) derivatives has been attributed to relativistic effects (increase in the effective nuclear charge when high-speed electrons are moving close to a heavy atomic nucleus).[2,3] This relativistic effect involves a contraction of the less-diffuse orbitals(s- and p- orbitals) and an expansion of the more difusse orbitals (d- and f- orbitals) and it reaches the maximum for gold.[2–4] The tendency in gold(I) to form metal-metal interactions with other Au(I) centers or other metals of similar charge (e.g. Ag(I), Cu(I), Tl(I)) is attributed to the sub-bonding interaction introduced through the stabilization of the filled 5d-orbital-based molecular orbitals with the empty molecular orbitals of appropriate symmetry derived from the 6s and 6p orbitals by configuration mixing.[1–3] Polynuclear homometallic gold(I) and heterometallic gold(I)-M (e.g. M = Ag(I), Cu(I), Tl(I)) derivatives constitute an important family of luminescent metal compounds.[1] The presence of gold in these derivatives enhances the spin-orbit coupling of the system, which in turn facilitates the access to triplet excited states by intersystem crossing. Relaxation of the triplet excited state by radioactive decay would usually result in phosphorescence with large Stokes shifts.[1a] Since the first reports[4,5] on the photoluminescence of [Au2(μ-dppm)2]2+, di and polynuclear gold(I) phosphane derivatives have been widely studied,[1,6] including organometallic compounds,[7] compounds with sulfur-[6g,n,8] and nitrogen-containing[9] ligands and chalcogenide centred gold derivatives.[10] In some cases the short gold(I)…gold(I) distances in these derivatives may not play a decisive role in determining the emission energy, as the auxiliary counter anion or solvent can dramatically affect their photophysical properties.[6h,g,m] Other luminescent polynuclear gold(I) complexes without phosphanes incorporating carbeniate,[11] sulfur ligands,[12] ylide,[13] stibinne,[14] and most recently N-heterocyclic carbene[15] ligands have been described. Examples of luminescent gold-containing polymers and gold nanoparticles are also known.[16]
Heterometallic di and polynuclear gold(I)-M (M = Ag, Cu) with[1c,17,18] and without[1c,19,20] phosphane ligands have also displayed interesting luminescent properties. These properties may be associated with different factors such as the nature of the ligand and the heterometal, or the presence or absence of metallophilic interactions. In general for Au-M (M = Ag, Cu) compounds with weak Au-M interactions (not clusters) the metallophilic interactions are mainly responsible for the photophysical properties observed. The number of polynuclear homometallic[4,6a,c,f,m–p,r,7b,c,e,f,8b,f,9a,c,11,14,15] or heterometallic[1c,17b–e,h,18b,c,19a,b,d,f,h,j,l,20c] gold(I) compounds luminescent in solution at room temperature is more limited. Most of these complexes are only brightly emissive in the solid state at room temperature.[e.g: 1a,6h] The measurements in solution are mostly performed in degassed solvents like CH2Cl2, CHCl3, CH3CN, Me2CO or THF. Examples of polynuclear gold(I) compounds luminescent in DMSO or aqueous solution at RT are limited to [Au2(μ-G).(μ-dmpe)](KBr)0.75.2H2O (G = guaninato dianion, dmpe = 1,2-bis(dimethylphosphano)ethane)[9b] and some N-heterocyclic dinuclear gold(I) carbene compounds.[15b] Interestingly, some of these carbene gold(I) derivatives which also displayed potential antitumor properties (targeting mitochondria) were used in luminescence studies of intracellular distribution.[15b] More recently, Au-Ag alkynyl phosphane aggregates (encapsulated by silica nanoparticles) which exhibit intense phosphorescence free from oxygen quenching, have been applied in two-photon imaging in human mesenchymal stem cells.[17b]
Gold(I)[21] and silver(I)[22] derivatives have been studied in the last few decades for their potential applications in medicine. While silver(I) derivatives are used mainly as antibacterial agents,[22,23] gold(I) compounds (especially those containing thiolates and phosphanes) have been used in the treatment of rheumatoid arthritis.[21a] More recently, gold(I)-phosphane and carbene complexes have been studied as potential antitumor,[15,24] antiparasitic[25] and antimicrobial agents.[26,27] Silver carbene derivatives have also displayed high activity against selected tumor cancer cells in vitro[27] and Gram-positive and Gram-negative bacteria.[27,28] It is however surprising that the biological activity of heterometallic gold-silver derivatives has been scarcely investigated[29] when a synergistic or cooperative effect of the two metals (as described for other heterometallic systems like Ti-Ru or Ti-Au in anticancer therapy)[30] could be anticipated.
We report here on the preparation of organo-heterometallic derivatives with mesityl and bis-phosphane bridging ligands of the general formula [Au2M(μ-mes)2(μ-LL)]A (M = Ag, A = ClO4−, L-L = dppe 2a, dppy 2b; M = Ag, A = SO3CF3−, L-L = dppe 3a, dppy 3b; M = Cu, A = PF6−, L-L = dppe 4a, dppy 4b) (Scheme 1) from new organometallic dinuclear complexes [Au2(μ-mes)2(μ-LL)] (L-L = dppe 1a, dppy 1b). These compounds display very short Au-Au and Au-M distances that imply metallophylic interactions and, as a result, are luminescent in solid state at room temperature and at 77 K, or in frozen solutions. The compounds seem to aggregate into polymers (Scheme 1) in the solid state or concentrated solutions. The compounds are soluble in DMSO and mixtures of DMSO /H2O and their antimicrobial activity against Gram-positive and Gram-negative bacteria and yeast has been evaluated. Most tested compounds display moderate to high antibacterial activity while heteronuclear Au2M derivatives with dppe (2a-4a) are the more active (MIC 10 to 1 μg/mL) and more active than the dinuclear Au2 parent compound (1a) against Gram-negative bacteria. Compounds containing silver, Au2Ag (2a, 3a) are also more active than silver salts (AgX; X = ClO4−, OSO2CF3−) against both Gram-negative and Gram-positive bacteria. Au2Ag compounds with dppy (2b, 3b) are also potent against fungi. Compound 2b is luminescent in diluted DMSO solutions at room temperature and could potentially be used in the future in studies of fluorescence microscopy to track these types of derivatives inside yeast or mammalian cells.
Scheme 1.
Preparation of the Di and Polynuclear Organometallic Gold(I)-M (Au2, {Au2Ag}n and {Au2Cu}n) Compounds.
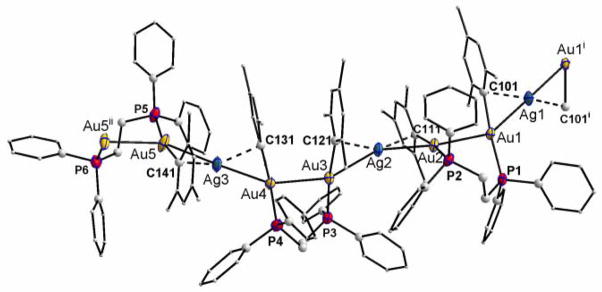
Figure 1.
Structure of the polymeric cation [(μ-Ag){Au2(μ-mes)2(μ-dppe)}]+n in compound 3a.
Results and Discussion
1. Chemistry and characterization
Some years back we and others reported on the use of mesityl gold complexes of the type [Au(mes)L] (L = AsPh3, PPh3) as precursors to polynuclear homo and heterometallic gold(I) derivatives.[31–34] The mesityl group (2,4,6-Me3C6H2) can act as a terminal ligand or as a bridge between two or more metal centres, affording different bonding modes (the most common one a three-centre-two-electron bond). Thus dinuclear Au2,[35] Au-Ag[31] and trinuclear Au2M (M = Ag, Cu)[32] gold(I) derivatives with mesityl bridging ligands were prepared by addition of weakly coordinated gold, silver or copper compounds (such as Ag(OSO2CF3), Ag(OClO3), [{Au(PPh3)}2(μ-Cl)2]ClO4 or [Cu(CNMe)4]PF6 to derivatives of the type [Au(mes)L] or [Ag(mes)]4.[35] Some of the compounds were structurally characterized and displayed supported metal-metal interactions (some of them can be considered formally as metal-metal bonds). The cluster [Au(mes)]5 was prepared by different ways[33,35–37] and addition of Ag+ and Cu+ ions to this and the related cluster [Au(trip)]6 (trip = 2,4,6-{(CH3)CH]3C6H2))[34] resulted in heterometallic gold(I) compounds with very interesting structural features and unsupported Au-Ag bonds like that of [Au6Ag(trip)6](CF3SO3).[34] More recently, the addition of silver perfluorocarboxylates to [Au(mes)]5 afforded heterometallic complexes of the type [AuAg4(RCO2)4(tht)x]n with supported Au…Ag interactions and the mesityl ligands bridging 3 centers in an unprecedented situation.[38] We also prepared Au(I) mesityl derivatives with the bidentate phosphane dppm (1,2-Bis(diphenylphosphano)methane)[31] and dppe (1,2-Bis(diphenylphosphano)ethane).[39] In the case of dppm we obtained the mononuclear derivative [Au(mes)(dppm)] which was used as a precursor to Au-Ag compounds with terminal mesityl and bridging dppm which did not display formal Au-Ag bonds.[40] In the case of dppe dinuclear [Au2(mes) 2(μ-dppe)] 1a was obtained instead.[39] The addition of AgA (A = OSO2CF3; OClO3) or [Cu(CNMe)4]PF6 to 1a lead to the formation of heterometallic compounds (Scheme 1) with mesityl bridging ligands of the general formula [Au2M(μ-mes)}2(μ-dppe)]A (M = Ag, A = ClO4−, 2a; A = SO3CF3−, 3a; M = Cu, A = PF6−, 4a). These cationic air-stable heterometallic compounds are soluble in DMSO and could potentially be used in biological studies. Besides, we noticed that they are brightly luminescent in the solid state under a common vis-UV lamp. Although the synthesis and partial characterization of 1a-4a had been described before by one of us,[39] the results were never published and crystallographic, luminescence and biological studies of these complexes had not been undertaken.
Complexes 1a–4a are air- and moisture-stable white (1a) or yellow solids (2a–4a). Acetone solutions of cationic compounds 2a–4a display conductivities typical of 1:1 electrolytes. The IR spectra show absorptions arising from the anions ClO4− (2a) at 1088 (br, vs), 623 (s) cm−1, CF3SO3− (3a) at 1262 (br), 1221 (s), 1154 (s) cm−1 and PF6− (4a) at 839 (br, vs) cm−1. The 31P{1H} NMR (CDCl3) of 1a shows a singlet at 42.5 ppm. In 2a (44.9 ppm), 3a (45.2 ppm) and 4a (44.0 ppm) the signal is down-field displaced from that of 1a as in other polynuclear gold phosphane complexes with mesityl bridges. The single signal indicates that all phosphorous in the molecule are chemically equivalent. The 1H NMR spectra of 2a–4a show three singlets for the mesityl ligands, slightly displaced from the resonances of the starting material 1a, and in a consistent ratio with the phenyl and methylene resonances of the ancillary ligands. There is only one type of mesityl ligand for every compound. The mass spectra (FAB+) for 1a does not show the parent peak but signals that can be assigned to polynuclear fragments of similar and higher molecular weight could be observed instead: [Au2(mes)(dppe)]+ [M – mes]+ at m/z = 911 (100%) and [Au3(mes)2(dppe)]+ [M + Au]+ at m/z = 1227 (42%). This is an indication that, the preparation of trinuclear derivatives from 1a, is feasible. The mass spectra (FAB+) show, for all the heterometallic compounds, the ion peak [M – A]+ with 100% intensity at m/z 1136 (2a, 3a) and at m/z 1092 (4a).
The structure of compound 3a has been determined by an X-ray diffraction study (Figure 1); selected bond lengths and angles are given in table 1.
Table 1.
Selected Bond lengths [Å] and angles [o] for 3a.[a]
| Au(1)-C(101) | 2.098(15) | Au(4)-P(4) | 2.295(4) |
| Au(1)-P(1) | 2.301(4) | Au(4)-Ag(3) | 2.8506(13) |
| Au(1)-Ag(1) | 2.7560(6) | Au(5)-C(141) | 2.086(15) |
| Au(1)-Au(2) | 2.9228(8) | Au(5)-P(5) | 2.306(9) |
| Au(2)-C(111) | 2.081(13) | Au(5)-Ag(3) | 2.8024(13) |
| Au(2)-P(2) | 2.300(4) | Au(5)-Au(5)#1 | 2.9951(12) |
| Au(2)-Ag(2) | 2.7807(12) | Ag(1)-C(101) | 2.312(13) |
| Au(3)-C(121) | 2.069(14) | Ag(1)-Au(1)#2 | 2.7560(6) |
| Au(3)-P(3) | 2.296(4) | Ag(2)-C(111) | 2.252(14) |
| Au(3)-Ag(2) | 2.7619(13) | Ag(2)-C(121) | 2.257(14) |
| Au(3)-Au(4) | 2.9226(8) | Ag(3)-C(131) | 2.339(13) |
| C(101)-Au(1)-P(1) | 174.0(4) | Ag(2)-Au(2)-Au(1) | 149.13(3) |
| C(101)-Au(1)-Ag(1) | 54.9(4) | C(121)-Au(3)-P(3) | 172.9(4) |
| P(1)-Au(1)-Ag(1) | 119.16(9) | C(121)-Au(3)-Ag(2) | 53.4(4) |
| C(101)-Au(1)-Au(2) | 95.9(4) | P(3)-Au(3)-Ag(2) | 119.66(10) |
| P(1)-Au(1)-Au(2) | 89.95(9) | C(121)-Au(3)-Au(4) | 97.1(4) |
| Ag(1)-Au(1)-Au(2) | 150.72(3) | P(3)-Au(3)-Au(4) | 89.85(10) |
| C(111)-Au(2)-P(2) | 171.5(4) | Ag(2)-Au(3)-Au(4) | 150.49(4) |
| C(111)-Au(2)-Ag(2) | 52.8(4) | C(131)-Au(4)-P(4) | 174.3(4) |
| P(2)-Au(2)-Ag(2) | 118.72(10) | C(131)-Au(4)-Ag(3) | 53.9(4) |
| C(111)-Au(2)-Au(1) | 96.5(4) | P(4)-Au(4)-Ag(3) | 122.15(11) |
| C(111)-Au(2)-Au(1) | 96.5(4) | C(131)-Au(4)-Au(3) | 93.9(3) |
| P(2)-Au(2)-Au(1) | 91.90(10) | P(4)-Au(4)-Au(3) | 90.83(10) |
Symmetry transformations used to generate equivalent atoms: #1 −x+2,y,−z+3/2 #2 −x+1,y,−z+1/2
The crystal structure of 3a consists of an “open” polymer of the type [(μ-Ag){Au2(μ-mes)2(μ-dppe)}]n(CF3SO3)n·1.6n(H2O). Crystals of 3a were highly unstable when removed from their mother liquor. The structure was solved by direct methods (more details in the SI section), which revealed the positions of the heavy atoms and of a subset of the C, O and F sites.
