Abstract
It is well established that refractive development is regulated by visual feedback. However, most optical treatment strategies designed to reduce myopia progression have not produced the desired results, primarily because some of our assumptions concerning the operating characteristics of the vision-dependent mechanisms that regulate refractive development have been incorrect. In particular, because of the prominence of central vision in primates, it has generally been assumed that signals from the fovea determine the effects of vision on refractive development. However, experiments in laboratory animals demonstrate that ocular growth and emmetropization are mediated by local retinal mechanisms and that foveal vision is not essential for many vision-dependent aspects of refractive development. On the other hand, the peripheral retina, in isolation, can effectively regulate emmetropization and mediate many of the effects of vision on the eye’s refractive status. Moreover, when there are conflicting visual signals between the fovea and the periphery, peripheral vision can dominate refractive development. The overall pattern of results suggests that optical treatment strategies for myopia that take into account the effects of peripheral vision are likely to be more successful than strategies that effectively manipulate only central vision.
Keywords: myopia, hyperopia, emmetropization, myopia progression, peripheral refraction, animal models
Because the effects of myopia on visual acuity can usually be mitigated (e.g., via spectacles), myopia is often considered to be a minor health care issue. However, for the following reasons, myopia is a significant public health concern and it is critical that we develop new treatment strategies for myopia.
First, myopia is a very common condition. While traditional estimates indicate that about 25% of the adult US population has myopia,1 in regions of East Asia, myopia has reached epidemic proportions and its prevalence now exceeds 80% in some highly educated groups.2–6 Moreover, there is growing evidence that the prevalence of myopia, including high myopia, is increasing rapidly in the USA1, 7–9, 10 and other non-Asian countries.11, 12 This rapid rise in myopia prevalence suggests that changing environmental factors are significantly influencing current patterns of refractive errors.13
Myopia is associated with ocular complications that can lead to permanent vision loss. Presumably because of structural changes associated with excessive axial elongation,14 myopic eyes have an increased risk of cataract,15–17 glaucoma,18, 19 chorioretinal degenerations and retinal detachments.20–22 As a result, myopia is a leading cause of permanent visual impairment.23
Myopia has a substantial economic burden on society. In the USA, we spend billions of dollars each year on refractions and optical corrections for myopia. In addition, considerable costs are associated with treating the eye diseases connected with myopia and managing the visual impairment and blindness that can result from these conditions.1, 24
Traditional optical treatment regimens for slowing myopia progression have had limited success. For example, under-correction strategies actually accelerate the rate of myopia progression25, 26 and although traditional multifocal lenses significantly reduce the rate of myopia progression, the magnitude of the treatment effects are generally small and not clinically meaningful.27–30
Fortunately, in recent years the optometric and vision science communities have made significant strides in our understanding of how vision can influence eye growth and refractive development. As a consequence I believe that in the near future we will have a variety of different optical treatment strategies that effectively reduce myopia progression. In this paper, I focus on one potential optical treatment strategy. Specifically, I will present the results from a series of studies of laboratory animals that I believe provide the foundation for a peripheral optical treatment strategy. For practical reasons, most of this research was conducted during the early infantile stage of ocular growth, a factor that potentially complicates extrapolations to older humans. However, the vision-dependent mechanisms that influence refractive development are active well into early adulthood, and appear, at least in laboratory animals, to operate in a qualitatively similar manner in young and old animals.31–34
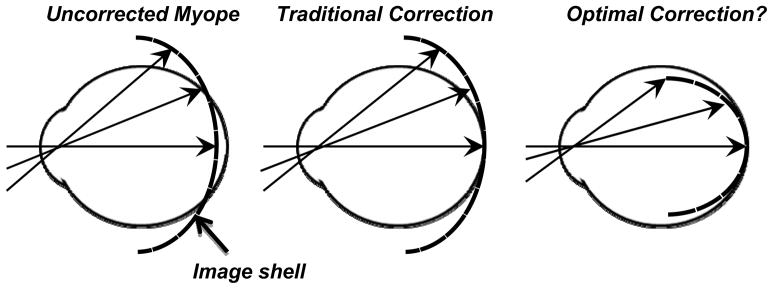
Figure 1 illustrates the basic goal of this peripheral optical treatment strategy. Primarily because myopic eyes are less oblate in shape than emmetropic eyes, the image shell for distant objects is flatter than the surface of the retina.35–38 As a consequence, when myopic eyes are corrected with traditional spectacle or contact lenses, the retinal image at the fovea is in focus; however, most of the retina experiences hyperopic defocus that generally increases in magnitude with eccentricity,39–46 a situation that arguably results in a strong signal for myopic growth.47–51 As shown in the right panel of Figure 1, the goals of the peripheral treatment strategy are to provide optimal central vision (i.e., an in-focus foveal image) and, at the same time, to increase the curvature of field of the image shell to eliminate peripheral visual signals that stimulate axial growth (i.e., hyperopic defocus) and/or to produce signals that reduce axial growth (i.e., in focus images or myopically focused images). This represents a significant departure from traditional negative-powered correcting lenses, which typically increase the degree of relative peripheral hyperopia in myopic eyes.52, 53
Figure 1.
Schematic of the optical goals of a potential peripheral treatment strategy to slow the progression of myopia. The left panel illustrates the position of the image shell for a distant object in a typical unaccommodated myopic eye. The middle panel shows that traditional correcting lenses provide an in focus foveal image but do not correct the relative hyperopia that usually occurs in the periphery. The right panel emphasizes that a fundamental goal of a peripheral treatment strategy would be to provide optimal central vision and at the same time eliminate peripheral visual signals that may stimulate growth and produce visual signals that normally reduce growth.
Why should we be concerned with peripheral refractive errors? With respect to controlling myopia progression, I believe that there are 5 primary reasons.
Ocular Growth and Refractive Development are Regulated by Visual Feedback
A very large body of evidence indicates that ocular growth and refractive development are regulated by visual feedback associated with the eye’s effective refractive state, in essence optical defocus. The observations that emmetropization does not occur in animals that are reared in total darkness,54 that from deprivation, by preventing meaningful visual feedback, results in unregulated, “open-loop” axial growth,55–58 and that, at least early in life, animals can recover from induced refractive errors59–63 support the idea that the eye uses visual feedback to regulate refractive development. However, the results that have come from what are commonly referred to as lens compensation experiments, a strategy that was pioneered by Frank Schaeffel,64, 65 have provided the strongest and most clinically meaningful evidence that optical defocus regulates ocular growth and refractive development.
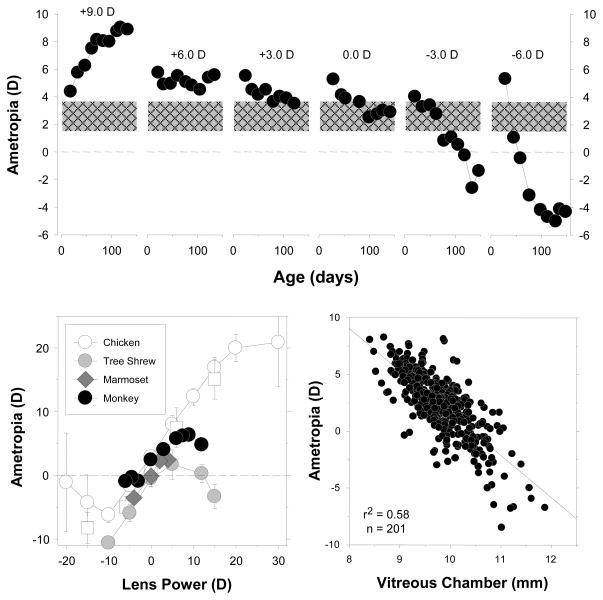
In lens compensation experiments, powered treatment lenses are employed to alter the eye’s effective refractive status by a known amount and the effects of these lenses on subsequent ocular and refractive development are assessed over time. The key finding, which is illustrated in Figure 2, is that the eyes of young animals can alter their refractive state in a manner that “compensates” for the optically imposed errors. In the top row of Figure 2, refractive error is plotted as a function of time for individual monkeys reared with positive-, negative- or zero-powered spectacle lenses over both eyes.61 As reflected by the first data point for each animal, shortly after birth infant monkeys are moderately hyperopic and, as illustrated by the control animal that was reared with zero-powered lenses over both eyes, emmetropization normally occurs quickly so that by 4–5 months of age most normal monkeys exhibit low degrees hyperopia. However, putting negative lenses on monkeys accelerates the normal reduction in hyperopia and causes negative-lens-reared monkeys to become myopic. The resulting degree of myopia is correlated with the power of the treatment lenses so that at the end of the treatment period when these animals viewed through their negative treatment lenses, their “corrected” refractive states are similar to the natural ametropias of normal monkeys. Conversely, positive lenses prevented the normal decrease in hyperopia and, as shown in the left most panel, it is possible to produce substantial amounts of absolute hyperopia in infant monkeys if the treatment lens powers are increased gradually over time during the lens-rearing period. The process of lens compensation is largely independent in the two eyes. For example, in response to securing different powered lenses in front of the two eyes, animals will develop anisometropias that compensate for the imposed imbalance.61, 66
Figure 2.