The remainder of the structure was located and refined in an alternating series of least-squares refinements and difference Fourier maps. The crystallographic asymmetric unit was found to include 2.5 units of the building block of the cationic polymer, [(μ-Ag){Au2(mes)2(μ-dppe)}]+, 2.5 triflate anions, CF3SO3−, and six water sites, of which four were assigned occupancies of 0.5. The section of the complex polymer in the chosen asymmetric unit is bounded at one end by a silver atom, Ag1, located on a crystallographic two-fold axis at (0.25, y, 0.5). Beginning at this point, the chain extends with two gold atoms, Au1 and Au2, each of which is ligated by one mesityl ligand and one P atom of a dppe ligand that bridges the two gold centers. The basic three-metal unit, Ag…Au…Au, is repeated (Ag2, Au3, Au4) with all three metal centers on general positions. Finally, the part of the chain in the asymmetric unit ends with Ag3 and Au5, which belong to a third link whose Au5…Au5ii component straddles a crystallographic two-fold axis at (0.75, y, 1.0). The dppe ligand that bridges the Au5…Au5ii unit is disordered about the two-fold axis. For the asymmetric unit we chose a chemically connected dppe, of which one P atom, P5, is bonded to Au5 with no symmetry applied and P6 is bonded to Au5ii (ii: 1.5-x, y, 2-z). The atoms of this dppe ligand, P5, P6 and C53 through C78, were assigned occupancies of 0.5 in line with the two-fold disorder. Two of the triflate sites were found to be fully occupied and the third, which includes atoms S3 and C153, was found near a crystallographic two-fold axis at (0.5, y, 0.25) and was assigned occupancy of 0.5. Six single-atom sites were located in intermolecular space and modeled as water sites. Based on considerations of the refined displacement parameters and chemically unreasonable contacts, four of these sites were refined with occupancies fixed at 0.5. These may not represent the exact stoichiometry of the crystal, and indeed we cannot guarantee that the stoichiometry remained constant in the brief time between when the highly unstable sample was removed from its mother liquor and when it was placed in the cold stream of the diffractometer.
Every silver center is bonded to the ipso carbon atoms of the mesityl groups and also bridges two {Au2(μ-dppe)} fragments with an Ag-Au distance which ranges from 2.7560(6) to 2.8506(13) Å (Table 1). The shorter distances (ca. 2.75 to 2.78 Å) are of the same order as those found in complexes with formal supported silver-gold bonds,[41] especially in the most closely related example with mesityl ligands [{Au(μ-mes)AsPh3}2Ag](ClO4)[32] (2.7758(8) Å). The longer distances Ag-Au found in 3a of 2.80 to 2.85 Å are of the same order of distances found in complexes where a formally nonbonding Ag….Au interaction has been proposed like in related mesityl complexes such as [{(Ph3P)Au(μ-mes)Ag(tht)}2](SO3CF3)2 [2.8245(6) Å][31] or [AuAg4(mes)(RCO2)4(tht)x]n (x = 1, R = CF3, CF2CF3, x = 3, CF2CF3)[38] which range from 2.8140(8) to 3.0782(6) Å (depending on the carboxylate). In some of these latter complexes one mesityl ligand is bridging one Au and two silver centers[38] and this is one of the reasons the Ag-Au distances are considerably longer. Thus, we can postulate appreciable silver-gold bonding interactions in 3a. In general the distances Ag-Au in compounds with supported silver-gold interactions are longer than those with unsupported ones and usually the derivatives with those supported gold-silver interactions do not display luminescence attributable to the metallophilic interactions. The distances Au-Au in 3a of 2.9226(8) and 2.9228(8) Å are quite short indicating a strong aurophilic interaction.[42] Similar and mostly longer distances have been found in luminescent polynuclear gold(I) derivatives with bis-phosphanes like [Au2(dppm)2]2+ (2.931(1)–2.962(1) Å depending on the counter ion),[5] [Au2(dmpe)2]2+ (dmpe = bis(dimethylphosphano)ethane; 2.9265(5)-2.974(3) Å depending on the counter ion),[6r] [Au3(dmmp)2]3+ (dmmp = bis(dimethylphosphanomethyl)methylphosphane; 2.962(1) and 2.981(1) Å),[6p] [Au2(dpephos)]2+ (dpephos: bis-(2-diphenylphosphano)phenylether); 2.9764(13)-3.0038 (6) Å depending on the counter ion),[6f] [Au2(xantphos)Cl2] (xantphos = 9,9-dimethyl-4,5-bis(diphenylphosphano)xanthene; 2.9947(4) Å), [6a] or [m-C6H4(OCH2CCAu)2(μ-dppm)] (3.049(1) Å).[7d] The Au2Ag derivatives described here (2a,b; 3a,b) which display quite short Ag-Au and Au-Au distances (as demonstrated for 3a) are pale yellow and brightly yellow emissive in solid state as described next. Gold atoms are in almost linear environments. The M-C bond lengths (Au-C distances range from 2.069(14) to 2.098(15) Å and Ag-C from 2.252(14) to 2.368(14) Å) are similar to those found in the mesityl heterometallic complexes mentioned above.[31,32,38]
We prepared the analogue di- (1b) and trinuclear (2b–4b) mesityl organometallic gold compounds with water soluble diphosphane dppy: 1,2-Bis(di-3-pyridylphosphano)ethane (Scheme 1). All complexes are air- and moisture-stable white (1b), pale yellow (2b–3b) or green solids (4b) which crystallize with molecules of water (see experimental). The heterometallic complexes 2b–4b are not soluble in CHCl3 or CH2Cl2 but they are soluble in CH3CN and DMSO. CH3CN solutions of cationic compounds 2b–4b display conductivities typical of 1:1 electrolytes. The IR spectra show absorptions arising from the anions ClO4− (2b) at 1082 (br, vs), 616(s) cm−1, CF3SO3-− (3b) at 1257 (br,vs), 1158 (m) cm−1 and PF6− (4b) at 839 (br, vs) cm−1. The 31P{1H} NMR (CDCN3) of 1b shows a singlet at 34.2 ppm. In 2b (32.9 ppm), 3b (32.9 ppm) and 4b (33.9 ppm) the broad signals are high-field displaced from that of 1b. However, in the 1H NMR spectra in CH3CN of 2b–4b the three singlets for the mesityl ligands are slightly displaced from the resonances of the starting material 1b (like in other heterometallic complexes with bridging mesityl ligands) and in a consistent ratio with the pyridyl and methylene resonances of the ancillary ligands. Like in the previous case only one type of phosphorous atom is observed in the 31P{1H} NMR spectra and only one type of mesityl ligand in the 1H NMR spectra for compounds 1b–4b in CH3CN. More detailed NMR experiments of these complexes are explained later on. The mass spectra (ESI+) for 1b shows the parent peak [M] at m/z: 1057 [100%] as well as signals that can be assigned to polynuclear fragments of similar molecular weight like: [Au2(mes)(dppy)]+ [M – mes]+ at m/z = 915 (14%). The mass spectra (ESI+) show, for all the heterometallic compounds the ion peak [M – A]+ with 100% intensity at m/z 1141 (2b, 3b) and at m/z 1097 (4b).
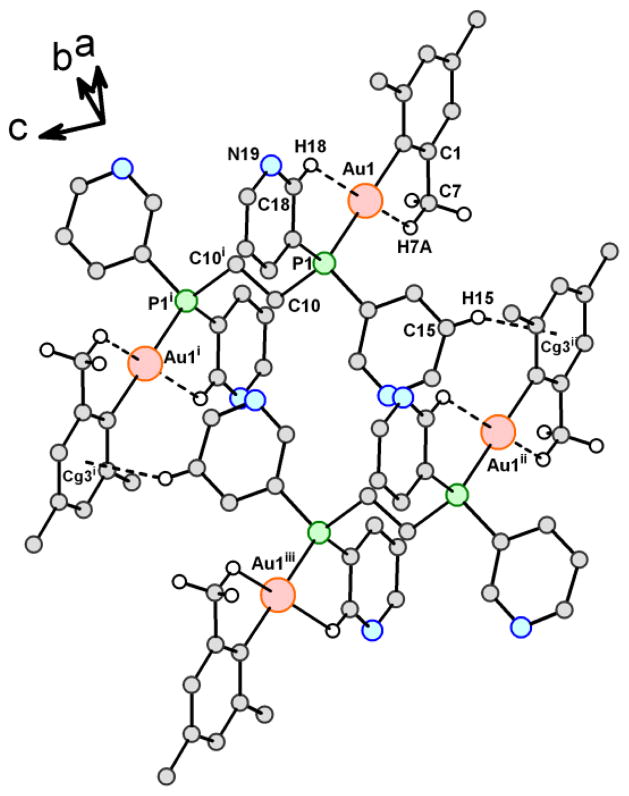
The structure of compound 1b has been determined by an X-ray diffraction study (Figure 2); selected bond lengths and angles are given in the legend of figure 2. The molecule is a dimer with two symmetric units (see Figure 2). The gold atoms are in a nearly linear environment (C(1)-Au(1)-P(1) 178.0(1)) and the distance Au-C is similar to the distances in other compounds where the mesityl group acts as a terminal ligand like in [Ag(μ-dppm)2{Au(mes)2}]ClO4 (2.083(10) and 2.080(9) Å).40 The distance Au-P is quite common for Au(I)-phosphane derivatives (including dinuclear complexes with bisphosphanes) and does not deserve further comment. No intra- or intermolecular gold-gold interactions are observed. Since the molecular conformation would be expected to be flexible, with unhindered rotation possible about either the Au1---P1 or Au1---C1 bond, it is pertinent to ask what factors contribute to the observed centric linear molecular topology, in which the torsion angle Au1…P1…P1′…Au1′ is 180° but in which the planes of the mesityl ligand and one of the pyridyl groups are parallel to each other to give a torsion angle C6---C1…P1---C17 of 2.9°. The extended topology mitigates for relief of steric effects, while the parallel orientations of the two rings brings the methyl group at C9 as close as possible to the C18---H18 bond of the pyridyl ring. We can identify both intra- and intermolecular interactions that serve to explain these observations.
Figure 2.
View of the structure of compound 1b (2 molecules) showing: a) the interactions of Au atoms and the H of a CH3 group from one mesityl ligand and an H from a pyridyl ring of the dppy ligand and b) One of the four C---H…π contacts in this molecule (two molecules of 1b, with complementary C15---H15…Cg3 contacts drawn as dashed lines. Symmetry codes (i): (-x, 1-y, 2-z); (ii): (x, −1+y, z); (iii): (−x, −y, 2-z)). Selected distances: Au(1)-C(1) 2.067(4); Au(1)-P(1) 2.295(1) Å. Selected angles: C(1)-Au(1)-P(1) 178.0(1); C(1)-Au(1)-C(2) 122.1 (3); C(1)-Au(1)-C(6) 119.5(3)°.
As the positions of hydrogen atoms are important to this discussion, we point out here that, while all H atoms were located at calculated positions and refined as riding atoms (with free rotation for the methyl groups), the final locations of the relevant H atoms were confirmed to be correct through the use of omit maps – difference Fourier maps in which the contribution of the atom or atoms in question is omitted from F(calc). Contoured maps were made for the two unique pyridyl rings to confirm their correct orientations, and for the methyl groups at C7 and C9. Most relevant is that the methyl group at C7 was thus confirmed to be correctly oriented. With that orientation, H7A makes its closest possible approach to Au1, 2.71 Å (no standard uncertainty is provided, since the local C---H distance was constrained during refinement). On the opposite side of Au1, H18 of the pyridyl ring that is parallel to the mesityl ligand, and the position of which is not in doubt, makes its closest possible approach to Au1, 2.89 Å. The observed molecular conformation brings the methyl group at C9 as close as possible to the C18---H18 unit of the nearby pyridyl group, although the H…H contacts thus formed are both greater than 2.6 Å and thus not indicative of steric problems. We note that an omit map for the methyl group at C9 showed that the H atoms are located at positions between two closely-spaced low maxima, with one H parallel to the plane of the mesityl phenyl ring but distal to Au1, and with the other two H atoms thus straddling the extended pyridyl plane containing the neighboring C18---H18 bond.
Whether the two Au1…H contacts are important in establishing the molecular conformation observed in the crystal is open to discussion, but these contacts must serve as stabilizing influences. The recent unambiguous observation by neutron diffraction of an O---H…Pt hydrogen bond with an H…Pt distance of 2.885(3) Å[43] demonstrated that such interactions are bona fide hydrogen bonds and are potentially important in molecular solids.[44] As for intermolecular contacts in this Van der Waals’ solid, the shortest ring…ring contact has a Cg…Cg distance of 4.695(3) Å; and we conclude that these are of no structure-directing importance. There are four C---H…π contacts, of which one has clearly a stablizing influence; one is within the limits normally considered for establishing the potential importance of such contacts, and two are borderline and of dubious importance (see Table in SI).
The nitrogen atoms in the pyridyl groups of the dppy phosphane could potentially coordinate metallic centers when 1b interacts with silver or copper salts. In the IR spectra of the Au2Ag complexes (2b and 3b) in solid state we observe broad signals at ca. 1640 cm−1 that seem to correspond to the coordination of metal fragments to the nitrogen of the 3-pyridyl group. This band appears in the IR spectra of the free phosphane dppy at 1568 cm−1 and in compound 1b (both as solids) at 1571 cm−1. In the IR spectra (of the solid 2b and 3b) we observe a broader band at 1576 (2b) or 1570 cm−1 (3b) and a new broad band at 1642 cm−1 for both complexes. Both similar bands are observed for the Au2Cu complex (4b), with that at 1575 cm−1 being weaker. The presence of these two bands could indicate the existence of two types of pyridyl groups in these compounds, with or without coordination to the metallic centers. Similar displacement of the pyridine band has been described in cyclo- and polyphosphazene with pyridine side groups after the coordination of the nitrogen atom to Au and Ag centers.[45] In our case we believe that these N-Ag or N-Cu interactions do not occur in solution since we do not observe a change in the 1H NMR signals of the protons from the pyridyl ring and we just observe one signal in the 31P{1H} NMR spectra for the compounds (in CD3CN or d6-DMSO). This signal is, however quite broad (as opposed to the sharp signal for 1b) which may indicate a fluxional behavior of the compounds at RT. These complexes (2b–4b) are not soluble in deuterated CHCl3, CH2Cl2, acetone, MeOH or THF. The low temperature 31P{1H} NMR for these complexes in CD3CN only displays one broad signal. Unfortunately with acetonitrile we only could get reliable data up to −35 °C due to the relatively high freezing point of this solvent. It is very reasonable to assume than in solution the polar solvent CH3CN or DMSO (media where the heterometallic complexes are soluble) may facilitate the metal decoordination to the nitrogen atom. A similar effect has been described for heterocyclic carbene silver compounds containing pendant pyridyl groups. In this case the interactions of the Ag atoms with the N of the pendant pyridyl groups are lost in CD3CN solutions.[19i]
In order to obtain more information we performed an X-ray analysis of crystals obtained while crystallizing compound 2b in a dichloromethane-acetone-DMSO solution by slow diffusion of n-hexane at −5°C. We have to mention that the crystals obtained (2c) are colorless and non luminescent, as opposed to compound 2b itself which is pale yellow and brightly yellow luminescent at RT in solid state and in solution. In the crystallization tube a yellow, luminescent non crystalline powder was also observed which may correspond to compound 2b, whose IR spectrum and powder-diffractogram are different to those of compound 2c, as it will be mentioned later.
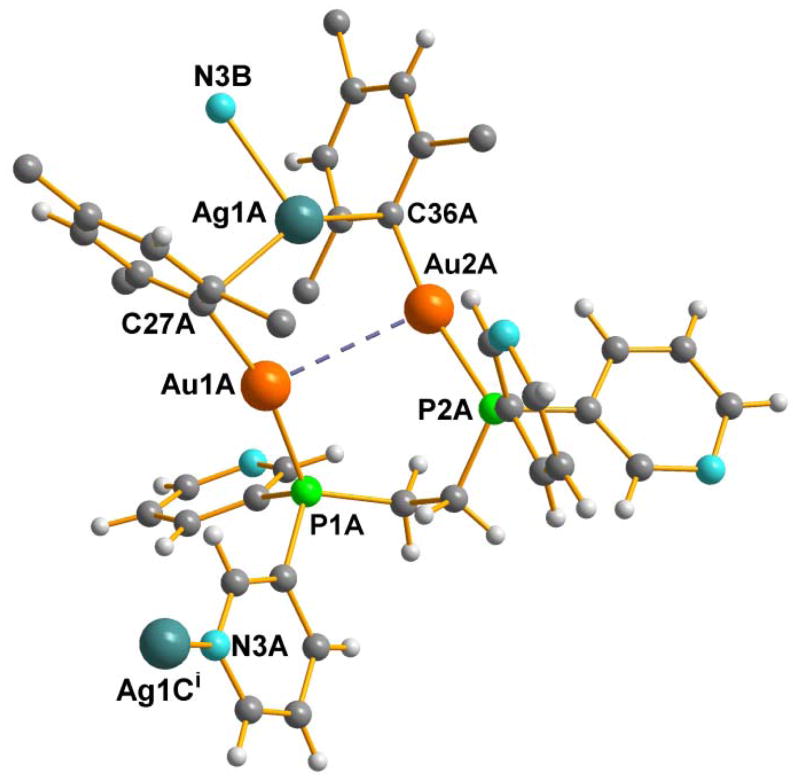
The structure of 2c was analyzed by single-crystal diffraction; and while the quality of the data permitted an unambiguous determination of the connectivity and general geometrical parameters, low resolution and weak diffraction prevented a full anisotropic refinement. A detailed description of the analysis and of the results is given in the supplementary material. The basic unit of the cationic polymer is a trinuclear (AgAu2) fragment of formula [AgAu2(μ-mes)2(μ-dppy)], which crystallizes with one equivalent of perchlorate, ClO4− and two equivalents of DMSO. But the crystallographic asymmetric unit is comprised of three links of the polymer chain along with the corresponding three units of ClO4− and 6 DMSO sites. One of this individual units is depicted in Figure 3. The individual units of the polymer, labeled “A,” “B,” and “C” in the atoms list, are shown in a figure in the SI. The A unit has a significantly shorter Au1…Au2 distance [Au1A…Au2A 3.3079(9) Å] than do the B and C units [Au1{B,C}…Au2{B,C} 3.4533(10), 3.4720(9) Å]. Within the asymmetric unit, the trinuclear core of the B fragment is rotated roughly 180° about the crystallographic c-axis with respect to the orientation of the A and C fragments. The latter are roughly parallel, although the difference in the Au1…Au2 distances between the A and C fragments ameliorates the severity of the pseudo-symmetry to at least some extent. The distances Au-Ag range from 2.822(1) to 2.998(1) Å with one Ag coordinated to two Au atoms in every individual unit (in unit A: Au1-Ag= 2.998(1) Å, Au2-Ag = 2.917(1) Å; in unit B: Au1-Ag= 2.933(1) Å, Au2-Ag = 2.917(1) Å; in unit C: Au1-Ag= 2.931(1) Å, Au2-Ag = 2.822(1) Å). These distances are similar or longer than the longest distances Ag-Au found in 3a of 2.80 to 2.85 Å. As commented before these values are of the same order of the distances found in complexes where a formally nonbonding Ag….Au interaction has been proposed. The Au-Au distances found in this structure are long enough for not being considered aurophilic interactions. The lack of metallophilic interactions may be the reason why these colorless crystals are not luminescent. There are two mesityl ligands acting as bridges between the Ag and the two Au atoms in every unit and the distances M-C are similar to those found in mesityl heterometallic complexes described before.[31,32,38] Importantly, in this case the silver atom of every unit is coordinated to the nitrogen atom of one pyridyl group of the dppy ligand of another unit (see Figure 3 and figures in the SI).
Figure 3.
One link of the polymeric cation {[Au2Ag(μ-mes)2(μ-dppy)]n}(n+), from compound 2c. The chain is propagated at Ag1A---N3B and at N3A---Ag1Ci. Symmetry code i: (1-x,-y,-0.5+z).
It seems plausible from the structure of 2c that the core {Au2Ag(μ-mes)2(μ–dppy)}+ is the same as in compound 2b in solid state and in solution. However, and as mentioned before, for 2b in solution an interaction between nitrogen atoms of the pyridyl groups and silver atoms is not observed.
For luminescent 2b in the solid state, it seems more reasonable that the compound may be a polymer which displays shorter Au-Au and/or Au-Ag interactions (as in 3a) as well as the coordination of Ag atoms to one or more N atoms from the pyridyl groups (as indicated by the IR spectrum of 2b and 3b). In fact, the powder-diffractogram of the luminescent and yellow compound 2b indicates that its structure is completely different to that of 2c (see supporting information). The IR spectrum of 2c is similar to that of 2b, (although it is not exactly the same) showing also a band at ca. 1654 cm−1 due to the coordination of the silver to the nitrogen of the 3-pyridyl group. Unfortunately we have not been able to obtain crystals of enough quality from 2b to assess their structure in the solid state. In the crystallization attempts to obtain monocrystals of 2b, white crystals of 2c are always obtained instead. What it seems clear is that in all these luminescent heterometallic complexes the mesityl is acting as a bridging ligand between the gold and other metal (Ag or Cu) centers in a 3c-2e− fashion and that the units {Au2M(μ-mes)2(μ–LL)} may assemble in the space through metallophilic interactions (Scheme 1). The compounds with dppy have the possibility of coordination of one or more nitrogen atoms to the silver or copper atom in the solid state. Different counterions and solvents may have an effect also in the structure in the solid state as they can also coordinate to the second metallic center. This effects may be the reason for differences found in the luminescence studies between compounds with dppe and dppy (a versus b) and even, in compounds with ddpy with different counterions (2b and 3b).
2. Luminescence Studies
We have studied the luminescence of all new compounds 1–4. Previously reported compound [Au2Cl2(μ-L-L)] (L-L = dppy 5b[46]) was also studied for comparative purposes. The luminescence of 5a (L-L = dppe[47]) in solid state at RT[8g,9a] had been reported before (data in Table 3).
Table 3.
Luminescent Spectral Data and Lifetime Measurement for the Compounds in Solid State and Solution.
| Compound | λmax, Solid (298 K) | λmax, Solid (77 K) | λmax, Solution (298 K) (DMSO)[a] | λmax, Solution (77 K) (DMSO)[b] | ||||
|---|---|---|---|---|---|---|---|---|
| λexc | λem [τ (μs)] | λexc | λem [τ(μs)] | λexc | λem [τ(μs)] | λexc | λem [τ (μs)] | |
| 5a [Au2Cl2(μ-dppe)] | 325[c] | 480[c] | -- | --- | ||||
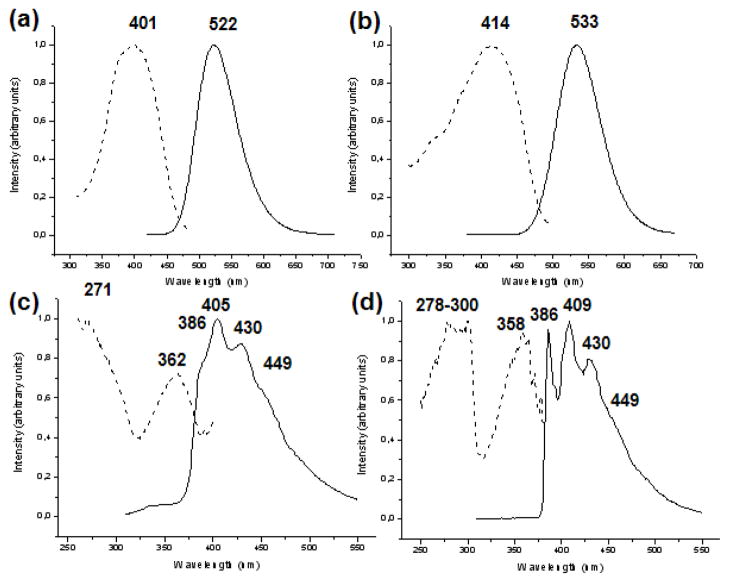
| 1a [Au2(mes)2(μ-dppe)] | 271 362 |
386 sh, 405[13], 430 [11], 449 sh | 278–300 358 |
386 [37],409[43], 430 [57], 449 sh | -- | --- | ||
| 2a [Au2Ag(μ-mes)2(μ-dppe)]ClO4 | 401 | 522 [18] | 381 | 527 [17] | -- | --- | 370 | 497 [15] |
| 3a [Au2Ag(μ-mes)2(μ-dppe)]SO3CF3 | 408 | 523 [15] | 386 | 527 [16] | -- | --- | 362 | 480 [18], 500 sh |
| 4a [Au2Cu(μ-mes)2(μ-dppe)]PF6 | 414 | 533 [12] | 405 | 546 [13] | -- | --- | 370 | 505 [16] |
| 5b [Au2Cl2(μ-dppy)] | 314 | 470 [7] | 327 | 447 [11] | -- | --- | 328 | 443 [11] |
| 1b [Au2(mes)2(μ-dppy)] | 415 | 505 [14] | 375 | 438 [44], 494 [13] | -- | --- | 347 | 426 [10] |
| 2b [Au2Ag(μ-mes)2(μ-dppy)]ClO4 | 315 398 |
359 [10], 520[16] 520 |
390 | 522 [12] | 339 | 524 [9] | 385 | 511 [19] |
| 3b [Au2Ag(μ-mes)2(μ-dppy)]SO3CF3 | 394 | 518 [14] | 388 | 518 [14] | -- | --- | 380 | 512 [16] |
| 4b [Au2Cu(μ-mes)2(μ-dppy)]PF6 | 440 | 563 [9] | 370 | 522 sh, 595 [10] | -- | --- | 367 | 482 [11] |
Data of wavelength are given in nm.
Data using DMSO as solvent at room temperature, 5 × 10−4 M.
Data in frozen solutions of DMSO, 5 × 10−4 M.
Data taken from reference [8g].
All new complexes are luminescent both at room temperature and at 77 K in solid state and in frozen solutions. Although complexes 2b and 3b are brightly luminescent under a common UV lamp (ex 365 nm) in concentrated solutions at RT, only 2b luminesces in a diluted DMSO solution (5 × 10−4 M) at RT. The excitation and emission data as well as the lifetimes for excited states are summarized in Table 3. The lifetimes are all relatively long (7–57 μs), which indicate the emission transitions are all forbidden and phosphorescent. The free phosphanes display a broad band with emission maxima at 433 (dppe) and 493 nm (dppy) in solid state at RT, upon excitation between 270–310 nm and 300–410m, respectively. Complexes 2a–4a, with dppe, are strongly luminescent in solid state or in frozen solutions (dichloromethane or DMSO). All of these complexes display a similar optical behavior both in solid state and in frozen solutions. The spectra show a simple excitation profile with its maximum located between 401 (2a) and 414 nm (4a), leading to a maximum emission band appearing between 522 (2a) and 533 nm (4a) in solid state at RT (see figure 4a and 4b), which is lightly red shifted when the temperature is lowered to 77 K. This last shift observed with decreasing temperature is a standard phenomenon in luminescent gold complexes with metallophillic interactions and has been related to a thermal contraction of the gold-gold interactions that leads to a reduction in the band gap energy.[48] This result is indicative of transitions influenced by the gold-gold or gold-heterometal interactions, as has also been observed in other gold(I)-heterometal compounds displaying Au-M interactions.[19e,19f,19l]
Figure 4.
Excitation (dash line) and emission (solid line) spectra in the solid state of: (a) compound 2a at room temperature (RT); (b) compound 4a at RT; (c) gold precursor 1a at RT; (d) gold precursor 1a at 77 K.
In contrast, the luminescence profile and features (excitation and emission maxima) of gold precursor 1a are very different, suggesting a different origin. For this dinuclear gold compound, an emission pattern of four peaks is observed (see figures 4c), which become a better resolved vibrational fine structure when the temperature is lowered to 77 K (figure 4d). This structured band is due to vibrations of phenyl groups (the energy difference between neighboring bands being ca. 1330 cm−1) and is similar to that showed in the emission spectrum of [AuCl(PPh3)][49] and other dinuclear gold complexes containing phosphane ligands,[4,9a,9c] whose emission was attributed to the ligand based π-π* transitions or to metal to ligand charge transfer (MLCT, Au 5d →PR3 π*). The energy of the emission for this complex 1a resembles that obtained for the free phosphane, dppe (433 nm). Thus, an intraligand transition modified by the coordination to gold is probably responsible for the luminescence in 1a. The same conclusion can be reasonably applied to 1b. Its spectrum shows very broad bands with emission maximum at 505 nm (excitation maximum at 415 nm), which is also similar to that of free phosphane, dppy (493 nm), and for which its crystalline structure show no intra- or intermolecular gold-gold interactions, as mentioned before.