Results from lens compensation experiments in infant monkeys. Top. Spherical-equivalent spectacle-plane ametropias plotted as function of age for individual monkeys reared with powered spectacle lenses in front of both eyes.61 The first and last data point for each animal represent the start and end of the lens-rearing period. The powers of the treatment lenses are given in each panel. The monkey shown in the left most plot was treated with progressively increasing powers of positive lenses beginning with +4.5 D and ending with +9 D lenses. All the other monkeys wore the same powered lenses throughout the treatment period. The cross-hatched area shows the mean (± 1 SD) ametropias for normal 4- to 5-month-old infant monkeys. The bottom left plot compares the results of lens compensation experiments in different species (chickens,120, 121 tree shrews,122 marmosets,70 monkeys61). The mean ametropia obtained at the end of the lens-rearing period is plotted as a function of the power of the treatment lenses. In the bottom right panel, the ametropia obtained at the end of the lens-rearing period is plotted as a function of vitreous chamber depth for individual experimental monkeys.74
It is noteworthy that the phenomenon of lens compensation has been observed in every animal species that has been studied in a systematic manner (e.g., chickens,64 fish,67 guinea pigs,68 tree shrews,69 marmosets70 and rhesus monkeys66). Moreover the results are qualitatively similar in these species. For example, in the bottom left graph in Figure 2, the mean refractive error at the end of the treatment period is plotted as a function of treatment lens power for 4 species commonly used in refractive error research. In each species, for a range of moderate powered treatment lenses the resulting ametropia was positively correlated with treatment lens power, i.e., treatment lenses produced predictable compensating changes in refractive error. This and other similarities between species (e.g., emmetropization, form deprivation myopia and the spatial and temporal integration properties of these vision-dependent mechanisms) indicate that the vision-dependent mechanisms that regulate refractive development have been largely conserved across species and consequently, it is likely that the results from laboratory animals can be extrapolated to humans with some degree of confidence. In this respect, when comparable data are available, the results from humans are qualitatively similar to those from laboratory animals. For example humans, like all animals species that have been studied in a systematic fashion, demonstrate the phenomenon of form deprivation myopia71 and anisometropic compensation.72
The lens-induced changes in refractive error are primarily axial in nature and come about principally as a result of changes in vitreous chamber growth rates. As shown in the lower right panel, at the end of the lens-rearing period, there is a strong correlation between refractive error and vitreous chamber depth. In animals subjected to monocular treatment regimens, interocular differences in vitreous chamber depth can explain upwards to 80–90% of the interocular differences in refractive error.60, 73 Changes in corneal power make a minor contribution to vision-induced changes in refractive error (e.g., r2 = 0.12); however, in monkeys, the remainder of the anterior segment is largely unaffected by vision-induced alterations in refractive error.74
The Vision-Dependent Mechanisms that Regulate Refractive Development Operate in a Regionally Selective Manner
Experiments that have employed reduction strategies to identify the visual system components that regulate refractive development have revealed that the dominant vision-dependent mechanisms are located within the eye. For example, surgically sectioning the optic nerve75–77 or pharmacologically blocking the conduction of action potentials in the optic nerve does not prevent the phenomenon of form-deprivation myopia.78 Similarly, interrupting signal transmission in the treated eye’s optic nerve does not prevent the recovery from form-deprivation myopia79 or the compensating responses to optically imposed defocus, although the resulting responses may be attenuated and there may be a shift in the end point for emmetropization.79–81 Moreover, experimental manipulations that eliminate the primary parasympathetic or sympathetic inputs to the eye also fail to prevent the phenomena of form deprivation myopia75 or lens compensation, although these vision induced responses may not be identical to those observed in intact eyes.81–83 In other words, the signals that the retinal image is degraded and/or defocused do not have to get out of the eye in order to produce vision-induced alterations in refractive error. In addition, the primary neural inputs to the eye and their associated physiological processes (e.g., accommodation) can be interrupted without eliminating the influence of vision-dependent mechanisms on refractive development.
More importantly, at least with respect to the potential influence of peripheral vision on central refractive development, the vision-dependent retinal mechanisms that dominate refractive development operate in a local, regionally selective manner. The most direct evidence for the local nature of these mechanisms come from experiments in which the visual signals for ocular growth have been varied in a systematic fashion across the visual field, an experimental strategy first employed by the Hodos84 and Wallman laboratories.59, 85
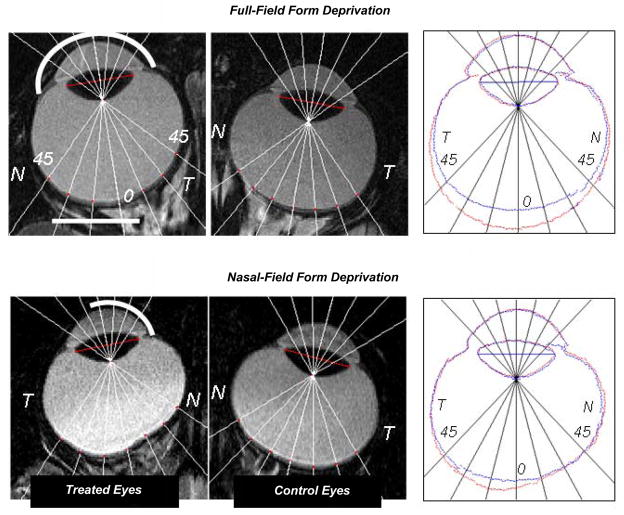
The results presented in Figure 3 demonstrate that, as in birds,59, 84, 85 tree shrews86 and guinea pigs,87 regional variations in visual experience can produce regionally selective alterations in ocular growth in primates. Specifically, Figure 3 shows the horizontal magnetic resonance images obtained at ages corresponding to the end of the treatment period for both eyes of representative monkeys treated with monocular, full-field form deprivation (top row) and monocular form deprivation that was restricted to the nasal visual field (bottom row). Simple inspection reveals that full-field form deprivation produced an obvious increase in the size of the treated eye. As emphasized in the right panel, which shows the superimposed outlines of the treated and control eyes, the anterior segments were similar in the two eyes. Full-field form deprivation resulted in relative increases in vitreous chamber depth that were greatest in the central retina and decreased in a relatively symmetric manner in the nasal and temporal retinas, i.e., the treated eye developed a longer axial length and became more prolate in shape.73 In contrast in the monkey reared with nasal-field form deprivation, the changes in vitreous chamber depth were asymmetrical across the horizontal retina. In particular, the increases in vitreous chamber depth were largely restricted to the temporal retina; the outlines of the treated and control eyes virtually overlapped over most of the nasal retina.88 Qualitatively similar results were obtained in monkeys reared with imposed nasal-field hyperopic defocus.89
Figure 3.
Magnetic resonance images obtained in the horizontal plane for the treated (left) and control eyes (middle) of monkeys reared with monocular full-field form deprivation73 and monocular nasal-field form deprivation.88 The nasal and temporal retinas are designated as N and T, respectively. In the right panels, the outlines for the treated (red) and fellow eyes (blue) have been superimposed after rotating the fellow eye images around the optic axes so that the nasal retinas (N) are shown to the right for both eyes. The superimposed images were aligned using the lines that connected the equatorial poles of the crystalline lenses as a reference (the red lines shown in the treated and fellow eye images in the left and middle columns).
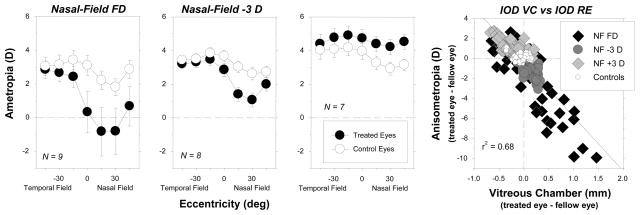
The regional alterations in vitreous chamber depth produced by hemifield treatment regimens predictably altered the pattern of peripheral refractions in the treated eyes. Figure 4 shows the average refractive errors plotted as a function of horizontal visual field eccentricity for the treated and control eyes of monkeys reared with nasal-field form deprivation, −3 D of nasal-field defocus, or +3 D of nasal-field defocus. In all three treatment groups, the treated-eye refractive errors obtained over the temporal visual field were similar to those for the fellow control eyes. However, for the monkeys reared with nasal-field form deprivation and nasal-field hyperopic defocus, the refractive errors in the nasal visual fields of the treated eyes were more myopic/less hyperopic than those for their fellow control eyes. For the monkeys reared with imposed nasal-field myopic defocus, the treated eyes exhibited relative hyperopic anisometropias that were greatest in magnitude in the nasal visual field. As illustrated in the right plot of Figure 4 in which interocular differences in refractive error are plotted as a function of interocular differences in vitreous chamber depth, there was a strong correlation between the changes in the pattern of peripheral refractions and the changes in eye shape.
Figure 4.
Average (±SE) spherical-equivalent refractive corrections obtained at the end of the lens-rearing period (about 4.5 months of age) plotted as a function of horizontal visual field eccentricity for monkeys reared with spectacle lenses that produced either form deprivation (leftmost),88 3 D of hyperopic defocus (second from left),89 or 3 D of myopic defocus in the nasal fields of the treated eyes (second from right). The treated and control eyes are represented by the filled and open symbols, respectively. In the right plot, the interocular differences in refractive error are plotted as a function of the interocular differences in vitreous chamber depth. Individual data are shown for the different visual field eccentricities. The solid line represents the best fitting regression line. NF FD = nasal field form deprivation; NF −3 D = nasal field hyperopic defocus; NF +3 D = nasal field myopic defocus; controls = normal monkeys.
The fact that the vision-dependent mechanisms that dominate refractive development operate in a regionally selective fashion suggests that it is unlikely that global mechanisms play a primary role in refractive development. For example, it is difficult to imagine how the act of accommodation or an increase in intraocular pressure could produce the regional changes in eye shape and refractive error shown in Figures 3 and 4. However, the existence of local acting vision-dependent mechanisms provides a way for peripheral vision to influence eye shape and axial length in a manner that is independent of central vision.
Visual Signals from the Fovea are Not Essential for Many Aspects of Vision-Dependent Growth
It has historically been assumed that visual signals from the fovea dominate refractive development.90 This is a logical assumption because resolution acuity is highest at the fovea and the fovea is the part of the retina that is most sensitive to optical defocus. In addition, accommodation is largely controlled by visual signals from the fovea. As a consequence, efforts to associate visual experience and refractive development, in particular, the development of myopia, have until recently concentrated almost exclusively on central vision and most of our optical treatment strategies have focused on manipulating image quality and/or the effective refractive state of the fovea.
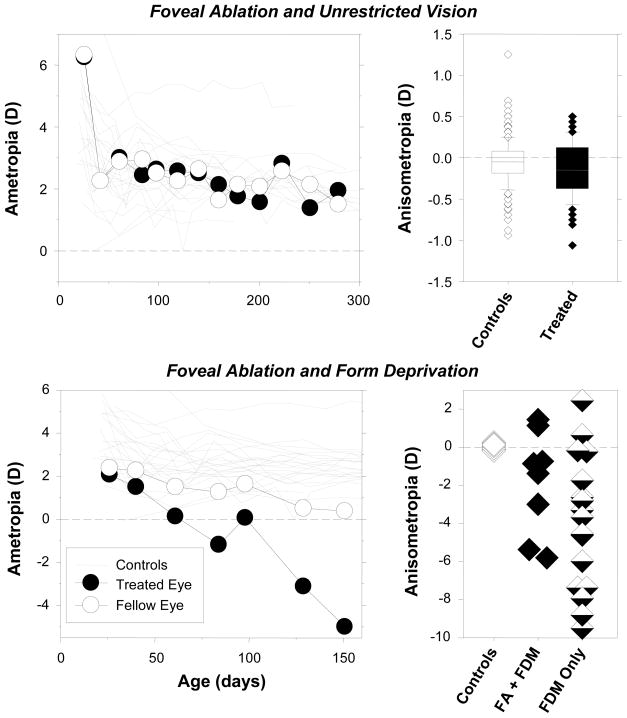
If visual signals from the fovea dominate vision-dependent ocular growth and refractive development, then it is reasonable to assume that eliminating these visual signals would alter refractive development. However, foveal signals can be eliminated without significantly interfering with many vision-dependent phenomena. For example, the top row of Figure 5 shows that eliminating visual signals from the fovea by laser photoablation does not alter the course of emmetropization in infant monkeys raised with unrestricted vision. The top left graph shows refractive error plotted as a function of age for a monkey that had the central 10° to 12° of the retina in one eye ablated using a thermal laser at 3 weeks of age. If foveal signals were critical for normal emmetropization, one would expect to see systematic interocular differences in refractive development, which could, for instance, be manifest as alterations in the time course, end point, or the effectiveness or efficiency of the emmetropization process. However, the course of emmetropization for the treated eye was very similar to that for the control eye and, as illustrated in the top right graph, which compares the interocular differences in refractive that occurred during emmetropization in normal monkeys and monocularly laser-treated monkeys, eliminating foveal visual signals does not appear to systematically alter normal refractive development.91
Figure 5.
Effects of foveal ablation on refractive development in animals reared with unrestricted vision (top row) and monocular form deprivation (bottom row).91 The left plots show spherical-equivalent refractive corrections plotted as a function of age for the treated (filled symbols) and control eyes (open symbols) of representative monkeys. The foveal ablations were performed at the onset of the observation period or at the onset of form deprivation. The right plots show the interocular differences in refractive error for individual animals. The top right graph includes data obtained throughout the observation period for 5 treated monkeys and 24 age-matched control monkeys. The horizontal lines in the box plots represent the medians, the bottoms and tops of the boxes represent the 25th and 75th percentiles. The whiskers that extend vertically from the tops and bottoms of the boxes represent the 90th and 10th percentiles, respectively. The diamond symbols represent outliers. The bottom right graph shows data obtained at the end of the lens-rearing period for individual animals. Controls = normal monkeys; FA + FDM = foveal ablation and form deprivation; FDM only = monocular form deprivation.
Visual signals from the fovea are not essential for the phenomenon of form-deprivation myopia.91 The bottom left plot of Figure 5 shows longitudinal refractions for a monocularly form-deprived monkey in which the fovea of the treated eye was ablated at the onset of form deprivation. Despite the absence of foveal signals, the treated eye systematically developed axial myopia during the period of form deprivation. As shown in the bottom right plot in Figure 5, at the end of the treatment period, the effects of form deprivation were similar in monkeys with foveal ablations and those with intact retinas. Although there is substantial inter-subject variability in the degree of FD myopia, there was a non-significant tendency for the refractive-error changes to be smaller in the eyes with foveal ablation. It would be expected that foveal signals would contribute to the overall signals for ocular growth in intact eyes and that eliminating the fovea would remove this contribution resulting in a somewhat smaller overall growth signal. However, it is clear that the fovea does not convey something that is unique or essential to the phenomenon of FDM.
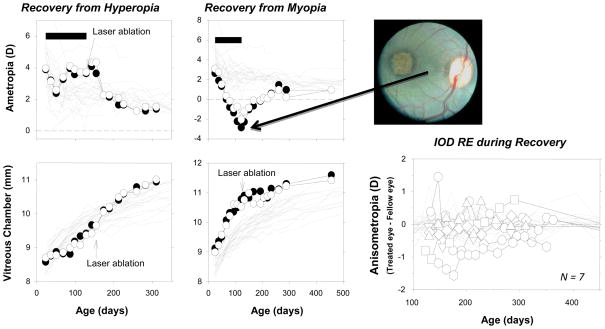
In the absence of visual signals from the fovea, eyes can recover from experimentally induced refractive errors,92 a process guided by visual feedback.63 Figure 6 shows longitudinal data from representative monkeys that wore treatment lenses over both eyes. In the animal represented in the left column, the treatment lenses slowed axial growth in both eyes and produced similar degrees of hyperopia. In the animal shown in the middle column, the experimental regimen accelerated axial growth and produced myopia in both eyes. At the end of the lens-rearing period, the fovea of one eye was ablated and the animals were allowed unrestricted vision. During the recovery period, both animals exhibited emmetropizing responses that very similar in the intact and laser-treated eyes. For the myopic animal, both eyes showed a dramatic decrease in vitreous chamber growth and both eyes became less myopic as the cornea and lens normally decreased in power, apparently following a genetically determined, preprogramed growth plan. Both eyes of the hyperopic animal exhibited an acceleration in vitreous chamber growth and the degree of hyperopia decreased systematically to normal levels. The key point, as emphasized in the right panel of Fig 6, there were no systematic differences in the recovery process in eyes with foveal ablations and those with intact retinas. These results suggest that the periphery, in isolation, can detect the presence of a refractive error and direct eye growth in a manner that eliminates that error.
Figure 6.
Effects of foveal ablations on recovery from experimentally induced refractive errors.92 Left. Spherical-equivalent, spectacle-plane refractive corrections (top) and mean vitreous chamber depths along the pupillary axis (bottom) are plotted as a function of age for the right eyes of individual control animals (thin gray lines) and the laser-treated (filled symbols) and non-lasered fellow eyes (open symbols) of two monkeys that wore binocular peripheral diffusers. The filled horizontal bars in the top plots indicate the lens-rearing periods. The laser photoablations were performed immediately after the lens-rearing period (top right). Right bottom. Interocular differences in refractive error plotted as a function of age for 7 monkeys with experimentally induced refractive errors that had the fovea of one eye ablated via laser photocoagulation at the end of the lens-rearing period. The first symbol for each animal represents the start of the recovery period. The thin gray lines represent data from the control monkeys.
Because of the prominent role of foveal vision in primates, it is somewhat uncomfortable to think that the fovea does not directly control refractive development. In this respect, it is important to recognize that 1) the vision-dependent mechanisms that dominate refractive development evolved in species without foveas (e.g., fish), i.e., species in which panoramic vision is more important than central vision, 2) these mechanisms operate very effectively even in eyes with comparatively low spatial resolution (e.g., chickens, tree shrews, and guinea pigs), and 3) the operating properties of the these mechanisms have been largely conserved across species. From this perspective, it is reasonable to expect the periphery to play a significant role in regulating eye growth and ocular shape in order to optimize refractive error across the visual field.
When Conflicting Signals Exist Between the Central and Peripheral Retina, Peripheral Visual Signals Can Dominate Central Refractive Development
It is likely that the relative contribution of visual signals from a given retinal location to overall eye growth vary with eccentricity and depend on various factors like the sensitivity of local retinal neurons to critical visual cues and the absolute number or density of retinal neurons (as well as eccentricity dependent variations in the biology of the choroid and sclera). Because the densities of cone photoreceptors, ganglion cells and certain other neurons are higher in the central retina and decrease dramatically with eccentricity,93 it seems reasonable to expect that foveal signals would generally override potentially conflicting signals from more peripheral locations. However, as illustrated in Figure 7 that is not necessarily the case.
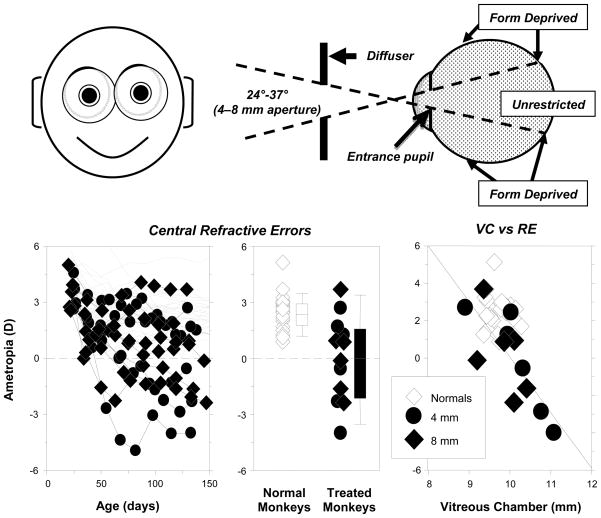
Figure 7.