On the other hand, unlike what we observed for 1a, the absorption spectra of the 2a-4a solid also differ from those of its solutions, which is also indicative that the absorption and emission properties of these heterometallic compounds are supramolecular in nature and that the extended metallic interactions in the solids are crucial for the observation of luminescence. The diffuse-reflectance UV (DRUV) spectrum of 1a in solid state displays bands with maxima at λ < 300 nm and upon its coordination to heterometallic atoms in 2a–4a, broad bands appear at ca. 400 nm (see also supporting information). These new bands lead to the observed emissions and could correspond to charge transfer processes between the metallic atom and the ligands or to metal centered transitions.[50]
In fact, none of the degassed solutions of compounds 2a–4a are luminescent at room temperature, neither in dichloromethane nor DMSO, in which the molecular self-aggregation of [Au2M(μ-mes)2(μ-LL)]+ units (M= Ag or Cu) through metallic interactions could be lost. Taking into account the spectroscopic data shown in the Chemistry Section, we assume that the simplest repeated unit in diluted solutions is [{Au2M(μ-mes)}2(μ-LL)]+, which wouldn’t therefore give an emission as a result of the interactions between metals. We have also carried out a study of the absorption and emission spectra at different concentrations (from 5 × 10−4 until 10−2 M) for 2a in order to detect a possible deviation of the Lambert-Beer’s Law, which would be consistent with the presence of molecular aggregation through aurophilic interactions in fluid solution, as was first detected by Laguna and coworkers,[19l] and which has also been observed in other Au-Ag complexes due to argento-aurophilic bonding.[19k, 19f] Unfortunately, we have not been able to obtain conclusive evidence from these studies at different concentrations. However, the observation of a similar luminescence spectrum for 2a–4a in frozen solution (dichloromethane or DMSO) and in solid state seems to be indicative that molecular aggregation through metallophilic atractions is implicated, as observed in solid state for 3a. In fact, the blue shift of the emission and excitation when measurements are carried out in frozen solution relative to that in solid state can be explained by a higher aggregation in solid state, as has also been observed in other gold(I)-heterometal compounds displaying extended Au-M interactions [19e, 51]
Complexes 2b–4b, with dppy, are also strongly luminescent in solid state or in frozen DMSO solutions. However, their behavior is not similar. The main facts we observed for these compounds are as follows:
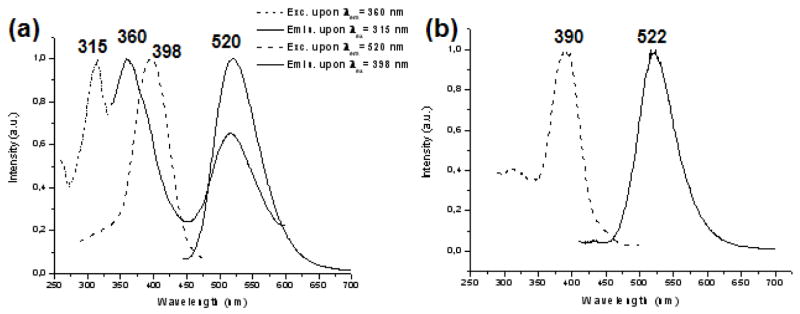
1) Complexes 3b and 4b show a similar behavior to those of analogue complexes 3a and 4a, which have dppe instead of dppy. The only observed difference is that for complex 4b the band seen at RT (range of emission 475–675 nm, with the maximum at 563 nm) unfolds into two others which are not so well defined when the measurement is taken at 77 K (at 522 and 595 nm). For these complexes, the absorption spectra of the solid also differ from those of the solutions. (In the supporting information section the DRUV spectra of compounds 1b–4b and the excitation and emission spectra of 3b and 4b in the solid state at RT are shown). These facts also indicate that the metallophilic interactions are crucial for the observation of luminescence. 2) Complex 2b behaves differently depending on the aggregation state and temperature. In the solid state at RT this compound exhibits two emissions, whose intensities depend on the excitation wavelength and temperature (see figure 5). Thus, at 315 nm, it exhibits two emission bands with the maxima at 360 and 520 nm, respectively. Excitation at a longer wavelength, 398 nm, gives only the emission band at 520 nm, which is similar to that observed in the rest of heterometallic complexes 2a–4a and 3b–4b, suggesting a similar origin. Thus, the metallophilic interactions between [Au2M(μ-mes)2(μ-LL)]+ units are probably responsible for the luminescence in these heterometallic complexes. In fact, as we have already mentioned, the colorless crystals 2c obtained from a solution of 2b show no gold-gold or gold-silver interactions and are not luminescent.
Figure 5.
Excitation and emission spectra in the solid state of: (a) compound 2b at RT; (b) compound 2b at LT.
A similar behavior at room temperature was observed by Yoshida et al[52a], which was attributed to the presence of two kinds of [Au(CN)2]n oligomers, the lower energy band being associated to the larger oligomers. Thus, the emission band at 360 nm might be assigned to oligomeric species including a small number of [Au2Ag(μ-mes)2(μ-dppy)]+ units such as dimer and trimer. The low energy band might originate from the larger oligomers. Upon freezing, 2b display intense luminescence with a maximum at 522 nm, which is comparable to the wavelength of the low energy band observed at RT. This result gives further confirmation that the low energy band originate from the extended chain structures.
Moreover, this complex 2b is the only one that luminesces in a diluted DMSO solution (5 × 10−4 M) at RT showing an emission band with a maximum at 524 nm (excitation maximum at 339 nm), which seems to indicate that for this complex the metallophilic interactions are not lost in a diluted solution. We have also carried out a study of the absorption and excitation spectra at different concentrations (from 5 × 10−5 to 10−3 M) for 2b in order to detect a possible deviation of the Lambert-Beer’s law. Thus, an increase of concentration from 5 × 10−4 to 10−3 M at RT produces the displacement of the excitation band to 366 nm. The absorption spectra of 2b in DMSO show that the weak shoulder appearing near 325 nm does not obey Lambert-Beer’s law, either, and appears at slightly lower energy when the concentration increases (see supporting information).[52b] This deviation from Lambert-Beer’s law is consistent with molecular aggregation in fluid solution because, as the number of metallophilic interactions increase the HOMO-LUMO gap is reduced. These results are consistent with an extended chain structure for the solid 2b, similar to that observed to 3a, and in fact, similar spectral changes have also been previously reported in other gold-silver complexes with extended chain structures with gold–gold or gold–silver interactions [19f, 19k, 19l and refs. therein] The observed emission energy in diluted solution (524 nm) is similar to that of lower energy observed for the solid (520 nm), which seems to indicate that the extended chain structure of 2b is kept in diluted solution.
In summary, all these results are consistent with an extended chain structure as that observed for 3a. However, from these data it is not possible to assign the nature of the transition associated because there are several possibilities, including metal to ligand charge transfer (MLCT) or metal centered (MC).[52c] Both of them have been used to explain the luminescent properties observed in other complexes with extended gold(I)-M interactions, which have emission and excitation maxima similar to those of our compounds. [19e,19f,19l,19m,20c]
3. Antimicrobial Activity
The antimicrobial activity of the new compounds (1–4) as well as that of previously described compounds 5a, 5b and the silver salts AgOClO3 and AgOSO2CF3 were evaluated against Gram-negative (Salmonella typhimurium and Escherichia coli), Gram-positive (Bacillus cereus and Staphyloccocus aureus) bacteria and yeast (Saccharomyces cerevisiae) (Table 4). A number of the compounds analyzed in this work exhibited Minimum Inhibitory Concentration (MIC) values in the 1 ug/ml – 100 ug/ml range (Table 4). None of the compounds tested showed significant activity against the eukaryotic S. cerevisiae at the concentrations tested. The compounds with the dppe ligand (a) had greater activity against bacteria than the compounds with the dppy ligand (b). The parent dinuclear compounds [Au2(mes)2(μ-LL)] 1a, 1b were insoluble or inactive against Gram-negative bacteria at concentrations of 100 μg/mL or lower. While compound 1a (with dppe) was toxic (10 μg/mL) for Gram-positive bacteria, 1b (with dppy) was inactive at concentrations of 100 μg/mL or lower. In the case of previously reported compounds [Au2Cl2(μ-LL)] 5a, 5b the derivative with the water soluble phosphane (dppy) was active (100 μg/mL) against Gram-negative and Gram-positive bacteria while 5a (with dppe) was inactive for all bacteria and yeast at the same concentration. The heretometallic compounds containing copper [Au2Cu(μ-mes)2(μ-LL)]PF6 4a, 4b were toxic for Gram-positive bacteria. Derivatives containing dppe (4a) were 10 times more active than those with dppy (4b) although their toxicity was similar to that of parent dinuclear compound 1a. 4b was more toxic to Gram-positive bacteria than parent compound 1b. The most toxic compounds were the heterometallic gold-silver compounds [Au2Ag(μ-mes)2(μ-LL)]A (2, 3). All these complexes were toxic to Gram-negative and Gram-positive bacteria. There were not significant differences depending on the counterion (the toxicity may come from the heterometallic Au2Ag cation). The derivatives with dppe (2a, 3a) were 10 (or even 100) times more active than those with dppy. However the derivatives with dppy (2b, 3b) were active against yeast at concentrations of 100 μg/mL.
Table 4.
Minimum inhibitory concentration (μg/mL) of compounds 1–5 and silver salts against microbial organisms.[a]
| Gram-negative | Gram-positive | Yeast | |||
|---|---|---|---|---|---|
| Compound | S. typhimurium | E. coli | B. cereus | S. aureus | S. cerevisiae |
| 1a [Au2(mes)2(μ-dppe)] | Ins[b] | Ins[b] | 10 | 10 | Ins[b] |
| 2a [Au2Ag(μ-mes)2(μ-dppe)]ClO4 | 10 | 10 | 1 | 10 | >100[c] |
| 3a [Au2Ag(μ-mes)2(μ-dppe)]SO3CF3 | 10 | 10 | 10 | 10 | >100[c] |
| 4a [Au2Cu(μ-mes)2(μ-dppe)]PF6 | Ins[b] | Ins[b] | 10 | 10 | Ins[b] |
| 5a [Au2Cl2(μ-dppe)] | >100[c] | >100[c] | >100[c] | >100[c] | >100[c] |
| AgOClO3 | 100 | 100 | 10 | 100 | 100 |
| AgOSO2CF3 | 100 | 100 | 10 | 100 | 100 |
| 1b [{Au(mes)}2(μ-dppy)] | >100[c] | >100[c] | >100[c] | >100[c] | >100[c] |
| 2b [Au2Ag(μ-mes)2(μ-dppy)]ClO4 | 100 | 100 | 100 | 100 | 100 |
| 3b [Au2Ag(μ-mes)2(μ-dppy)]SO3CF3 | 100 | 100 | 100 | 100 | 100 |
| 4b [Au2Cu(μ-mes)2(μ-dppy)]PF6 | >100[c] | >100[c] | 100 | 100 | >100[c] |
| 5b [{AuCl}2(μ-dppy)] | 100 | 100 | 100 | 100 | >100[c] |
Compounds were dissolved in DMSO.
Ins - The compound was insoluble in aqueous solution above 10 ug/ml.
Concentrations greater than 100 ug/ml were not tested because the compounds would not have been soluble.
Since we had observed that the heterometallic compounds decoordinate the “naked” metallic center over time in DMSO solutions, we tested the activity of the silver salts AgOClO3 and AgOSO2CF3 for comparison. We did not evaluate the activity of the air, moisture and light sensitive Cu(I) derivative [Cu(CH3CN)4]PF6 since it would have decomposed in DMSO solution immediately upon addition. The silver salts were toxic to Gram-positive and Gram-negative bacteria and yeast (100 μg/mL) and especially to Gram- positive bacteria B. cereus (10 μg/mL). The toxicity was the same for the Au2Ag derivatives with dppy (2a, 3a). However compounds Au2Ag with dppe resulted more toxic (10 times) than the silver salts for all Gram-positive and Gram-negative bacteria pointing out that, in this case, the toxicity does not come from the dissociation of the silver perclorate or triflate from the heterometallic derivative. The heterometallic compounds were also more toxic to Gram-negative bacteria than parent dinuclear compounds 1a and 1b. In this case it is obvious that there is a synergistic or cooperative effect between the gold and silver metals. The toxicity for parent dinuclear compound (1a) is similar for Gram-positive bacteria but the compound is not active for Gram-negative bacteria at concentrations of 100 μg/mL or lower. The cationic heterometallic Au2Ag compounds 2a, 3a allow for a better solubilization in the cell culture media and display high toxicity against Gram-negative bacteria (10 μg/mL) and Gram-positive bacteria (10 μg/mL). Remarkably, the complex with ClO4− (2a) displays a much higher toxicity for Gram-positive bacteria B. cereus (1 μg/mL) that any other of the compounds (parent dinuclear gold, silver salts or other heterometallic derivatives) tested.
The organometallic dinuclear gold(I) compound [Au2(mes)2(μ-dppe)] 1a resulted more active against Gram-positive bacteria and also more active than previously described coordination derivative [Au2Cl2(μ-dppe)] 5a. The fact that gold compounds are more toxic for Gram-positive bacteria than Gram-negative or fungi has been noted previously with auranofin[53a] and some other gold(I) phosphane derivatives including some described by us.[24e,26a,53b–f] However the nature of other ancillary ligands coordinated to the gold centers (besides the phosphanes) plays a decisive role. For instance a more complicated pattern is found for gold-thiol-phosphane derivatives and the antimicrobial potency can be higher for Gram-negative or fungi depending on the combination thiol-phosphane.[53a] We thought that the incorporation of water soluble phosphane dppy in the polynuclear gold(I) compounds would increase the solubility in DMSO and mixtures DMSO/water of the organometallic analogue compound and thus the toxicity against microorganisms. We found however that water-soluble derivative [Au2Cl2(μ-dppy)] 5b was more toxic than novel organometallic compound [Au2(mes)2(μ-dppy)] 1b as opossed to what was seen for dppe (5a vs 1a). Related complexes of gold(I) with dppe and pyridyl-substituted diphosphanes like [Au(LL)2]Cl (LL= dppe, dppy) had been described in the literature as cytotoxic agents against a panel of cisplatin-resistant human ovarian carcinoma cell lines.[54] Their citotoxicity was strongly dependent upon their lipophilicity being more cytotoxic and hepatotoxic those compounds more lipophilic (with dppe).[54a]
As mentioned before, the heterometallic Au2Ag compounds with dppe (2a, 3a) were the most active. They showed strong activity, MIC of 10 ug/ml or less, against both Gram-positive and Gram-negative bacteria. Dinuclear silver(I)-oxygen bonding complexes derived from camphanic acic ligands have displayed a wide spectrum of effective antimicrobial activity.[23d] Some N-heterocyclic carbene Ag(I) complexes inhibit more efficiently the growth of Gram-positive bateria including B. subtilis,[28b–d] Gram-negative E. coli,[28c–g] and P. aeruginosa[28c,e,f] and even antibiotic resistant strains of S. aureus.[28a,d] Binuclear and polymeric silver(I) complexes with a tridentate heterocyclic N- and S- ligand 8-(9pyridin-3-yl)methylthio) quinoline were good inhibitors of Gram-positive bacteria and some Gram-negative bacteria (P. aeruginosa) but they were very poor against E. coli.[55] The behaviour of the gold-silver compounds 2a, 3a and AgA (A = OClO3 −, OSO2CF3 −) is similar to that of silver-carbene derivatives,[28] silver fluorinated tris(pyrazolyl)borate complexes[56] and AgNO3 and silver(I) sulfadiazine[56] inhibiting grow of both Gram-negative and Gram-positive bacteria. However in the case of the tris(pyrazolyl)borate complexes it was demonstrated that the effect on Gram-positive species was due to the ligand whereas the activity for Gram-negative species was truly due to the silver ion.[56] In our case it seems that the activity displayed is due to the presence of the metals. The MIC of 2a and 3a for Gram-negative E. coli and Gram-positive S. aureus of 10 μg/mL is very low, in the order of those obtained recently for highly active antimicrobial silver nanoparticles (12.5 μg/mL)[22b] and some silver heterocyclic carbenes (1–32 μg/mL)[28d–g] for the same microorganisms. The value of 1 μg/mL of 2a (nanomolar range) for Gram-positive B. cereus is quite remarkable.
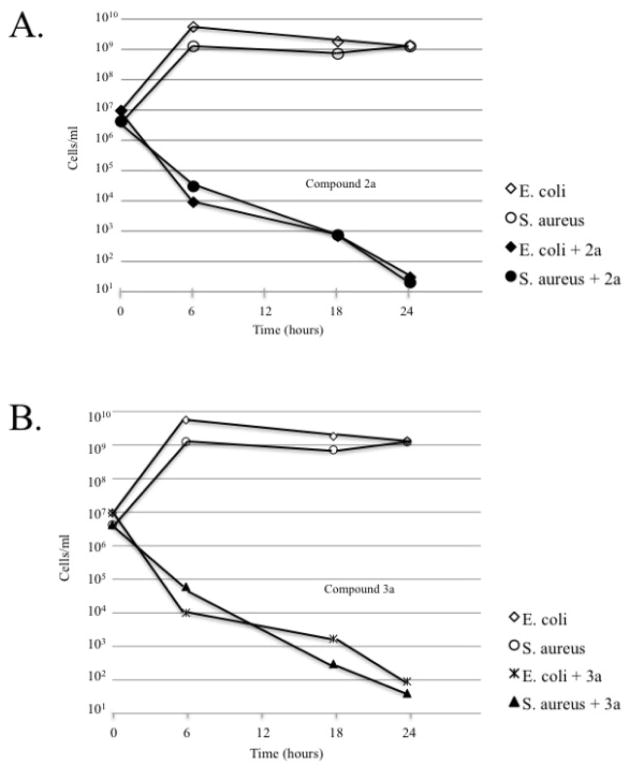
We tested the ability of these two compounds in Minimun Bactericidal Concentration (MBC) assays against E. coli and S. aureus (Figure 6). Both 2a and 3a showed strong bactericidal activity, reducing cell counts by several orders of magnitude. We did not observe complete killing within 24 hours with 2a and 3a which suggests that either longer exposure times may be required to get complete killing or that the availability of active compound has dropped sharply in the 24 hours of the assay. We cannot rule out the development of some resistance within the bacterial population although this is very unlikely given the short exposure time to the compounds. Overall the heterometallic Ag2Au compounds behave as highly active antibacterial agents for Gram-positive and Gram-negative bacteria with toxicity against Gram-negative bacteria much higher than that of the parent dinuclear Au2 compound or the AgA salts alone.
Figure 6.
The minimal bactericidal concentration was determined by growing E. coli or S. aureus in the presence of 2a (Fig 5a) or 3a (Fig 5b) at 10 ug/ml over 24 hours. At each time point dilutions of the cell cultures were plated onto Mueller Hinton plates and the number of colony forming units were used to calculate the cell concentration. The results show that nearly all bacteria were killed by the 2a or 3a compounds by 18 hours. Control cells incubated in the absence of compounds 2a or 3a continued to grow as expected.
Conclusion
In conclusion we have demonstrated that gold(I) organometallic complexes containing mesityl ligands and bridging bidentate phosphanes can serve as excellent precursors to highly luminescent heterometallic Au2M (M = Ag, Cu) derivatives and that the luminescence displayed, whether in the solid state or in frozen solutions, results from the formation of aggregated species that self-associate through metallophilic attractions. In solution, where the ions are dispersed, the complexes are non luminescent except compounds 2b and 3b in concentrated solutions which can be seen photoluminescent when excited with a hand-held UV lamp. The compounds display moderate to high antibacterial activity while heteronuclear Au2M derivatives with dppe (2a-4a) are very active (MIC 10 to 1 μg/mL) and more active than the dinuclear Au2 parent compound (1a) against Gram-negative bacteria. They are also more active than silver salts (AgX; X = ClO4−, OSO2CF3−) against both Gram-negative and Gram-positive bacteria. Au2Ag compounds with dppy (2b, 3b) are also potent against fungi. The new trinuclear Au2Ag compounds possess peculiar chemicophysical properties with respect to their precursors responsible for the observed biological effects. Compound 2b is also luminescent in diluted DMSO solutions at room temperature and could potentially be used in the future in studies of fluorescence microscopy to track these types of derivatives inside yeast or mammalian cells. These preliminary results warrant further studies on the synthesis of different luminescent biologically active Au-Ag heterometallic complexes.
Experimental Section
1. Synthesis and Characterization of the Polynuclear Metal Complexes
Solvents were purified by use of a PureSolv purification unit from Innovative Technology, Inc.; all other chemicals were used as received. Elemental analyses were carried out on a Perkin-Elmer 2400-B microanalyser. Infrared spectra (4000-400 cm−1) were recorded on a Nicolet 380 FT-IR infrared spectrophotometer on KBr pellets. The 1H, 13C{1H} and 31P{1H} NMR spectra were recorded in CDCl3, CD3CN or d6-DMSO solutions at 25 °C on a Bruker 400 and spectrometer (δ, ppm; J, Hz); 1H and 13C{1H} were referenced using the solvent signal as internal standard while 31P{1H} was externally referenced to H3PO4 (85%). The mass spectra (FAB+, matrix: 3-nitrobenzylalcohol) were recorded from CH2Cl2 solutions on a VG Autopsec spectrometer and the mass spectra (ESI+, matrix: DCTB) on a MALDI-TOF MICROFLEX (Bruker) spectrometer. Conductivity was measured in an OAKTON pH/conductivity meter in (CH3)2CO and CH3CN solutions. Compounds [Au(mes)(AsPh3)], [31] 5a,[33] 5b,[34] and water-soluble phosphane dppy[57] were prepared as previously reported. All other chemicals and solvents were purchased from Sigma-Aldrich and Strem Chemicals.
[Au2(mes)2(μ-LL)] (LL = dppe 1a; dppy 1b)
To dppe (0.50 mmol, 0.199 g) or water-soluble phosphine dppy (0.24 mmol, 0.098 g) in 15ml of CH2Cl2, [Au(mes)(AsPh3)][31] (0.5 mmol, 0.622 g, to obtain 1a or 0.489 mmol, 0.304 g to obtain 1b) was added and the mixture stirred for 20 min at rt. The solution was then evaporated to 3 mL and 20 mL of n-hexane was added. A compound precipitated which was filtered and dried in vacuo to afford pure 1a (white solid) or 1b (pale yellow solid). 1a: Yield: 0.491 g, 91%. Anal. Calc. for C44H46P2Au2 (1030): H, 4.55; C, 51.75; found H, 4.50; C, 51.3; MS(FAB+): [M/Z, (100%)]: 911 [M – mes]+. 31P{1H} NMR (CDCl3): δ = 42.0 ppm, (d6-dmso): δ= 42.7 ppm. 1H NMR (CDCl3): 2.30 (p-Me), δ = 2.64 (o-Me), 2.85 ((CH2)2), 6.97 (m-H) ppm. 13C{1H} (CDCl3): δ = 21.1 (p-Me), 24.2 ((CH2)2), 26.8 (o-Me), 126.6 (Mes, C3, C5), 127.8 (Mes, C1), 129.4 (Ph, C4), 131.5 (Ph, C3, C5), 133.3 (Ph, C2, C6), 135.5 (Ph, C1), 145.8 (Mes, C2, C4, C6) ppm. Conductivity (acetone): 4.8 S cm2 mol−1(neutral). 1b: Yield: 0.162 g, 93%. Anal. Calc. for C40H42N4P2Au2·H2O (1052): H, 4.21; C, 45.64; N, 5.33; found H, 3.89; C, 45.27; N, 5.75. MS(ESI+) [M/Z, (100%)]: 1057 [M + Na]. 31P{1H} NMR (CD3CN) δ = 34.4 ppm; (d6-DMSO): δ = 34.7 ppm. 1H NMR (CD3CN): δ = 2.23 (p-Me), 2.48 (o-Me), 3.02 ((CH2)2), 6.83 (m-H), 7.48 (H3), 8.09 (H4), 8.74 (H2), 9.01 (H6) ppm; (d6-dmso) : δ = 2.16 (p-Me), 2.34 (o-Me), 2.51 ((CH2)2), 6.71 (m-H), 7.57 (H3), 8.23 (H4), 8.76 (H2), 9.08 (H6) ppm. 13C{1H} (CDCl3): δ = 21.3 (p-Me), 23.7 ((CH2)2), 27.2 (o-Me), 124.4 (Py, C3), 127.0 (Mes, C3), 136.4 (Py, C5), 140.9 (Py, C4), 145.4 (Mes, C1), 153.0 (Py, C2), 153.7 (Py, C6) ppm. Conductivity (CH3CN): 13.2 S cm2 mol−1(neutral).
[Au2Ag(μ-mes)2(μ-LL)]ClO4 (LL = dppe 2a; dppy 2b)
To a solution of [Au(mes)(μ-dppe)] 1a (0.20 mmol, 0.206 g) for the preparation of 2a or [{Au(mes)}2(μ-dppy)] 1b (0.15 mmol, 0.155 g) for the preparation of 2b in 20 mL of CH2Cl2 was added to a solution of AgClO4 (0.20 mmol, 0.415 g 2a, 0.15 mmol, 0.031g 2b) in 10 mL of Et2O. The solution was stirred at 0°C protected from the light for 20 min. The solvent was then reduced to 2–3 mL and 20 mL of n-hexane was added. A yellow solid precipitated and was filtered and dried in vacuo to afford pure 2a (yellow solid) or 2b (pale yellow solid). 2a: Yield 0.204 g, 82%. Anal. Calc. for C44H46P2Au2AgClO4 (1136): H, 3.75; C, 42.69; found H, 3.45; C, 42.25. MS(FAB+) [M/Z, (%)]: 1136 [M]+. IR: ν(ClO4−) = 1088 (br, vs), 623(s) cm−1. 31P{1H} NMR (CDCl3) δ = 44.9 ppm. (d6-DMSO): δ = 46.9 ppm. 1H NMR(CDCl3): δ = 2.30 (p-Me), 2.34 (o-Me), 3.07 ((CH2)2), 7.04 (m-H) ppm. 13C{1H} NMR(CDCl3): δ = 21.0 (p-Me), 23.2 ((CH2)2), 27.2 (o-Me), 127.9 (Mes, C3, C5), 129.6 (Ph, C2, C4, C6), 130.1 (Mes, C1), 133.6 (Ph, C3, C5), 136.6 (Ph, C1), 145.3 (Mes, C4), 154.3 (Mes, C2, C6) ppm. Conductivity (acetone): 160 S cm2 mol−1 (1:1 electrolyte). 2b: Yield: 0.135 g, 72%. Anal. Calc. for C40H42N4P2Au2AgClO43H2O (1296): H, 3.73; C, 37.07; N, 4.32; Found H,3.34; C, 36.86; N,4.23. MS(FAB+) [M/Z, (%)]: 1141 [M]+. ν(ClO4−) = 1082 (br, vs), 616(s) cm−1. 31P{1H} NMR (CD3CN): δ = 32.7 ppm; 1H NMR (CD3CN): δ = 2.41 (o-Me), 2.24 (p-Me), 6.86 (m-H), 3.14 ((CH2)2), 7.53 (H3), 8.12 (H4), 8.74 (H2), 9.04 (H6) ppm. 13C{1H} NMR (CDCN3): δ = 20.8 (o-Me), 21.3 (p-Me), 26.6 ((CH2)2), 127.0 (Mes, C3, C5), 124.3 (Py, C3), 133.3 (Mes, C2), 141.1 (Py, C4), 153.0 (Py, C2), 153.4 (Py, C6) ppm. Conductivity (CHCN3): 121.6 S cm2 mol−1 (1:1 electrolyte).