Effects of peripheral form deprivation on refractive development.92 Top. Schematic illustrating the effects of the treatment lenses. Bottom left. Spherical-equivalent refractive corrections measured along the pupillary axis plotted as a function of age for the right eyes of individual control animals (thin gray lines) and treated monkeys (filled symbols) reared with diffusers with 4 mm (diamonds) and 8 mm apertures (circles). Bottom middle. Refractive errors for treated (diamonds, 4 mm apertures; circles, 8 mm apertures) and control animals at ages corresponding to the end of the period of peripheral form deprivation. The open and filled bars indicate the median and the 10th, 25th, 50th, and 90th percentiles for the control and treated monkeys, respectively. Bottom right. Vitreous chamber depth plotted as a function of spherical-equivalent refractive error for treated (diamonds, 4 mm apertures; circles, 8 mm apertures) and control animals at ages corresponding to the end of the treatment period. The line represents the results from the regression analysis of the data from the treated monkeys.
Figure 7 presents a comparison of central refractive development between normal monkeys and those reared wearing binocular diffuser spectacles that had either 4 or 8 mm central apertures centered on the pupils of each eye.92 When viewing through the treatment lenses, the central 24°–37° of the retina received unrestricted vision while the remaining parts of the visual field experienced form deprivation. In essence, the central retina experienced visual signals that normally support emmetropization, while the peripheral retina received visual signals that normally result in axial myopia. As illustrated in the bottom left panel of Figure 7, the longitudinal refractive-error profiles of the experimental animals were very different from those observed in normal monkeys. At the end of the lens-rearing period, 8 of the 12 experimental monkeys showed central refractive errors that were less hyperopic/more myopic than 95% of the normal monkeys (bottom middle panel). These myopic changes in the treated monkeys were associated with increases in the axial depth of the vitreous chamber (bottom right panel).
The results shown in Figure 7 demonstrate that visual signals from the periphery can override visual signals from the central retina and alter central refractive development. Stated in another way, the centrally generated signals associated with in-focus images and myopic defocus, i.e., signals that normally stop axial growth, were not effective in retarding the axial myopia produced by peripheral form deprivation. There are several potential explanations for the relative dominance of the peripheral retina. As suggested by Wallman and Winawer,94 if there is summation or integration of growth signals across the posterior globe, spatial summation factors may allow the peripheral retina to dominate central refractive development, particularly if the relative strengths of growth/stop signals are dependent on the absolute number of specific signaling neurons. For example, although the density of many retinal neurons is highest in the central retina, the absolute numbers are low because the central retina represents a relatively small part of the total retina. Specifically, the diameter of the fovea and parafovea (the area of retina with the highest density of cones and ganglion cells) is only about 2.5 mm, which represents the central 8°–9° of the retina.95 However, together the fovea and parafovea make up less than 0.5% of the total retinal area. The absolute numbers of retinal neurons are much higher in the periphery simply because the peripheral retina is so large in comparison to the fovea. This may be particularly true for certain neuron types (e.g., dopaminergic amacrine cells) that appear to be directly involved in the vision-dependent biochemical cascade96 controlling refractive development and that have relatively flat density profiles across the retina.93
In addition, because of the geometry of the globe, the periphery could influence central axial length and refractive development via the actions of peripheral mechanisms acting in a local, regionally selective manner.46 Visual signals that influence ocular growth and refractive error cause the sclera to expand or to resist expansion in a tangential direction.97 In this respect, signals to increase growth (e.g., hyperopic defocus) would result in tangential expansion in the periphery that would also affect the axial position of the posterior retina and, thus, the central refractive error.
It is also possible that the vision-dependent mechanisms that regulate ocular growth are distributed across the retina in manner that favors the periphery. For instance, although it has generally been assumed that cone photoreceptors are primarily involved in the detection of visual signals for eye growth, studies in knock-out mice suggest that rod photoreceptors, which are preferentially distributed in the mid-periphery, are important for the detection of signals that are involved in emmetropization and the phenomenon of form-deprivation myopia.98 In the chicken, bullwhip neurons, a sparse population of retinal neurons located primary in the periphery, have been shown to regulate equatorial eye growth in a vision dependent manner.99
Refractive Errors Can Vary with Eccentricity and Peripheral Optical Errors Can Alter Central Refractive Development
In addition to the expected increase in radial astigmatism with eccentricity, the eye’s spherical-equivalent refractive error can vary substantially with eccentricity, 39–41, 43, 46, 100 i.e., the visual signal for eye growth can vary with eccentricity. Several observations in humans suggest that the pattern of peripheral refraction could have a significant impact on emmetropization at the fovea and, in particular, could play a role in the onset and progression of myopia. For example, the pattern of peripheral refractive error varies systematically with the central refractive error. Specifically, in comparison to emmetropic and hyperopic eyes, eyes with central axial myopia are less oblate/more prolate in shape35, 101 and typically exhibit relative peripheral hyperopia in the horizontal meridian (i.e., the periphery is less myopic than the fovea)40, 43, 102 that increases in magnitude with the degree of central myopia.35, 38 Since central vision effectively controls accommodation and dictates the plane of fixation, the periphery of prolate-shaped eyes would often experience hyperopic defocus, which arguably could serve as a stimulus for the onset and progression of myopia.51, 94
Longitudinal studies in humans indicate that peripheral hyperopia often precedes the onset of central myopia and can be a risk factor for the onset and progression of myopia. For example, Hoogerheide et al.47 found that young, emmetropic adults who exhibited peripheral hyperopic errors for both the tangential and sagittal image shells were much more likely to develop myopia during pilot training than those individuals who exhibited simple myopic astigmatism in the periphery. Similarly, Schmid50 found a significant correlation between retinal steepness (specifically more prolate-shaped eyes) and myopic shifts in central refraction in children; however, this relationship was only significant for the temporal retina. On the other hand, Mutti et al.103 examining a single eccentricity in the temporal retina failed to find a consistent relationship between peripheral hyperopia and myopia onset. They did observe a significant relationship between the degree of relative peripheral hyperopia and myopia progression, but the overall influence of the peripheral hyperopia was weak.
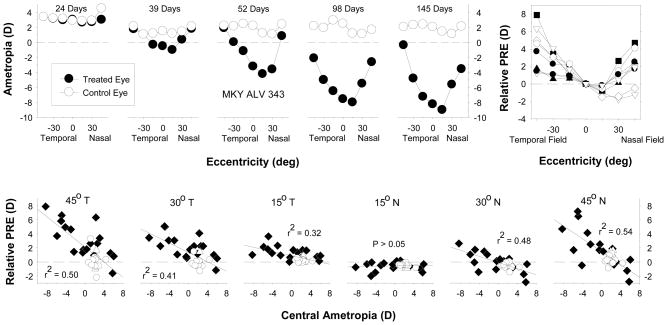
It will be difficult to determine the relative contribution of peripheral hyperopia to myopia onset and progression in humans because the relationship between central and peripheral refraction is complex. For example, in myopic eyes, relative peripheral hyperopia is common in the horizontal meridian, but not the vertical meridian.36 Little is known about how these conflicting peripheral signals between different parts of the eye influence axial growth and, in this respect, most human studies have investigated only a very limited number of eccentricities and they rarely take into account that traditional spectacle lenses actually increase the degree of relative peripheral hyperopia experienced by myopic individuals.52, 53 Moreover, consider the results shown in Figure 8, which demonstrate that, in addition to producing central axial myopia, form deprivation alters the pattern of peripheral refractions in infant monkeys resulting in relative peripheral hyperopia (top row). The degree of relative peripheral hyperopia generally increases with the degree of central axial myopia, but the strength of this relationship varies with eccentricity (bottom row) and there are asymmetries in the degree of relative hyperopia between the nasal and temporal visual fields. These changes in peripheral refraction come about because the treated eyes become less oblate/more prolate in shape during the development of form-deprivation myopia. With respect to the relationship between central myopia and peripheral hyperopia, these results are important because the diffusers employed in these experiments virtually eliminate defocus signals. Thus, the presence of relative peripheral hyperopia, even though it was observed prior to the onset central myopia in many animals,73 could not have contributed to onset or progression of axial myopia. Consequently, the relationship between relative peripheral hyperopia and central myopia is not always casual in nature. Instead, it is likely that the treated eyes of the form-deprived monkeys became more prolate in shape during axial elongation as a consequence of inherent biological constraints on the growth of the eye. This relationship makes it difficult to evaluate the contribution of peripheral hyperopia to myopia progression because the magnitude of the peripheral error would depend on the baseline degree of central myopia. For example, if you were investigating the hypothesis that the degree of peripheral hyperopia influences the rate of myopia progression, based on the data in Figure 8, one would predict that high myopes would progress at a faster rate than low myopes. However, this potential relationship would be masked by many factors that influence one’s absolute refractive state.
Figure 8.
Effects of form deprivation on the pattern of peripheral refractive errors.73 Top. Spherical-equivalent refractive corrections that were obtained at different times during the treatment period for a representative diffuser-reared monkey plotted as a function of visual field eccentricity. The plot on the left was obtained at the onset of the treatment period; the ages for the subsequent measures are shown in each plot. The filled and open symbols represent the treated and fellow eyes, respectively. Top right. Relative interocular differences in spherical-equivalent refractive corrections (treated eye - fellow eye) plotted as a function of eccentricity along the horizontal meridian for individual diffuser-reared monkeys that exhibited central axial myopia. Bottom row. Relative peripheral refractive corrections (peripheral – central refraction) for the treated eyes of individual normal (open circles) and form-deprived monkeys (filled diamonds) plotted as a function of the central ametropia for different horizontal field eccentricities. The solid lines represent the best-fitting regression lines.
In addition, comparisons of the degree of peripheral hyperopia with the rate of myopic progression would also be confounded by the fact that vision-induced axial elongation rates vary substantially from one individual to the next. For example, consider the range of results shown in Figure 5 (bottom right plot). In these experiments, each of the form-deprived animals with intact retinas wore the same strength diffusers and the onset and duration of form deprivation was well controlled. Although the majority of the treated eyes developed axial myopia, the degree of myopia varied over nearly a 10 D range and several monkeys actually developed hyperopic errors (also see the bottom left plot in Figure 7). It would be very difficult to take these individual differences in response rate into account when assessing the relationship between the degree of peripheral hyperopia and myopia progression because they would be essentially unknown.