[Au2Ag(μ-mes)2(μ-LL)]SO3CF3 (LL = dppe 3a; dppy 3b)
To a solution of [Au(mes)(μ-dppe)] 1a (0.20 mmol, 0.206 g) for the preparation of 3a or a solution of [{Au(mes)}2(μ-dppy)] 1b (0.10 mmol, 0.103 g) for the preparation of 3b in 20 mL of CH2Cl2 was added a solution of AgSO3CF3 (0.20 mmol, 0.514 g, 3a or 0.10 mmol, 0.026 g, 3b) in 10 mL of Et2O. The reaction mixture was stirred at 0°C protected from the light for 20 min (3a) or 90 min (3b). The solvent was then reduced to 2–3 ml and 20 mL of n-hexane was added. A yellow solid precipitated and was filtered and dried in vacuo to afford pure 3a (yellow solid) or 3b (pale yellow solid). 3a: Yield: 0.198 g, 76%. Anal. Calc. for C45H46P2Au2Ag SO3F3 (1286): H, 3.60; C, 41.97; S, 2.49; found H, 3.6; C, 41.85: S, 2.9. MS(FAB+) [M/Z, (%)]: 1136 [M]+. IR: ν(SO3CF3−) = 1262 (br), 1221 (s), 1154 (s) cm−1. 31P{1H} NMR (CDCl3): δ = 45.2 ppm. (d6-DMSO): δ = 47.0 ppm. 1H NMR (CDCl3): δ = 2.35 (p-Me), 2.65 (o-Me), 3.13 (P-(CH2)2-P), δ=7.06 (m-H) ppm. 13C{1H} NMR(CDCl3): δ = 21.4 (p-Me), 27.4 (o-Me), 30.9 ((CH2)2), 127.3 (Mes, C3, C5), 129.7 (Ph, C3, C5), 132.1 (Ph, C4), 133.6 (Ph, C2, C6) (Mes, C1 and Ph, C1 not showing) ppm. Conductivity: 107 S cm2mol−1 (1:1 electrolyte). 3b: Yield: 0.105 g, 82%. Anal. Calc. for C41H42N4P2Au2AgSO3F34H2O (1362): H, 3.55; C, 36.16; N, 4.11, found H, 3.11; C, 35.74: N, 3.96. MS(FAB+) [M/Z, (%)]:1141 [M]+. IR: ν(SO3CF3−) = 1257 (br,vs), 1158 (m) cm−1. 31P{1H} NMR (CD3CN): δ = 32.5 ppm. 1H NMR (CD3CN): δ = 2.25 (p-Me), 2.40 (o-Me), 3.16 ((CH2)2), 6.89 (m-H), 7.53 (H3), 8.13 (H4), 8.75 (H2), 9.05 (H6) ppm (3-pyridyl). 13C{1H} NMR (CDCN3): δ = 20.4 (p-Me), 20.7 ((CH2)2), 25.7 (o-Me), 127.0 (Mes, C3, C5), 124.6 (Py, C3), 141.4 (Py, C4), 153.1 (Py, C2), 153.7 (Py, C6) ppm. Conductivity (CHCN3): 181.0 S cm2 mol−1 (1:1 electrolyte).
[Au2Cu(μ-mes)2(μ-LL)]PF6 (LL = dppe 4a; dppy 4b)
To a 20 mL of CH2Cl2 solution of [Au(mes)(μ-dppe)] 1a (0.20 mmol, 0.206 g) for the preparation of 4a or [Au(mes)(μ-dppy)] 1b (0.10 mmol, 0.103 g) for the preparation of 4b, [Cu(CH3CN)4]PF6 (0.21 mmol, 0.078 g, 4a; 0.10 mmol, 0.037 g, 4b) was added and stirred for 20 min at r.t. The solvent was then reduced to 2–3 mL and 20 mL of n-hexane was added to obtain a precipitate which was filtered and dried in vacuo to afford pure 4a (greenish yellow solid) or 4b (green solid). 4a: Yield 0.162 g, 62%. Anal. Calc. for C45H46P3Au2F6Cu (1092): H, 3.74; C, 42.65; found H, 3.75; C, 42.25. MS(FAB+) [M/Z, (%)]:1092 [M]+. IR: ν(PF6−) = 834 (br, vs) cm−1. 31P{1H} NMR (CDCl3): δ = 44.0, −144.1 ppm (PF6, sept); (d6-DMSO): 42.6, −144.1 ppm (PF6, sept);. 1H NMR (CDCl3): δ = 2.31 (p-Me), 2.69 (o-Me), 3.03 ((CH2)2), 7.01 (m-H) ppm. 13C{1H} NMR(CDCl3): δ = 21.6 (p-Me), 22.2 ((CH2)2), 27.3 (o-Me), 127.5 (Mes, C3, C5), 128.3 (Ph, C3, C5), 129.2 (Mes, C1), 129.9 (Ph, C4), 132.4 (Ph, C2, C6), 133.4 (Ph, C1), 144.5 (Mes, C4), 154.0 (Mes, C2, C6) ppm. Conductivity (acetone): 130 S cm2 mol−1 (1:1 electrolyte). 4b: Yield: 0.09 g, 90%. Anal. Calc. for C40H42N4P3Au2F6Cu.5H2O (1333): H, 3.93; C, 36.03; N, 4.20. found H, 3.48 C, 35.72, N, 4.13. MS(FAB+) [M/Z, (%)]:1097 [M]+. IR: ν(PF6−) = 839 (br, vs) cm−1. 31P{1H} NMR (CD3CN): δ = 34.1 ppm; 1H NMR(CD3CN): δ = 2.22 (o-Me), 2.43 (p-Me), 6.81 (m-H), 3.05 ((CH2)2), 7.49 (H3), 8.11 (H4), 8.73 (H2), 9.01 (H6) ppm. 13C{1H} (CDCN3): δ = 20.0 (o-Me), 20.0 (p-Me), 26.1 ((CH2)2), 126.2 (Mes, C3, C5), 124.6 (Py, C3), 141.1 (Py, C4), 152.6 (Py, C2), 153.7 (Py, C6) ppm. Conductivity (CHCN3): 170.4 S cm2 mol−1 (1:1 electrolyte).
2. Single-Crystal X-ray Diffraction Studies
Data for 1b (CCDC 841439), 2c (CCD 841440) and 3a (CCDC 841438) were collected by using a Xcalibur S3 diffractometer (graphite monochromated Mo-Kα radiation, λ = 0.71073 Å, φ-ω scans). These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
1b: Crystals of 1b (yellow prisms with approximate dimensions 0.42 × 0.26 × 0.17 mm) where obtained when a solution of 1b in CH2Cl2 was crystallized by slow diffusion of n-hexane at −5 °C. As the crystals were found to be unstable when removed from their mother liquor, a single crystal of 1b was harvested using a Mitegen support with a drop of perfluorinated oil and then placed directly on the diffractometer at a temperature of 173 K. 2c: Crystals of 2c (colorless prisms with approximate dimensions 0.16 × 0.03 × 0.02 mm) where obtained when a solution of 2b in dichloromethane-acetone-DMSO solution by slow diffusion of n-hexane at −5°C. As the crystals were found to be highly unstable when removed from their mother liquor, a single crystal of 2c was harvested using a Mitegen support with a drop of perfluorinated oil and then placed directly on the diffractometer at a temperature of 100 K. 3a: Crystals of 3a (yellow sphenoids with approximate dimensions 0.39 × 0.18 × 0.12 mm) where obtained when a solution of 3a in CH2Cl2 was crystallized by slow diffusion of n-hexane at −5 °C. As the crystals were found to be highly unstable when removed from their mother liquor, a single crystal of 3a was harvested using a Mitegen support with a drop of perfluorinated oil and then placed directly on the diffractometer at a temperature of 150 K. The diffraction pattern was considered to be acceptable, although weak. The data for 1b, 2c and 3a were gathered and processed using the usual procedures for the Xcalibur S3 diffractometer (graphite monochromated Mo-Kα radiation, λ = 0.71073 Å, φ-ω scans) including multi-scan corrections for systematic errors. The structure was solved by direct methods, which revealed the positions of the heavy atoms and of a subset of the C, O and F sites. The remainder of the structure was located and refined in an alternating series of least-squares refinements and difference Fourier maps. Details of the solution and refinements for 1b and 3a compound are presented in Table 5 and in the SI while the data and full analysis of the results for 2c can be found in the SI.
Table 5.
Crystal data and structure refinement for complexes 1b and 3a.
| Compound | 1b [Au2(mes)2(μ-dppy)] | 3a [(μ-Ag){Au2(mes)2(μ-dppe)}]n(CF3SO3)n·1.6n(H2O) |
|---|---|---|
| formula | C40H42Au2N4P2 | (C225H246Ag5Au10F15O23P10S5)n |
| Fw | 1034.65 | n·6582.23 |
| T [K] | 173(1) | 150(1) |
| λ (MoKα)[Å] | 0.71073 | 0.71073 |
| crystal system | triclinic | monoclinic |
| Space group | P1 | I2/a (No. 15) |
| a [Å] | 8.3949(3) | 28.859(3) |
| b [Å] | 9.4059(3) | 39.0504(8) |
| c [Å] | 12.2451(4) | 22.9486(10) |
| α [o] | 88.941(3) | --- |
| β [o] | 86.401(3) | 91.662(7) |
| χ[o] | 79.104(3) | --- |
| V [Å]3 | 947.567 | 25851 |
| Z | 1 | 4 |
| Dcalcd (g cm−3) | 1.813 | 1.687 |
| μ (mm−1) | 7.850 | 6.186 |
| R(Fo)[a] | 0.0296 [for 5303 data with Fo2 > 2σ(Fo2)] | 0.0631 [for 15200 data with Fo2 > 2σ(Fo2)] |
| Rw(Fo2)[b] | 0.0651 | 0.1812 |
R(Fo) (= R1) = Σ | |Fo| – |Fc| | / Σ |Fo|.
Rw(Fo2) (= wR2) = {Σ [ w(Fo2–Fc2)2 ] / Σ [ w(Fo2)2 ] }1/2
The room-temperature powder diffraction pattern for Compound 2b was measured by the X-ray Diffraction and Fluorescence Service of the University of Zaragoza, using a Rigaku DMax 2500 using graphite-monochromated Cu radiation. Data were recorded from 5 – 60° 2-theta with a step size of 0.03°. The powder diffraction pattern for compound 2c was simulated using the program Platon, with the simulation based on the single-crystal diffraction analysis of 2c at T = 100 K. The difractograms are collected in the SI.
3. Luminescence studies
Absorption spectra in solution were recorded with a Unicam UV-4 spectrophotometer. Diffuse-reflectance UV (DRUV) spectra were recorded in the same equipment using a Spectralon RSA-UC-40 Labsphere integrating sphere. The solid samples were mixed with silica gel and placed in a homemade cell equipped with a quartz window. The intensities were recorded in Kubelka-Munk units: log[R/(1−R)2], where R= reflectance.
Steady-state photoluminescence spectra were recorded with a Jobin-Yvon Horiba Fluorolog FL-3-11 spectrofluorimeter using band pathways of 3 nm for both excitation and emission. Phosphorescence lifetimes were recorded with a Fluoromax phosphorimeter accessory containing a UV xenon flash tube at a flash rate between 0.05 and 25 Hz. The lifetime data were fit using the Jobin-Yvon software package and the Origin 7.0 program. The solid samples were prepared by mixing the compounds with silica gel.
4. Microbial Toxicity Assays
Bacteria and yeast were stored as glycerol stocks at −80°C and streaked onto Meuller-Hinton plates prior to each experiment. Colonies from these newly prepared plates were inoculated into 5 ml of media (tryptic soy broth for E. coli, S. salmonella, S. aureus and B. cereus and YPD broth for S. cerevisiae) and grown overnight at 37°C (30°C for S. cerevisiae and 25°C for B. cereus). The overnight cultures were diluted to an OD600 <0.01 (ThermoSpectronic, Genesys 8 spectrophotometer) in 2 ml of fresh media in sterile culture tubes. The compounds (1–5, AgClO4 and AgSO3CF3) were brought up in DMSO to a concentration of 1 mg/ml and immediately added into the cell culture tubes at 1 μg/ml, 10 μg/ml or 100 μg/ml. Control tubes for each cell type were inoculated with DMSO alone. The culture tubes were then placed at the appropriate temperature in a shaking incubator (Lab-Line Orbital Shaker), at ~200 RPM. Cell growth was monitored by taking OD600 readings at several time points over the course of 24 hours. The minimal inhibitory concentration (MIC) was determined to be the concentration at which there was negligible increase in the OD600 value from the initial reading after 24 hours. All samples were tested in triplicate. The bactericidal activities of compounds 2a and 3a against S. aureus and E. coli were determined by plating dilutions of bacterial cultures on to Mueller-Hinton plates at each time point and counting the resulting number of colonies.
Supplementary Material
Acknowledgments
We thank the financial support of a grant from the National Institute of General Medical Sciences, SC2GM082307 (M.C.). The Spanish team thanks the MICINN “Spanish Ministry of Science and Innovation” for finantial support: CTQ2008-01784 (J.J.) and MAT2008-04350 and CONSOLIDER-INGENIO in Molecular Nanoscience, CSD 2007-00010 (L.R.F). We thank undergraduate student Chaya Levine for her assistance with some of the experiments.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemeurj.org/ or from the author.