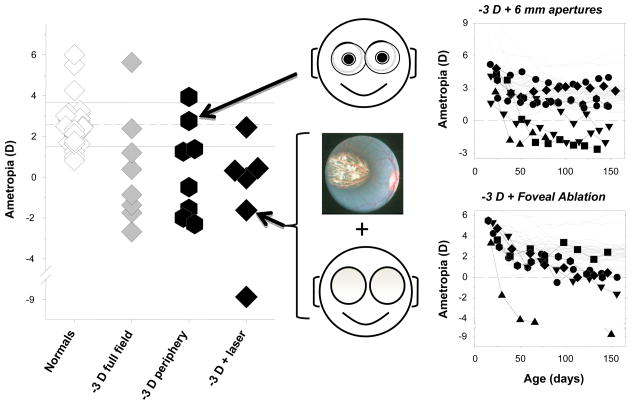
It appears that the potential role of peripheral hyperopia in the genesis and progression of myopia can probably best be addressed in laboratory animals. In this respect, the results illustrated in Figure 9 demonstrate that optically imposed peripheral hyperopia can produce central axial myopia in infant monkeys. First, rearing monkeys with negative spectacle lenses that provided unrestricted central vision and 3 D of relative peripheral hyperopia consistently produced central axial myopia and the range and degree of central myopia was comparable to that produced by full-field, −3 D lenses. Qualitatively similar results have been reported in chickens reared wearing negative spectacle lenses with central apertures.48 Second, isolating relative hyperopia in the periphery using negative lenses combined with laser ablation of the central retina also results in central axial myopia.51
Figure 9.
Effects of relative peripheral hyperopia on refractive development.51 Left. Spherical-equivalent refractive corrections obtained at ages corresponding to the end of the lens-rearing period for monkeys that were reared with unrestricted vision, wearing −3 D lenses that covered the entire visual field, wearing −3 D lenses with 6 mm apertures that produced relative peripheral hyperopia, and wearing full-field −3 D spectacle combined with foveal ablation. Right. Refractive error plotted as a function of age for normal monkeys (thin gray lines) and monkeys reared with −3 D lenses with 6 mm apertures (filled symbols, top) or full-field −3 D lenses and foveal ablations (filled symbols, bottom).
Thus, in terms of designing an optical treatment strategy to reduce myopia progression, it seems prudent to develop correcting strategies that eliminate peripheral hyperopic errors that, as shown above, can promote myopia progression. However, even if peripheral hyperopic defocus is not the primary cause of myopic progression, designing correction strategies that produce visual signals in the periphery that normally slow or stop axial growth (e.g., in-focus images or myopic defocus) is a promising approach. The data represented in the left column of Figure 6 show that myopic defocus isolated to the peripheral retina is very effective in reducing axial growth in infant monkeys. Similarly, Liu and Wildoset48 have recently demonstrated that optically imposed peripheral myopia greatly reduces axial growth in chickens. However, in terms of optimal peripheral corrections, it is important to recognize that there is much still to be learned about the effects of peripheral vision on central refractive development. In particular, it will be important to determine the optimal peripheral refractive state to reduce axial growth and the optimal peripheral treatment zone to control central axial elongation. For example, to optimize peripheral treatment strategies, it will be important to understand whether, as Schmid’s data suggests,50 the effectiveness of peripheral visual signals varies between semi-meridians and whether compensating for these meridional differences enhances treatment efficacy.
Results from Clinical Studies Indicate That Peripheral Treatment Strategies Are Effective in Slowing Myopia Progression
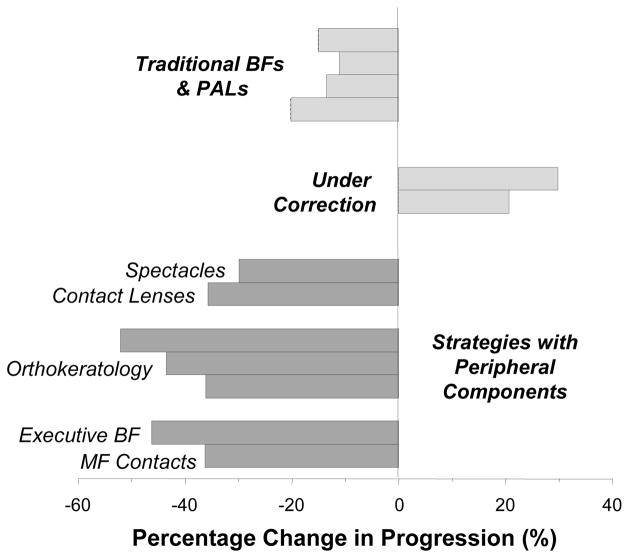
Clinical trials have demonstrated conclusively that correcting lenses can alter the rate of myopia progression in children. Figure 10 summarizes the changes in progression rate observed in some recent studies. The results have been segregated into groups based on the stated intent of the treatment strategy and whether the treatment lenses would have a substantial effect on peripheral refractive errors.
Figure 10.
Relative efficacy of optical treatment strategies for slowing myopia progression. For most of the studies, refractive-error data were used to calculate the percentage difference in progression rate between the treated and control groups. For the orthokeratology trials measures of vitreous chamber depth or axial length were employed to calculate the differences in axial elongation rate between the treated and control groups. The data for traditional bifocals and progressive addition lenses were obtained from references 27, 29, 30, 104. The results for under-correction strategies were obtained from references 25, 26. The results for strategies which included significant peripheral components were obtained from 107 for spectacle lenses, 108 for contact lenses, 112–114 for orthokeratology, 28 for executive bifocals, and 115 for multi-focal contact lenses.
An aim of the studies that employed traditional bifocals or progressive addition lenses was to decrease the level of accommodation during near work and/or to improve the quality of foveal vision, for example, by eliminating the degree of central hyperopic defocus associated with a lag of accommodation.27, 29, 30, 104 Although it is unclear how successful these treatment lenses were in accomplishing these goals,105 all of the studies found a statistically significant reduction in myopia progression relative to traditional spectacles. Although from a scientific perspective the results demonstrated that spectacle lenses could alter the course of myopia progression, the overall treatment effects were not clinically meaningful, possibly because the treatment zones of the lenses influenced a relatively small proportion of the visual field.
Recent studies employing distance under-correction strategies were based on observations in laboratory animals that showed that myopic defocus can slow axial elongation and produce hyperopic shifts in refraction. However, in children, under-correction strategies have failed to slow myopia progression26 and, in some cases, have been found to actually increase myopia progression relative to traditional single-vision spectacle prescription strategies.25 At first glance, these results seem to directly contradict the findings from laboratory animals. However, it is important to recognize that the overall optical effects of under-correcting a myopic human eye are likely to be very different from those imposed by a positive lens on the normal eyes of young animals. For example, with respect to distant targets, under-correction regimens will impose a relatively small amount of myopic defocus in the central retina (typically 0.5 to 0.75 D). Given the magnitude of relative peripheral hyperopia exhibited by most myopic humans, these small degrees of distance under-correction are very unlikely to eliminate the large amounts of hyperopic defocus in the periphery. In contrast, in animal studies, the powers of positive treatment lenses are generally much higher than the degree of under-correction. Moreover, the patterns of peripheral refractions in laboratory animals are likely to be very different from those of myopic children. For example, the majority of infant monkeys exhibit moderate amounts of central hyperopia and small amounts of relative peripheral myopia.106 As a consequence, positive treatment lenses employed with animals will typically impose relative myopic defocus on distant objects in both the central and peripheral retina, i.e., conditions that normally reduce the rate of axial elongation.
Correcting lenses that specifically target the pattern of peripheral refractions can reduce the rate of myopia progression. Recently, the Vision Cooperative Research Centre (Sydney, Australia) completed the initial clinical trials of new contact lenses and spectacle lenses that were designed to increase the curvature of the peripheral image shell while fully correcting central refractive errors, in essence, reducing the degree of relative peripheral hyperopia while maintaining clear central vision. Twelve-month data obtained for the new spectacle lens designs indicate that, relative to traditional spectacle lenses, 2 of 3 new designs (which differed in the amount and spatial distribution of the peripheral power zones) had no effect on progression rates. The third design, which had the smallest unrestricted central zone, reduced progression by an insignificant 17% in children 6–16 years of age. However, in a subgroup of younger children (6–12 years of age) with at least one myopic parent (i.e., children with relatively fast progression rates), this new lens significantly reduced myopia progression by 30%.107 The experimental contact lens designs were more successful. Specifically, in 7- to 14-year-old children, contact lenses that reduced relative peripheral hyperopia decreased the average rate of myopia progression by 36% over a 12-month period.108 It is likely that the contact lens designs were more effective than the best spectacle lens design because the optical treatment zones in the contact lenses were positioned closer to the line of sight and because, with contact lenses, the treatment zone remains centered during eye movements. Although these preliminary results provide proof that peripheral optical manipulations can slow myopia progression, much longer treatment periods will be necessary to prove clinical efficacy and much work is needed to optimize these lens designs.
Over-night orthokeratology optically corrects myopia by flattening the central cornea. For moderate degrees of central myopia, the primary changes in corneal topography are usually restricted to the central ±20° of the visual field. As a consequence, the normal pattern of peripheral refractions is changed dramatically. Specifically, beyond the area of central flattening, the peripheral refractions typically change from the relative peripheral hyperopia found at baseline to relative peripheral myopia,109–111 in essence establishing a peripheral refractive state that normally reduces axial growth in laboratory animals. In this respect, the initial small-scale clinical trials indicated that orthokeratology slows axial elongation in myopic children by about 50% over a two-year period.112, 113 These promising early results have recently been confirmed in a larger, prospective study that found a 36% reduction in axial elongation over a two-year period.114 Interestingly, in this study, the larger reductions in progression rate were observed in individuals with higher degrees of central myopia, possibly reflecting the fact that orthokeratology strategies would be expected to produce the highest degrees of relative peripheral myopia in highly myopic children.
Promising results have also been obtained using treatment lenses that produce relative myopic defocus in the peripheral and central fields simultaneously. For example, Cheng et al.28 found that executive bifocal spectacles reduced myopia progression by 46% on average over a two-year period in 8–13 year-old children and Anstice and Phillips,115 using multifocal contact lenses that had optical profiles similar to traditional center-distant bifocal contact lenses, reported a 36% reduction in myopia progression in an 8-month trial. Although the primary intent in both studies was to manipulate central vision, in both cases the treatment lenses also produced relative myopic defocus over a very large part of the periphery. With the executive bifocal, when children were viewing through the distance portion of the lens, the near add produced relative myopic shifts over approximately half the visual field. In this respect, the larger treatment effects observed with executive bifocals versus progressive addition lenses may reflect the executive bifocal’s effectively larger treatment zone, i.e., relative myopic shifts were produced over a larger portion of the visual field. With the Anstice and Phillips’ multifocal contact lens, children postured their accommodation for the distance portions of the lenses at all distances and, thus, experienced superimposed relative myopic defocus essentially across the entire visual field.
Inspection of Figure 10 shows that optical treatment strategies that influence the eye’s effective refractive state over a substantial part of the peripheral field are likely to be more effective than traditional bifocals and progressive addition lenses and that peripheral treatment strategies can produce clinically meaningful reductions in the myopia progression. With respect to these peripheral treatment strategies, it is important to note that there is another commonality in all of these designs. In addition to treating a large part of the visual field, each of these strategies produces simultaneous myopic defocus, which animal studies show is a strong stimulus for reducing axial growth116–118. For example, with the Anstice and Phillips’ contact lenses, the myopic defocus is spatially superimposed on the in-focus central retinal image. With the Vision CRC contact lenses and spectacle lenses and with orthokeratology, the myopic defocus is spatially restricted to the periphery, but it exists simultaneously with clear central vision.
As originally suggested by research on laboratory animals, it seems likely that any lens design that imposes myopic defocus across a substantial part of the eye will be effective in slowing the rate of myopia progression. The more consistently the imposed myopic defocus is maintained over time and across fixation distances, the more likely it is to be effective. Because central vision controls accommodation, peripheral treatment strategies, like that illustrated in Figure 1, can consistently produce relative myopic shifts over time and they offer a number of other advantages. First, peripheral treatment strategies produce an anti-myopia treatment effect while maintaining optimal central vision, in contrast to strategies that impose simultaneous myopic defocus across the central retina. Second, peripheral treatment strategies, by reducing the degree of peripheral hyperopic defocus, can actually produce measurable improvements in peripheral vision, a potentially valuable side benefit.119 Third, peripheral treatment strategies can be implemented using all of our traditional optical treatment methods (i.e., spectacles, contact lenses, orthokeratology and corneal laser surgery). This is an advantage because it will be important to develop effective anti-myopia designs in all of these modalities to meet patient needs. At the least, we should design correcting lenses that do not induce peripheral optical conditions that may actually promote myopia progression (e.g., like many traditional negative spectacle lenses).
While I believe that we are on the verge of having a number of optical treatment options that do effectively slow myopia progression, it is important to note that many of the clinical results just outlined are preliminary in nature and these new lens designs must be investigated in larger and longer trials. Moreover, there are still many issues associated with these optical treatment strategies that must be resolved. For example, the optimal peripheral image shell manipulations required to slow myopia progression is not known. In this respect, it seems likely that as we develop a better understanding of the role of the periphery in regulating eye growth, we can increase the effectiveness of peripheral treatment strategies. For example, to date, all of the optical treatment strategies have employed a single power profile; no attempts have been made to optimize the peripheral optical manipulations to take into account the substantial inter-subject differences in the pattern of peripheral refractions or the significant intra-ocular variations in peripheral refraction. It will be important to improve the efficacy of these potential optical treatment strategies, because optical treatment strategies will not eliminate existing myopic errors (except possibly in very young children). Instead, they will probably only slow subsequent progression, which is a strong argument for employing these treatment options at as early an age as practical. But given the increasing prevalence of myopia, having an optical treatment strategy that produces a clinically meaningful reduction in myopia progression would have huge public health benefits.
Acknowledgments
This work was supported by grants from the National Eye Institute (EY 03611, EY 07551) and funds from the Vision Cooperative Research Centre and the Greeman-Petty Professorship, UH Foundation. I would not have been selected to receive the Charles F. Prentice Medal if I had not had the good fortune to work with outstanding colleagues. Dr. Li-Fang Hung, my partner in this refractive development project, has been involved in this research program from its inception and over the last 20 years has made major contributions to all aspects of this research. Drs. Chea-su Kee, Ramkumar Rammamirtham, and Juan Huang, former or current graduate students in my lab, made important contributions to the results presented in this paper. My two long-time collaborators, Drs. Ron Harwerth (who has also served as my primary mentor and role model throughout my career) and Yuzo Chino gave me the opportunity to be involved in their research on visual system development, which provided me the background that allowed my lab to pursue the effects of visual experience on refractive development. Dr. Brien Holden, whom I met by chance at an AAO meeting, and all the fantastic scientists associated with the Vision CRC and the Brien Holden Vision Institute have done the difficult and critical job of taking results from the laboratory and translating them to the clinical setting.
Footnotes
The author is a co-author on patents related to peripheral optical treatment strategies for slowing myopia progression.
References
- 1.Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Arch Ophthalmol. 1983;101:405–7. doi: 10.1001/archopht.1983.01040010405011. [DOI] [PubMed] [Google Scholar]
- 2.Lin LL, Shih YF, Hsiao CK, Chen CJ. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann Acad Med Singapore. 2004;33:27–33. [PubMed] [Google Scholar]
- 3.Hosaka A. Population studies—myopia experience in Japan. Acta Ophthalmol Suppl. 1988;185:37–40. doi: 10.1111/j.1755-3768.1988.tb02659.x. [DOI] [PubMed] [Google Scholar]
- 4.Woo WW, Lim KA, Yang H, Lim XY, Liew F, Lee YS, Saw SM. Refractive errors in medical students in Singapore. Singapore Med J. 2004;45:470–4. [PubMed] [Google Scholar]
- 5.Wu MM, Edwards MH. The effect of having myopic parents: an analysis of myopia in three generations. Optom Vis Sci. 1999;76:387–92. doi: 10.1097/00006324-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Tay MT, Au Eong KG, Ng CY, Lim MK. Myopia and educational attainment in 421,116 young Singaporean males. Ann Acad Med Singapore. 1992;21:785–91. [PubMed] [Google Scholar]
- 7.Vitale S, Ellwein L, Cotch MF, Ferris FL, 3rd, Sperduto R. Prevalence of refractive error in the United States, 1999–2004. Arch Ophthalmol. 2008;126:1111–9. doi: 10.1001/archopht.126.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz J, Tielsch JM, Sommer A. Prevalence and risk factors for refractive errors in an adult inner city population. Invest Ophthalmol Vis Sci. 1997;38:334–40. [PubMed] [Google Scholar]
- 9.Wang Q, Klein BE, Klein R, Moss SE. Refractive status in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1994;35:4344–7. [PubMed] [Google Scholar]
- 10.The Framingham Offspring Eye Study Group. Familial aggregation and prevalence of myopia in the Framingham Offspring Eye Study. Arch Ophthalmol. 1996;114:326–32. doi: 10.1001/archopht.1996.01100130322017. [DOI] [PubMed] [Google Scholar]
- 11.Rose K, Smith W, Morgan I, Mitchell P. The increasing prevalence of myopia: implications for Australia. Clin Experiment Ophthalmol. 2001;29:116–20. doi: 10.1046/j.1442-9071.2001.00389.x. [DOI] [PubMed] [Google Scholar]
- 12.Bar Dayan Y, Levin A, Morad Y, Grotto I, Ben-David R, Goldberg A, Onn E, Avni I, Levi Y, Benyamini OG. The changing prevalence of myopia in young adults: a 13-year series of population-based prevalence surveys. Invest Ophthalmol Vis Sci. 2005;46:2760–5. doi: 10.1167/iovs.04-0260. [DOI] [PubMed] [Google Scholar]
- 13.Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005;24:1–38. doi: 10.1016/j.preteyeres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Fong DS, Epstein DL, Allingham RR. Glaucoma and myopia: are they related? Int Ophthalmol Clin. 1990;30:215–8. doi: 10.1097/00004397-199030030-00009. [DOI] [PubMed] [Google Scholar]
- 15.Lim R, Mitchell P, Cumming RG. Refractive associations with cataract: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 1999;40:3021–6. [PubMed] [Google Scholar]