References
- 1.a) Yam VWW, Cheng ECC. Chem Soc Rev. 2008;37:1806–1813. doi: 10.1039/b708615f. [DOI] [PubMed] [Google Scholar]; b) Barbieri A, Accorsi G, Aramaroli N. Chem Commun. 2008:2185–2193. doi: 10.1039/b716650h. [DOI] [PubMed] [Google Scholar]; c) Fernández EJ, Laguna A, López-de-Luzuriaga JM. Dalton Trans. 2007:1969–1981. doi: 10.1039/b702838p. [DOI] [PubMed] [Google Scholar]; d) Vogler A, Kunkely H. Coord Chem Rev. 2001;219–221:489–507. [Google Scholar]; e) Schmidbaur H. Chem Soc Rev. 1995;24:391–400. [Google Scholar]
- 2.Pyykko P, Zhao Y. Angew Chem Int Ed Engl. 1991;30:604–605. [Google Scholar]
- 3.a) Pyykko P. Angew Chem Int Ed. 2004;43:4412–4456. doi: 10.1002/anie.200300624. [DOI] [PubMed] [Google Scholar]; b) Pyykko P. Science. 2000;290:64–65. [Google Scholar]; c) Pyykko P, Mendizábal F. Inorg Chem. 1998;37:3018–3025. [Google Scholar]; d) Pyykko P. Chem Rev. 1997;97:597–636. doi: 10.1021/cr940396v. [DOI] [PubMed] [Google Scholar]
- 4.King C, Wang J-C, Khan MNI, Fackler JP., Jr Inorg Chem. 1989;28:2145–2149. [Google Scholar]
- 5.Che C-M, Kwong HL, Yam VW-W, Cho KC. J Chem Soc, Chem Commun. 1989:885–886. [Google Scholar]
- 6.a) Partyka DV, Updegraff JB, II, Zeller M, Hunter AD, Gray TC. Dalton Trans. 2010;39:5388–5397. doi: 10.1039/b920717a. [DOI] [PubMed] [Google Scholar]; b) Pan QJ, Zhou X, Guo YR, Fu HG, Zhang HX. Inorg Chem. 2009;48:2844–2854. doi: 10.1021/ic801687w. [DOI] [PubMed] [Google Scholar]; c) Pan QJ, Zhou X, Fu HG, Yu HT. Eur J Inorg Chem. 2006:1050–1059. [Google Scholar]; d) Bardají, M., de la Cruz, M.T., Jones, P.G., Laguna, A., Martínez, J., Villacampa, M.D. Inorg. Chim. Acta 2005, 358, 1365–1372. e) Pawlosky V, Kunkely H, Vogler A. Inorg Chim Acta. 2004;357:1309–1312. [Google Scholar]; f) Pintado-Alba, A., de la Riva, H., Nieuwhuyzen, M., Bautista, D., Raithby, P.R., Sparkes, H.A., Teat, S.J., López-de-Luzuriaga, J.M., Lagunas, M.C. Dalton Trans. 2004, 3459–3467; g) Bardají, M., Jones, P.G., Laguna, A., Villacampa, M.D., Villaverde, N. Dalton Trans. 2003, 4529–4536. h) Fu WF, Chan KC, Cheung KK, Che CM. Chem Eur J. 2001;7:4656–4664. doi: 10.1002/1521-3765(20011105)7:21<4656::aid-chem4656>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]; i) Calhorda MJ, Crespo O, Gimeno MC, Jones PG, Laguna A, López-de-Luzuriaga JM, Pérez JL, Ramon MA, Veiros LF. Inorg Chem. 2000;39:4280–4285. doi: 10.1021/ic000136y. [DOI] [PubMed] [Google Scholar]; j) Brandys MC, Puddephatt RJ. J Am Chem Soc. 2001;123:4839–4840. doi: 10.1021/ja010128w. [DOI] [PubMed] [Google Scholar]; k) Leung KH, Phillips DL, Tse MC, Che CM, Miskpwski VM. J Am Chem Soc. 1999;121:4799–4803. [Google Scholar]; l) Fu WF, Chan KC, Miskowski VM, Che CM. Angew Chem Int Ed Engl. 1999;38:2783–2785. [PubMed] [Google Scholar]; m) Field JS, Grieve J, Haines RJ, May N, Zulu MM. Polyhedron. 1998;17:3021–3029. [Google Scholar]; n) Tang SS, Chang CP, Lin IJB, Liou LS, Wang JW. Inorg Chem. 1997;36:2294–2300. doi: 10.1021/ic9607245. [DOI] [PubMed] [Google Scholar]; o) Yam VWW, Lee WK. J Chem Soc, Dalton Trans. 1993:2097–2100. [Google Scholar]; p) Yam VW-W, Lai T-F, Che C-MJ. Chem Soc, Dalton Trans. 1990:3747–3752. [Google Scholar]; q) Che C-M, Kwong H-L, Poon C-K, VW-W J Chem Soc, Dalton Trans. 1990:3215–3219. [Google Scholar]; r) Jaw HRC, Savas MM, Rogers RD, Mason WR. Inorg Chem. 1989;28:1028–1037. [Google Scholar]
- 7.a) Crespo O, Gimeno MC, Laguna A, Ospino I, Aullon G, Oliva JM. Dalton Trans. 2009:3807–3813. doi: 10.1039/b820803d. [DOI] [PubMed] [Google Scholar]; b) Yam VWW, Cheung KL, Yip SK, Cheung KK. J Organomet Chem. 2003;681:196–209. [Google Scholar]; c) McArdle CP, Irwin MJ, Jennings MC, Vittal JJ, Puddephatt RJ. Chem Eur J. 2002;8:723–734. doi: 10.1002/1521-3765(20020201)8:3<723::AID-CHEM723>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]; d) Bardají M, Jones PG, Laguna A. J Chem Soc, Dalton Trans. 2002:3624–3629. [Google Scholar]; e) Hunks WJ, McDonald MA, Jennings MC, Puddephatt RJ. Organometallics. 2000;19:5063–5070. [Google Scholar]; f) Yam VWW, Choi SWK. J Chem Soc, Dalton Trans. 1994:2057–2059. [Google Scholar]
- 8.a) Arias J, Bardají M, Espinet P. Inorg Chem. 2008;47:1597–1606. doi: 10.1021/ic702252j. [DOI] [PubMed] [Google Scholar]; b) Ho SYH, Cheng ECC, Tiekinink ERT, Yam VWW. Inorg Chem. 2006;45:8165–8174. doi: 10.1021/ic0608243. [DOI] [PubMed] [Google Scholar]; c) Song L, Xia SQ, Hu SM, Du SW, Wu XT. Polyhedron. 2005;24:831–836. [Google Scholar]; d) Zhang HX, Pan QJ. Inorg Chem. 2004;43:593–601. doi: 10.1021/ic0300159. [DOI] [PubMed] [Google Scholar]; e) Bardají M, Laguna A, Jones PG, Fischer AK. Inorg Chem. 2000;39:3560–3566. doi: 10.1021/ic9914342. [DOI] [PubMed] [Google Scholar]; f) Yam VWW, Cheng ECC, Cheung KK. Angew Chem Int Ed. 1999;38:197–199. [Google Scholar]; g) Jones WB, Yuan J, Narayanaswamy R, Young MA, Elder RC, Bruce AE, Bruce MRM. Inorg Chem. 1995;34:1996–2001. [Google Scholar]
- 9.a) Kim P-SG, Hu Y, Brandys MC, Burchell TJ, Puddephatt RJ, Sham TK. Inorg Chem. 2007;46:949–957. doi: 10.1021/ic0609352. [DOI] [PubMed] [Google Scholar]; b) Colacio E, Crespo O, Cuesta R, Kivekas R, Laguna AJ. Inorg Biochem. 2004;98:595–600. doi: 10.1016/j.jinorgbio.2004.01.013. [DOI] [PubMed] [Google Scholar]; c) Brandys MC, Jennings MC, Puddephatt RJ. J Chem Soc, Dalton Trans. 2000:4601–4606. [Google Scholar]
- 10.Gimeno MC, Laguna A. Chem Soc Rev. 2008;37:1952–1966. doi: 10.1039/b708618k. refs. therein. [DOI] [PubMed] [Google Scholar]
- 11.Vickery JC, Olmstead MM, Fung EY, Balch AL. Angew Chem Int Ed Engl. 1997;36:1179–1181. [Google Scholar]
- 12.Mansour MA, Connick WB, Lachicotte RJ, Gysling HJ, Eisenberg R. J Am Chem Soc. 1998;120:1329–1330. [Google Scholar]
- 13.a) Pan QJ, Zhou X, Fu HG, Zhang HX. Organometallics. 2008;27:2474–2482. [Google Scholar]; b) Lee YA, Eisenberg R. J Am Chem Soc. 2003;125:7778–7779. doi: 10.1021/ja034560k. [DOI] [PubMed] [Google Scholar]
- 14.Bojan VR, Fernández EJ, Laguna A, López-de-Luzuriaga JM, Monge M, Olmos ME, Silvestru C. J Am Chem Soc. 2005;127:11564–11565. doi: 10.1021/ja053237+. [DOI] [PubMed] [Google Scholar]
- 15.a) dit Dominique FJB, Gornitzka H, Sournia-Saquet A, Hemmert C. Dalton Trans. 2009:340–352. doi: 10.1039/b809943j. [DOI] [PubMed] [Google Scholar]; b) Ray L, Shaikh MM, Ghosh Inorg Chem. 2008;47:230–240. doi: 10.1021/ic701830m. [DOI] [PubMed] [Google Scholar]; c) Barnard PJ, Wedlock LE, Baker MV, Berners-Price SJ, Joyce DA, Skelton BW, Steer JH. Angew Chem Int Ed. 2006;45:5966–5970. doi: 10.1002/anie.200601526. [DOI] [PubMed] [Google Scholar]; d) Barnard PJ, Baker MV, Berners-Price SJ, Skelton BW, White AH. Dalton Trans. 2004:1038–1047. doi: 10.1039/b316804b. [DOI] [PubMed] [Google Scholar]
- 16.Two selected examples: Rawashdeh-Omary MA, López-de-Luzuriaga JM, Rashdan MD, Elbjeirami O, Monge M, Rodríguez-Castillo M, Laguna AJ. Am Chem, Soc. 2009;131:3824–3825. doi: 10.1021/ja809585n.and refs. therein. Zheng J, Petty JF, Dickson RM. J Am Chem Soc. 2003;125:7780–7781. doi: 10.1021/ja035473v.and refs. therein.
- 17.Luminescent gold-silver compounds containing phosphine ligands: Crespo O, Gimeno MC, Laguna A, Lahoz FJ, Larraz C. Inorg Chem. doi: 10.1021/ic201245q. In press.Koshevoy IO, Lin Y-C, Chen YC, Kartutunen AJ, Haukka M, Chou P-T, Tunik SP, Pakkanen TA. Chem Commun. 2010:1440–1442. doi: 10.1039/b920001k.Wang QM, Jia JH. J Am Chem Soc. 2009;131:16634–16635. doi: 10.1021/ja906695h.Xie ZL, Wei QH, Zhang LY, Chen ZN. Inorg Chem Commun. 2007;10:1206–1209.Crespo O, Gimeno MC, Laguna A, Larraz C, Villacampa MD. Chem Eur J. 2007;13:235–246. doi: 10.1002/chem.200600566.Wang QM, Lee YA, Crespo O, Deaton J, Tang C, Gyslin HJ, Gimeno MC, Larraz C, Villacampa MD, Laguna A, Eisenberg R. J Am Chem Soc. 2004;126:9488–9489. doi: 10.1021/ja048091d.Crespo O, Fernandez EJ, Gil M, Gimeno MC, Jones PG, Laguna A, López-de-Luzuriaga JM, Olmos ME. J Chem Soc, Dalton Trans. 2002:1319–1326.
- 18.Luminescent gold-copper compounds containing phosphine ligands: refs. 17 e,g; Calhorda MJ, Ceamanos C, Crespo O, Gimeno MC, Laguna A, Larraz C, Vaz PD, Villacampa MD. Inorg Chem. 2010;49:8255–8269. doi: 10.1021/ic100413x.Koshevoy IO, Lin YC, Karttunen AJ, Chou PT, Vainiotalo P, Tunik SP, Haukka M, Pakkanen TA. Inorg Chem. 2009;48:2094–2102. doi: 10.1021/ic801987t.Lin YC, Chou PT, Koshevoy IO, Pakkamen TA. J Phys Chem A. 2009;113:9270–9276. doi: 10.1021/jp9040452.Koshevoy IO, Karttunen AJ, Tunik SP, Haukka M, Selinanov SI, Melnikov AS, Serdobintsev PV, Khodorkovskiy MA, Pakkanen TA. Inorg Chem. 2008;47:9478–9488. doi: 10.1021/ic801073k.Koshevoy IO, Koskinen L, Haukka M, Tunik SP, Serdobintsev PY, Melnikov AS, Pakkanen TA. Angew Chem Int Ed. 2008;47:3942–3945. doi: 10.1002/anie.200800452.Hao L, Mansour MA, Lachicotte RJ, Gysling HJ, Eisenberg R. Inorg Chem. 2000;39:5520–5529. doi: 10.1021/ic000396f.
- 19.Luminescent gold-silver compounds which do not contain phosphine ligands: Laguna A, Lasanta T, López-de-Luzuriaga JM, Monge M, Naumov P, Olmos ME. J Am Chem Soc. 2010;132:456–457. doi: 10.1021/ja909241m.Fernández EJ, Hardacre C, Laguna A, Lagunas MC, López-de-Luzuriaga JM, Monge M, Montiel M, Olmos ME, Puelles RC, Sánchez-Forcada E. Chem Eur J. 2009;15:6222–6233. doi: 10.1002/chem.200900167.Fernández EJ, López-de-Luzuriaga Monge M, Olmos ME, Puelles RC, Laguna A, Mohamed AA, Fackler JP., Jr Inorg Chem. 2008;47:8069–8076. doi: 10.1021/ic800452w.Yip SK, Chan CL, Lam WH, Cheung KK, Yam VWW. Photochem Photobiol Sci. 2007;6:365–371. doi: 10.1039/b612334a.Fernández EJ, Jones PG, Laguna A, López-de-Luzuriaga JM, Montiel M, Olmos ME, Puelles RC. Organometallics. 2007;26:5931–5939.Fernandez EJ, Laguna A, López-de-Luzuriaga JM, Monge M, Montiel M, Olmos ME, Rodríguez-Castillo M. Organometallics. 2006;25:3639–3646.Wang QM, Lee YA, Crespo O, Deaton J, Tang C, Gyslin HJ, Gimeno MC, Larraz C, Villacampa MD, Laguna A, Eisenberg R. J Am Chem Soc. 2004;126:9488–9489. doi: 10.1021/ja048091d.Catalano VJ, Moore AL. Inorg Chem. 2005;44:6558–6566. doi: 10.1021/ic050604+.Colis JCF, Larochelle C, Fernández EJ, López-de-Luzuriaga JM, Monge M, Laguna A, Tripp C, Patterson H. J Phys Chem B. 2005;109:4317–4323. doi: 10.1021/jp045868g.Catalano VJ, Malwitz MA, Etogo AO. Inorg Chem. 2004;43:5714–5724. doi: 10.1021/ic049604k.Rawashdeh-Omary MA, Omary MA, Fackler JP., Jr Inorg Chim Acta. 2002;334:376–384.Fernández EJ, Gimeno MC, Laguna A, López-de-Luzuriaga JM, Monge M, Pyykko P, Sundholm D. J Am Chem Soc. 2000;122:7287–7293.Burini A, Bravi R, Fackler JP, Jr, Galassi R, Grant TA, Omary MA, Pietroni BR, Staples RJ. Inorg Chem. 2000;39:3158.
- 20.Luminescent gold-copper compounds which do not contain phosphine ligands: refs. 19 d,e., Fernández EJ, Laguna A, López-de-Luzuriaga JM, Monge M, Montiel M, Olmos ME, Rodríguez-Castillo M. Dalton Trans. 2009:7509–7518. doi: 10.1039/b900768g.Catalano VJ, Moore AL, Shearer J, Kim J. Inorg Chem. 2009;48:11362–11375. doi: 10.1021/ic901914n.Fernández EJ, Laguna A, López-de-Luzuriaga JM, Monge M, Montiel M, Olmos ME. Inorg Chem. 2005;44:1163–1165. doi: 10.1021/ic048346o.