- 16.Leske MC, Wu SY, Nemesure B, Hennis A. Risk factors for incident nuclear opacities. Ophthalmology. 2002;109:1303–8. doi: 10.1016/s0161-6420(02)01094-1. [DOI] [PubMed] [Google Scholar]
- 17.Wong TY, Klein BE, Klein R, Tomany SC, Lee KE. Refractive errors and incident cataracts: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 2001;42:1449–54. [PubMed] [Google Scholar]
- 18.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106:2010–5. doi: 10.1016/s0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 19.Wong TY, Klein BE, Klein R, Knudtson M, Lee KE. Refractive errors, intraocular pressure, and glaucoma in a white population. Ophthalmology. 2003;110:211–7. doi: 10.1016/s0161-6420(02)01260-5. [DOI] [PubMed] [Google Scholar]
- 20.Curtin BJ, Karlin DB. Axial length measurements and fundus changes of the myopic eye. I. The posterior fundus. Trans Am Ophthalmol Soc. 1970;68:312–34. [PMC free article] [PubMed] [Google Scholar]
- 21.The Eye Disease Case-Control Study Group. Risk factors for idiopathic rhegmatogenous retinal detachment. Am J Epidemiol. 1993;137:749–57. [PubMed] [Google Scholar]
- 22.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:381–91. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu JH, Cheng CY, Chen SJ, Lee FL. Visual impairment in a Taiwanese population: prevalence, causes, and socioeconomic factors. Ophthalmic Epidemiol. 2001;8:339–50. doi: 10.1080/09286586.2001.11644261. [DOI] [PubMed] [Google Scholar]
- 24.Vitale S, Cotch MF, Sperduto R, Ellwein L. Costs of refractive correction of distance vision impairment in the United States, 1999–2002. Ophthalmology. 2006;113:2163–70. doi: 10.1016/j.ophtha.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Chung K, Mohidin N, O’Leary DJ. Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Res. 2002;42:2555–9. doi: 10.1016/s0042-6989(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 26.Adler D, Millodot M. The possible effect of undercorrection on myopic progression in children. Clin Exp Optom. 2006;89:315–21. doi: 10.1111/j.1444-0938.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- 27.Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000;77:395–401. doi: 10.1097/00006324-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Cheng D, Schmid KL, Woo GC, Drobe B. Randomized trial of effect of bifocal and prismatic bifocal spectacles on myopic progression: two-year results. Arch Ophthalmol. 2010;128:12–9. doi: 10.1001/archophthalmol.2009.332. [DOI] [PubMed] [Google Scholar]
- 29.Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, Leske MC, Manny R, Marsh-Tootle W, Scheiman M. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- 30.Edwards MH, Li RW, Lam CS, Lew JK, Yu BS. The Hong Kong progressive lens myopia control study: study design and main findings. Invest Ophthalmol Vis Sci. 2002;43:2852–8. [PubMed] [Google Scholar]
- 31.Smith EL, III, Bradley DV, Fernandes A, Boothe RG. Form deprivation myopia in adolescent monkeys. Optom Vis Sci. 1999;76:428–32. doi: 10.1097/00006324-199906000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Zhong X, Ge J, Nie H, Smith EL., III Compensation for experimentally induced hyperopic anisometropia in adolescent monkeys. Invest Ophthalmol Vis Sci. 2004;45:3373–9. doi: 10.1167/iovs.04-0226. [DOI] [PubMed] [Google Scholar]
- 33.Papastergiou GI, Schmid GF, Laties AM, Pendrak K, Lin T, Stone RA. Induction of axial eye elongation and myopic refractive shift in one- year-old chickens. Vision Res. 1998;38:1883–8. doi: 10.1016/s0042-6989(97)00347-7. [DOI] [PubMed] [Google Scholar]
- 34.Siegwart JT, Jr, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–15. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- 35.Atchison DA, Jones CE, Schmid KL, Pritchard N, Pope JM, Strugnell WE, Riley RA. Eye shape in emmetropia and myopia. Invest Ophthalmol Vis Sci. 2004;45:3380–6. doi: 10.1167/iovs.04-0292. [DOI] [PubMed] [Google Scholar]
- 36.Atchison DA, Pritchard N, Schmid KL. Peripheral refraction along the horizontal and vertical visual fields in myopia. Vision Res. 2006;46:1450–8. doi: 10.1016/j.visres.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Singh KD, Logan NS, Gilmartin B. Three-dimensional modeling of the human eye based on magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2006;47:2272–9. doi: 10.1167/iovs.05-0856. [DOI] [PubMed] [Google Scholar]
- 38.Logan NS, Gilmartin B, Wildsoet CF, Dunne MC. Posterior retinal contour in adult human anisomyopia. Invest Ophthalmol Vis Sci. 2004;45:2152–62. doi: 10.1167/iovs.03-0875. [DOI] [PubMed] [Google Scholar]
- 39.Atchison DA, Pritchard N, White SD, Griffiths AM. Influence of age on peripheral refraction. Vision Res. 2005;45:715–20. doi: 10.1016/j.visres.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 40.Ferree CE, Rand G. Interpretation of refractive conditions in the peripheral field of vision: a further study. Arch Ophthalmol. 1933;9:925–38. [Google Scholar]
- 41.Ferree CE, Rand G, Hardy C. Refraction for the peripheral field of vision. Arch Ophthalmol. 1931;5:717–31. [Google Scholar]
- 42.Ferree CE, Rand G, Hardy C. Refractive asymmetry in the temporal and nasal halves of the visual field. Am J Ophthalmol. 1932;15:513–22. [Google Scholar]
- 43.Millodot M. Effect of ametropia on peripheral refraction. Am J Optom Physiol Opt. 1981;58:691–5. doi: 10.1097/00006324-198109000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Millodot M, Lamont A. Letter: Refraction of the periphery of the eye. J Opt Soc Am. 1974;64:110–11. doi: 10.1364/josa.64.000110. [DOI] [PubMed] [Google Scholar]
- 45.Rempt F, Hoogerheide J, Hoogenboom WP. Peripheral retinoscopy and the skiagram. Ophthalmologica. 1971;162:1–10. doi: 10.1159/000306229. [DOI] [PubMed] [Google Scholar]
- 46.Seidemann A, Schaeffel F, Guirao A, Lopez-Gil N, Artal P. Peripheral refractive errors in myopic, emmetropic, and hyperopic young subjects. J Opt Soc Am (A) 2002;19:2363–73. doi: 10.1364/josaa.19.002363. [DOI] [PubMed] [Google Scholar]
- 47.Hoogerheide J, Rempt F, Hoogenboom WP. Acquired myopia in young pilots. Ophthalmologica. 1971;163:209–15. doi: 10.1159/000306646. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Wildsoet C. The effect of two-zone concentric bifocal spectacle lenses on refractive error development and eye growth in young chicks. Invest Ophthalmol Vis Sci. 2011;52:1078–86. doi: 10.1167/iovs.10-5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mutti DO, Hayes JR, Mitchell GL, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48:2510–9. doi: 10.1167/iovs.06-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmid GF. Association between retinal steepness and central myopic shift in children. Optom Vis Sci. 2011;88:684–90. doi: 10.1097/OPX.0b013e3182152646. [DOI] [PubMed] [Google Scholar]
- 51.Smith EL, III, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009;49:2386–92. doi: 10.1016/j.visres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin Z, Martinez A, Chen X, Li L, Sankaridurg P, Holden BA, Ge J. Peripheral defocus with single-vision spectacle lenses in myopic children. Optom Vis Sci. 2010;87:4–9. doi: 10.1097/OPX.0b013e3181c078f1. [DOI] [PubMed] [Google Scholar]
- 53.Tabernero J, Vazquez D, Seidemann A, Uttenweiler D, Schaeffel F. Effects of myopic spectacle correction and radial refractive gradient spectacles on peripheral refraction. Vision Res. 2009;49:2176–86. doi: 10.1016/j.visres.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Guyton DL, Greene PR, Scholz RT. Dark-rearing interference with emmetropization in the rhesus monkey. Invest Ophthalmol Vis Sci. 1989;30:761–4. [PubMed] [Google Scholar]
- 55.Smith EL, III, Harwerth RS, Crawford ML, von Noorden GK. Observations on the effects of form deprivation on the refractive status of the monkey. Invest Ophthalmol Vis Sci. 1987;28:1236–45. [PubMed] [Google Scholar]
- 56.Smith EL, III, Hung LF. Form-deprivation myopia in monkeys is a graded phenomenon. Vision Res. 2000;40:371–81. doi: 10.1016/s0042-6989(99)00184-4. [DOI] [PubMed] [Google Scholar]
- 57.Tigges M, Tigges J, Fernandes A, Eggers HM, Gammon JA. Postnatal axial eye elongation in normal and visually deprived rhesus monkeys. Invest Ophthalmol Vis Sci. 1990;31:1035–46. [PubMed] [Google Scholar]
- 58.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–8. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- 59.Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–63. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 60.Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., III Recovery from form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3361–72. doi: 10.1167/iovs.04-0080. [DOI] [PubMed] [Google Scholar]
- 61.Smith EL, III, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–35. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 62.Troilo D, Nickla DL. The response to visual form deprivation differs with age in marmosets. Invest Ophthalmol Vis Sci. 2005;46:1873–81. doi: 10.1167/iovs.04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wildsoet CF, Schmid KL. Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact or sectioned optic nerves. Vision Res. 2000;40:3273–82. doi: 10.1016/s0042-6989(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 64.Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–57. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- 65.Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991;31:717–34. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]
- 66.Hung LF, Crawford ML, Smith EL., III Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1:761–5. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- 67.Shen W, Sivak JG. Eyes of a lower vertebrate are susceptible to the visual environment. Invest Ophthalmol Vis Sci. 2007;48:4829–37. doi: 10.1167/iovs.06-1273. [DOI] [PubMed] [Google Scholar]
- 68.Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–27. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 69.Shaikh AW, Siegwart JT, Jr, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76:308–15. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 70.Whatham AR, Judge SJ. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Res. 2001;41:267–73. doi: 10.1016/s0042-6989(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 71.Rabin J, Van Sluyters RC, Malach R. Emmetropization: a vision-dependent phenomenon. Invest Ophthalmol Vis Sci. 1981;20:561–4. [PubMed] [Google Scholar]
- 72.Phillips JR. Monovision slows juvenile myopia progression unilaterally. Br J Ophthalmol. 2005;89:1196–200. doi: 10.1136/bjo.2004.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang J, Hung LF, Ramamirtham R, Blasdel TL, Humbird TL, Bockhorst KH, Smith EL., III Effects of form deprivation on peripheral refractions and ocular shape in infant rhesus monkeys (Macaca mulatta) Invest Ophthalmol Vis Sci. 2009;50:4033–44. doi: 10.1167/iovs.08-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., III Nature of the refractive errors in rhesus monkeys (Macaca mulatta) with experimentally induced ametropias. Vision Res. 2010;50:1867–81. doi: 10.1016/j.visres.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raviola E, Wiesel TN. An animal model of myopia. N Engl J Med. 1985;312:1609–15. doi: 10.1056/NEJM198506203122505. [DOI] [PubMed] [Google Scholar]
- 76.Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res. 1987;6:993–9. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- 77.Wildsoet C, Pettigrew JD. Experimental myopia and anomalous eye growth patterns unaffected by optic nerve section in chickens: evidence for local control of eye growth. Clin Vis Sci. 1988;3:99–107. [Google Scholar]
- 78.Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis Neurosci. 1994;11:143–53. doi: 10.1017/s0952523800011184. [DOI] [PubMed] [Google Scholar]
- 79.Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 1991;31:1237–50. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- 80.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–94. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- 81.Wildsoet C. Neural pathways subserving negative lens-induced emmetropization in chicks--insights from selective lesions of the optic nerve and ciliary nerve. Curr Eye Res. 2003;27:371–85. doi: 10.1076/ceyr.27.6.371.18188. [DOI] [PubMed] [Google Scholar]
- 82.Schaeffel F, Troilo D, Wallman J, Howland HC. Developing eyes that lack accommodation grow to compensate for imposed defocus. Vis Neurosci. 1990;4:177–83. doi: 10.1017/s0952523800002327. [DOI] [PubMed] [Google Scholar]
- 83.Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36:1023–36. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- 84.Hodos W, Kuenzel WJ. Retinal-image degradation produces ocular enlargement in chicks. Invest Ophthalmol Vis Sci. 1984;25:652–9. [PubMed] [Google Scholar]
- 85.Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–7. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- 86.Norton TT, Siegwart JT. Local myopia produced by partial visual-field deprivation in tree shrew. Soc Neurosci Abstr. 1991;17:558. [Google Scholar]
- 87.McFadden SA. Partial occlusion produces local form deprivation myopia in the guinea pig eye. Invest Ophthalmol Vis Sci. 2002;43:E-Abstract 189. [Google Scholar]
- 88.Smith EL, III, Huang J, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2009;50:5057–69. doi: 10.1167/iovs.08-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith EL, III, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010;51:3864–73. doi: 10.1167/iovs.09-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stone RA, Flitcroft DI. Ocular shape and myopia. Ann Acad Med Singapore. 2004;33:7–15. [PubMed] [Google Scholar]
- 91.Smith EL, III, Ramamirtham R, Qiao-Grider Y, Hung LF, Huang J, Kee CS, Coats D, Paysse E. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci. 2007;48:3914–22. doi: 10.1167/iovs.06-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith EL, III, Kee CS, Ramamirtham R, Qiao-Grider Y, Hung LF. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46:3965–72. doi: 10.1167/iovs.05-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodieck RW. The First Steps in Seeing. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- 94.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–68. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 95.Bishop PO. Binocular vision. In: Moses RA, Hart WM, editors. Adler’s Physiology of the Eye: Clinical Application. 8. St. Louis: Mosby; 1987. pp. 619–89. [Google Scholar]
- 96.Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A. 1989;86:704–6. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 98.Jabbar SB, Faulkner AE, Schmid GF, Schaeffel F, Abey J, Iuvone PM, Pardue MT. Rod photoreceptor contributions to refractive development and form deprivation myopia in mice. Invest Ophthalmol Vis Sci. 2010;51:E-Abstract 1726. [Google Scholar]
- 99.Fischer AJ, Ritchey ER, Scott MA, Wynne A. Bullwhip neurons in the retina regulate the size and shape of the eye. Dev Biol. 2008;317:196–212. doi: 10.1016/j.ydbio.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 100.Lotmar W, Lotmar T. Peripheral astigmatism in the human eye: experimental data and theoretical model predictions. J Opt Soc Am. 1974;64:510–3. doi: 10.1364/josa.64.000510. [DOI] [PubMed] [Google Scholar]
- 101.Atchison DA, Pritchard N, Schmid KL, Scott DH, Jones CE, Pope JM. Shape of the retinal surface in emmetropia and myopia. Invest Ophthalmol Vis Sci. 2005;46:2698–707. doi: 10.1167/iovs.04-1506. [DOI] [PubMed] [Google Scholar]
- 102.Mutti DO, Sholtz RI, Friedman NE, Zadnik K. Peripheral refraction and ocular shape in children. Invest Ophthalmol Vis Sci. 2000;41:1022–30. [PubMed] [Google Scholar]
- 103.Mutti DO, Sinnott LT, Mitchell GL, Jones-Jordan LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K. Relative peripheral refractive error and the risk of onset and progression of myopia in children. CLEERE Study Group. Invest Ophthalmol Vis Sci. 2011;52:199–205. doi: 10.1167/iovs.09-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hasebe S, Ohtsuki H, Nonaka T, Nakatsuka C, Miyata M, Hamasaki I, Kimura S. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Invest Ophthalmol Vis Sci. 2008;49:2781–9. doi: 10.1167/iovs.07-0385. [DOI] [PubMed] [Google Scholar]
- 105.Berntsen DA, Mutti DO, Zadnik K. The effect of bifocal add on accommodative lag in myopic children with high accommodative lag. Invest Ophthalmol Vis Sci. 2010;51:6104–10. doi: 10.1167/iovs.09-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hung LF, Ramamirtham R, Huang J, Qiao-Grider Y, Smith EL., III Peripheral refraction in normal infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2008;49:3747–57. doi: 10.1167/iovs.07-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sankaridurg P, Donovan L, Varnas S, Ho A, Chen X, Martinez A, Fisher S, Lin Z, Smith EL, 3rd, Ge J, Holden B. Spectacle lenses designed to reduce progression of myopia: 12-month results. Optom Vis Sci. 2010;87:631–41. doi: 10.1097/OPX.0b013e3181ea19c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holden BA, Sankaridurg P, Lazon P, Smith EL, III, Sastry S, Chen X, Kwan J, Martinez A, Ho A, Ge J. Reduction in the rate of progress of myopia with a contact lens designed to reduce relative peripheral hyperopia. Invest Ophthalmol Vis Sci. 2010;51:E-abstract 2220. doi: 10.1167/iovs.11-7260. [DOI] [PubMed] [Google Scholar]
- 109.Queirós A, González-Méijome JM, Jorge J, Villa-Collar C, Gutiérrez AR. Peripheral refraction in myopic patients after orthokeratology. Optom Vis Sci. 2010;87:323–9. doi: 10.1097/OPX.0b013e3181d951f7. [DOI] [PubMed] [Google Scholar]
- 110.Kang P, Swarbrick H. Peripheral refraction in myopic children wearing orthokeratology and gas-permeable lenses. Optom Vis Sci. 2011;88:476–82. doi: 10.1097/OPX.0b013e31820f16fb. [DOI] [PubMed] [Google Scholar]
- 111.Charman WN, Mountford J, Atchison DA, Markwell EL. Peripheral refraction in orthokeratology patients. Optom Vis Sci. 2006;83:641–8. doi: 10.1097/01.opx.0000232840.66716.af. [DOI] [PubMed] [Google Scholar]
- 112.Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005;30:71–80. doi: 10.1080/02713680590907256. [DOI] [PubMed] [Google Scholar]
- 113.Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol. 2009;93:1181–5. doi: 10.1136/bjo.2008.151365. [DOI] [PubMed] [Google Scholar]
- 114.Kakita T, Hiraoka T, Oshika T. Influence of overnight orthokeratology on axial elongation in childhood myopia. Invest Ophthalmol Vis Sci. 2011;52:2170–4. doi: 10.1167/iovs.10-5485. [DOI] [PubMed] [Google Scholar]
- 115.Anstice NS, Phillips JR. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology. 2011 doi: 10.1016/j.ophtha.2010.10.035. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 116.Diether S, Wildsoet CF. Stimulus requirements for the decoding of myopic and hyperopic defocus under single and competing defocus conditions in the chicken. Invest Ophthalmol Vis Sci. 2005;46:2242–52. doi: 10.1167/iovs.04-1200. [DOI] [PubMed] [Google Scholar]
- 117.Tse DY, Lam CS, Guggenheim JA, Lam C, Li KK, Liu Q, To CH. Simultaneous defocus integration during refractive development. Invest Ophthalmol Vis Sci. 2007;48:5352–9. doi: 10.1167/iovs.07-0383. [DOI] [PubMed] [Google Scholar]
- 118.Wildsoet CF, Collins MJ. Competing defocus stimuli of opposite sign produce opposite effects in eyes with intact and sectioned optic nerves in the chick. Invest Ophthalmol Vis Sci. 2000;41:S738. [Google Scholar]
- 119.Holden BA, Sankaridurg P, Lazon P, Ho A, Smith EL, III, Chen X, Lin J, Naduvilath T, Ge J. Central and peripheral visual performance of a novel contact lens designed to control progression of myopia. Invest Ophthalmol Vis Sci. 2011;52:E-Abstract 6518. doi: 10.1167/iovs.11-7260. [DOI] [PubMed] [Google Scholar]
- 120.Irving EL, Callender MG, Sivak JG. Inducing ametropias in hatchling chicks by defocus--aperture effects and cylindrical lenses. Vision Res. 1995;35:1165–74. doi: 10.1016/0042-6989(94)00235-e. [DOI] [PubMed] [Google Scholar]
- 121.Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, Christensen AM. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- 122.Siegwart JT, Norton TT. Refractive and ocular changes in tree shrews raised with plus or minus lenses. Invest Ophthalmol Vis Sci. 1993;34(Suppl):1208. [Google Scholar]