- 21.Recent reviews: Pacheco EA, Tiekink ERT, Whitehouse MW. In: Biomedical applications of gold and gold compounds. Corti C, Holliday R, editors. Gold, CRC Press; 2010. Pacheco EA, Tiekink ERT, Whitehouse MW. Gold compounds and their applications in medicine. In: Mohr F, editor. Gold Chemistry. Wiley-VCH; 2009. pp. 283–319. and refs. therein Nobili S, Mini E, Landini I, Gabbiani C, Casini A, Messori L. Med Res Revs. 2009;30:550–580. doi: 10.1002/med.20168.Bindoli A, Rigobello MP, Scutari G, Gabbiani C, Casini A, Messori L. Coord Chem Revs. 2009;253:1692–1707.Ott I. Coord Chem Revs. 2009;253:1670–1681.Abdou HE, Mohamed AA, Fackler JP, Jr, Burini A, Galassi R, López-de-Luzuriaga JM, Olmos ME. Coord Chem Revs. 2009;253:1661–1669.Milanic V, Dou QP. Coord Chem Revs. 2009;253:1649–1660. doi: 10.1016/j.ccr.2009.01.032.Bruijnincx PCA, Sadler PJ. Current Opinion in Chemical Biology. 2008;12:197–206. doi: 10.1016/j.cbpa.2007.11.013.Barnard PJ, Berners-Price S. Coord Chem Revs. 2007;251:1889–1902.
- 22.Recent reviews and selected examples: Ag nanoparticles Ray M, Yadav A, Gade A. Biotech Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002.and refs. therein Fernández EJ, Garcia-Barrasa J, Laguna A, López-de-Luzuriaga JM, Monge M, Torres C. Nanotech. 2008;19:185602, 6. doi: 10.1088/0957-4484/19/18/185602.Ag compounds: Liu F, Amis R, Hwang E, Varela-Ramírez A, Aguilera RJ, Ovalle R, Contel M. Molecules. 2011;16:6701. doi: 10.3390/molecules16086701.Castellano JJ, Shafii SM, Ko F, Donate G, Wright TE, Mannari RJ. Int Wound J. 2007;4:114–122. doi: 10.1111/j.1742-481X.2007.00316.x.
- 23.Selected examples: Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Burns. 2007;33:139–148. doi: 10.1016/j.burns.2006.06.010.Melayie A, Sun Z, Hindi K, Milsted A, Ely D, Reneker DH, Tessier CA, Youngs WJ. J Am Chem Soc. 2005;127:2285–2291. doi: 10.1021/ja040226s.Tessier CA, Youngs WJ. J Med Chem. 2004;47:973–977. doi: 10.1021/jm030262m.Kasuga NC, Sugie A, Nomiya K. Dalton Trans. 2004:3732–3740. doi: 10.1039/B411859F.
- 24.Recent examples: N-heterocyclic gold(I) carbenes Rubbiani R, Kitanovick I, Alborzinia H, Can S, Kitanovick A, Onambele LA, Stefanopoulou M, Geldmacher Y, Sheldrick WS, Wolber G, Prokop A, Wolf S, Ott I. J Med Chem. 2010;53:8608–8618. doi: 10.1021/jm100801e.Hickey JL, Ruhayel RA, Barnard PJ, Baker MV, Berners-Price S, Filipovska A. J Am Chem Soc. 2008;138:12570–12571. doi: 10.1021/ja804027j.Phosphine gold(I) derivatives: Gandin V, Fernandes AP, Rigobello MP, Dani B, Sorrentino F, Tisato F, Bjornsted M, Bindoli A, Stutaro A, Rella R, Marzano C. Biochem Pharmacol. 2010;79:90–101. doi: 10.1016/j.bcp.2009.07.023.Vergara E, Casini A, Sorrentino F, Zava O, Cerrada E, Rigobello MP, Bindoli A, Laguna M, Dyson PJ. ChemMedChem. 2010;5:96–102. doi: 10.1002/cmdc.200900370.Ellie BT, Levine C, Ubarretxena-Belandia I, Varela-Ramírez A, Aguilera R, Ovalle R, Contel M. Eur J Inorg Chem. 2009:3421–3430. doi: 10.1002/ejic.200900279.and refs. therein.
- 25.Most recent review: Navarro M. Coord Chem Revs. 2009;253:1619–1626.
- 26.Selected recent examples: Fillat MF, Gimeno MC, Laguna A, Latorre E, Ortego L, Villacampa MD. Eur J Inorg Chem. 2011:1487–1495.Eiter LC, Hall NV, Day CS, Saluta G, Kucera GL, Bierbach U. J Med Chem. 2009;52:6519–6522. doi: 10.1021/jm9012856.Jackson-Rosario S, Cowart D, Myers A, Tarrien R, Levine RL, Scott RA, Shelf WTh. J Biol Inorg Chem. 2009;14:507–519. doi: 10.1007/s00775-009-0466-z.Nomiya K, Yamamoto S, Noguchi R, Yokoyama H, Kasuga NC, Ohyama K, Kato C. J Inorg Biochem. 2003;95:208–220. doi: 10.1016/s0162-0134(03)00125-9.
- 27.Ray S, Mohan R, Singh JK, Samantaray MK, Shaikh MM, Panda D, Ghosh P. J Am Chem Soc. 2007;129:15042–15053. doi: 10.1021/ja075889z. [DOI] [PubMed] [Google Scholar]
- 28.a) Panzner MJ, Deeraksa A, Smith A, Wright BD, Hindi KM, Kascatan-Nebioglu A, Torres AG, Judy BM, Hovis CE, Hilliard JK, Mallett RJ, Cope E, Estes DM, Cannon CL, Leid JG, Youngs WJ. Eur J Inorg Chem. 2009:1739–1745. doi: 10.1002/ejic.200801159. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Melaiye A, Simons RS, Milsted A, Pingitore F, Wesdemiotis C, Tessier CA, Youngs WJ. J Med Chem. 2004;47:973–977. doi: 10.1021/jm030262m. [DOI] [PubMed] [Google Scholar]; c) Melaiye A, Sun ZH, Hindi K, Milsted A, Ely D, Reneker DH, Tessier CA, Youngs WJ. J Am Chem Soc. 2005;127:2285–2291. doi: 10.1021/ja040226s. [DOI] [PubMed] [Google Scholar]; d) Roland S, Jolivat C, Cresteil T, Eloy L, Bouhours P, Hequet A, Mansuy V, Vanucci C, Paris JM. Chem Eur J. 2011;17:1442–1446. doi: 10.1002/chem.201002812. [DOI] [PubMed] [Google Scholar]; e) Kascatan-Nebioglu A, Melaiye A, Hindi K, Durmus S, Panzner MJ, Hogue LA, Mallett RJ, Hovis CE, Coughenour M, Crosby SD, Milsted A, Ely DL, Tessier CA, Cannon CL, Youngs WJ. J Med Chem. 2006;49:6811–6818. doi: 10.1021/jm060711t. [DOI] [PubMed] [Google Scholar]; f) Hindi KM, Siciliano TJ, Durmus S, Panzner MJ, Medvetz DA, Reddy DV, Hogue LA, Hovis CE, Hilliard JK, Mallet RJ, Tessier CA, Cannon CL, Youngs WJ. J Med Chem. 2008;51:1577–1583. doi: 10.1021/jm0708679. [DOI] [PubMed] [Google Scholar]
- 29.Barrio E, Casas JS, Couce MD, Laguna A, López-de-Luzuriaga JM, Monge M, Sánchez A, Sordo J, Varela JM, Vázquez López EM. Eur J Inorg Chem. 2011:1322–1333. [Google Scholar]
- 30.Pelletier F, Comte V, Massard A, Wenzel M, Toulot S, Richard P, Picquet M, Le Gendre P, Zava O, Edafe F, Casini A, Dyson PJ. J Med Chem. 2010;53:6923–6933. doi: 10.1021/jm1004804.and refs. therein. Wenzel M, Bertrand B, Eymin MJ, Comte V, Harvey JA, Richard P, Groessl M, Zava O, Amrouche H, Harvey PD, Le Gendre P, Picquet M, Casini A. Inorg Chem. 2011;50:9472–9480. doi: 10.1021/ic201155y.González-Pantoja JF, Stern M, Jarzecki AA, Royo E, Robles-Escajeda E, Varela-Ramírez A, Aguilera RJ, Contel M. Inorg Chem. 2011;50:11099–11110. doi: 10.1021/ic201647h.
- 31.Contel M, Jiménez J, Jones PG, Laguna A, Laguna M. J Chem Soc, Dalton Trans. 1994:2515–2518. [Google Scholar]
- 32.Contel M, Garrido J, Gimeno MC, Jones PG, Laguna A, Laguna M. Organometallics. 1996:4939–4943. [Google Scholar]
- 33.Laguna M, Garrido J, Contel M. Inorg Synth. 2000;33:181–184. [Google Scholar]
- 34.Cerrada E, Contel M, Valencia AD, Laguna M, Gelbrich T, Hursthouse MB. Angew Chem Int Ed. 2000;39:2353–2356. doi: 10.1002/1521-3773(20000703)39:13<2353::aid-anie2353>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 35.Usón R, Laguna Fernández EJ, Ruíz Romero ME, Jones PG, Lautner J. J Chem Soc, Dalton Trans. 1989:2127–2131. [Google Scholar]
- 36.Gambarotta S, Floriani C, Chiesi-Villa A, Guastini C. J Chem Soc, Chem Commun. 1983:1304–1306. [Google Scholar]
- 37.Meyer EM, Gambarotta S, Floriani C, Chiesi-Villa A, Guastini C. Organometallics. 1989;8:1067–1079. [Google Scholar]
- 38.Fernández EJ, Laguna A, López-de-Luzuriaga JM, Montiel M, Olmos ME, Pérez J, Puelles RC. Organometallics. 2006;25:4307–4315. [Google Scholar]
- 39.Contel M. PhD. Public University of Navarra; Spain: 1996. [Google Scholar]
- 40.Contel M, Garrido J, Gimeno MC, Jiménez J, Jones PG, Laguna A, Laguna M. Inorg Chim Acta. 1997;254:157–161. [Google Scholar]
- 41.Selected review: Bardají M, Laguna M. Eur J Inorg Chem. 2003:3069–3079.and refs. therein
- 42.Schmidbaur H, Schier A. Chem Soc Rev. 2008;37:1931–1951. doi: 10.1039/b708845k. and refs. therein. [DOI] [PubMed] [Google Scholar]
- 43.Rizzato S, Bergs J, Mason SA, Albinati A, Kozelka J. Angew Chem. 2010;122:7602–7605. doi: 10.1002/anie.201001892. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:7440–7443. doi: 10.1002/anie.201001892. [DOI] [PubMed] [Google Scholar]
- 44.Falvello LR. Angew Chem Int Ed. 2010;49:10045–10047. doi: 10.1002/anie.201002928. [DOI] [PubMed] [Google Scholar]
- 45.a) Carriedo GA, García-Alonso FJ, García-Alvarez JL, Díaz C, Valenzuela ML, Yutronic-Saez N. Polyhedron. 2002;21:2587–2592. [Google Scholar]; b) Jiménez J, Laguna A, Benouazzane M, Sanz JA, Díaz C, Valenzuela ML, Jones PG. Chem Eur J. 2009;15:13509– 13520. doi: 10.1002/chem.200902180. [DOI] [PubMed] [Google Scholar]
- 46.Berners-Price SJ, Bowen RJ, Hambley TW, Healy PC. J Chem Soc Dalton Trans. 1999:1337–1346. [Google Scholar]
- 47.Cariati F, Naldini L, Simonetta G, Malatesta L. Inorg Chim Acta. 1967;1:315–318. [Google Scholar]
- 48.Forward JM, Fackler JP, Jr, Assefa Z. In: Optoelectronic Properties of Inorganic Compounds; Roundhill DM, Fackler JP Jr, editors. Plenum Press; New York: 1999. pp. 195–226. [Google Scholar]
- 49.Kim P-SG, Hu YF, Puddephatt RJ, Sham TK. Phys Scr. 2005;T115:545–547. [Google Scholar]
- 50.The correlation between the nuclearity enhancement and the metal-centered transition energy is well known [19l and refs. therein] because as the number of Au-Au interactions increases, the HOMO-LUMO gap is reduced.
- 51.a) Wang S, Fackler JP, Jr, king C, Wang JC. J Am Chem Soc. 1988;110:308. [Google Scholar]; b) Wang S, Garzón G, king C, Wang JC, Fackler JP., Jr Inorg Chem. 1989;28:4616. [Google Scholar]
- 52.Yoshida Y, Fujii J, Saito G, Hiramatsu T, Sato N. J Mater Chem. 2006;16:724–727.b) Similar energy values have been attributed to Au-Au singlet-triplet (5dσ*→6pz) transitions.[19f]. c) An interligand charge transfer is highly unlikely due to the relatively low energy of the observed emission.
- 53.a) Novelli F, Recine M, Sparatore F, Juliano C. Il Farmaco. 1999:232–236. doi: 10.1016/s0014-827x(99)00019-1. [DOI] [PubMed] [Google Scholar]; b) Nomiya K, Noguchi R, Ohsawa K, Tsuda K, Oda M. J Inorg Biochem. 2000;78:363–370. doi: 10.1016/s0162-0134(00)00065-9. [DOI] [PubMed] [Google Scholar]; c) Nomiya K, Noguchi R, Shigeta T, Kondoh Y, Tsuda K, Ohsawa K, Kasuga NC, Oda M. Bull Chem Soc Jpn. 2000;73:1143–1152. [Google Scholar]; d) Nomiya K, Noguchi R, Oda M. Inorg Chim Acta. 2000;298:24–32. [Google Scholar]; e) Henderson W, Nicholson BK, Tienkink ERT. Inorg Chim Acta. 2006;359:204–214. [Google Scholar]; f) Marques LL, de Oliveira GM, Lang ES, de Campos MMA, Gris LRS. Inorg Chem Commun. 2007;10:1083–1087. [Google Scholar]
- 54.Liu JJ, Galettis P, Farr A, Maharaj L, Samarasinha H, McGechan AC, Baguley BC, Bowen RJ, Berners-Price S, Mckeage MJ. J Inorg Biochem. 2008;102:303–310. doi: 10.1016/j.jinorgbio.2007.09.003.Berners-Price SJ, Bowenm RJ, Galettis P, Healy PC, McKeage MJ. Coord Chem Rev. 1999:185–186. 823–836.and refs. therein.
- 55.Zhang J-A, Pan M, Zhang J-Y, Zhagn H-K, Fan Z-J, Kang B-S. Polyhedron. 2009;28:145–149. [Google Scholar]
- 56.van Waasbergen LG, Fajdetic I, Fianchini M, Dias HVR. J Inorg Biochem. 2007;101:1180–1183. doi: 10.1016/j.jinorgbio.2007.05.002. and refs therein. [DOI] [PubMed] [Google Scholar]
- 57.Bowen RJ, Graner AC, Berners-Price SJ, Jenkis ID, Sue RE. J Org Chem. 1998;554:181–184. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.