In mouse models of systemic lupus erythematosus, antibodies that cross-react with double-stranded DNA and the NR2A subunit of the NMDAR cause apoptosis of NR2A-expressing neurons within the brainstem of developing female fetuses, resulting in a gender bias.
Abstract
Systemic lupus erythematosus (SLE), a disease of women during childbearing years, is characterized by the production of double-stranded DNA antibodies. A subset of these antibodies, present in 40% of patients, cross-reacts with the NR2A and NR2B subunits of the N-methyl-d-aspartate receptor (NMDAR). In this study, we show that, in mouse models, these antibodies cause a loss of female fetus viability by inducing apoptosis of NR2A-expressing neurons within the brainstem late in fetal development; gender specificity derives from a time-dependent increased expression of NR2A in female brainstem or increased vulnerability of female fetal neurons to signaling through NR2A-containing NMDARs. This paradigm is consistent with available data on the sex ratio of live births of women with SLE. It represents a novel mechanism by which maternal autoantibodies can severely affect fetal health in a gender-specific fashion and raises the question of how many maternal antibodies affect brain development or exhibit gender-specific fetal effects.
During fetal development, transplacental transmission of maternal antibody provides perinatal immunity to many infectious agents. However, transmission of maternal autoantibodies to the fetus can also cause transient or long-lasting pathologies. During the second trimester of pregnancy, maternal antibody can access the fetal brain, resulting in a 50–70-fold increase in IgG in fetal compared with maternal brains (Lee et al., 2009).
DNA/N-methyl-d-aspartate receptor (NMDAR) cross-reactive antibodies (DNRAbs) are elicited in mice immunized with a peptide mimetope of DNA (DWEYSVWLSN) multimerized on a poly-lysine backbone (MP; Putterman and Diamond, 1998; Khalil et al., 2001). They bind activated NMDARs to amplify calcium influx, thereby increasing NMDAR-mediated synaptic responses at low concentration and causing excitotoxicity at high concentration (Faust et al., 2010). We recently demonstrated that immunizing pregnant mice with a multimeric form of a peptide mimetope of DNA (MP) to produce DNRAbs, which were transferred into fetuses and caused neuronal death in fetal neocortex, led to in utero cortical abnormalities and selective cognitive deficits in male offspring (Lee et al., 2009). Interestingly, pregnant dams consistently birthed more male than female offspring.
RESULTS AND DISCUSSION
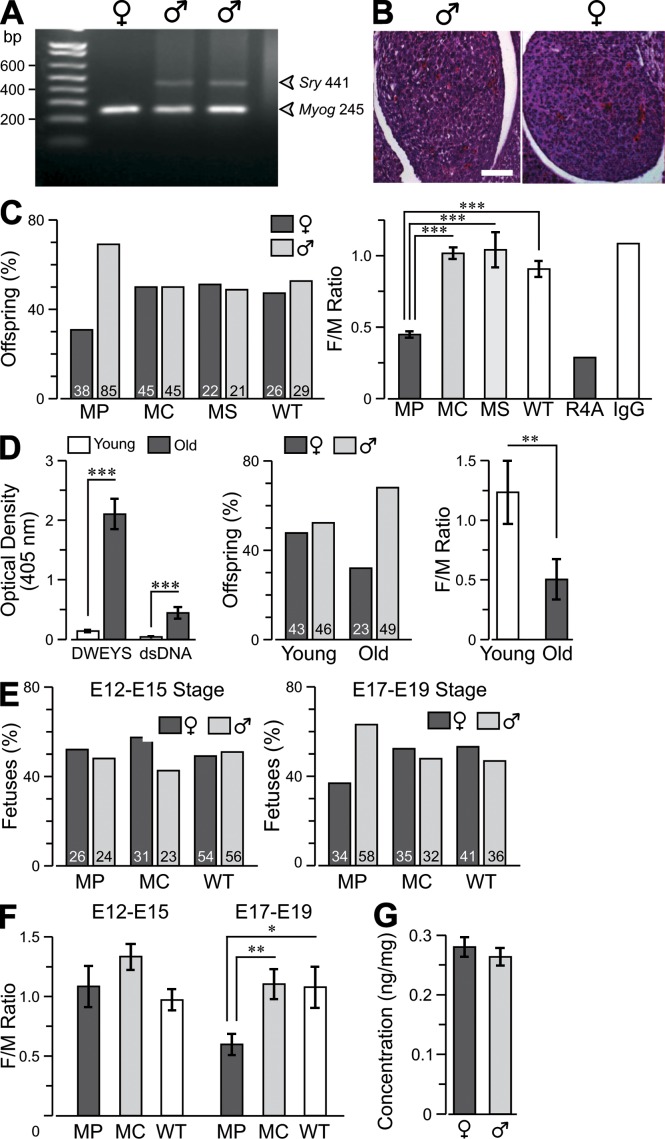
To pursue this observation, we immunized female BALB/c mice with MP, poly-lysine backbone alone (MC), or scrambled peptide (WSDYEVWLSN) on a poly-lysine backbone (MS). Detection of the Sry gene from the Y chromosome (Koopman et al., 1990) allowed unequivocal determination of male offspring that was confirmed by gonad histology (Fig. 1, A and B). MP dams produced litters that were 31% female, whereas litters from MC and MS dams were 50% or 51% female, respectively, similar to WT mice (Fig. 1 C; analysis of variance [ANOVA], F = 27.7, P < 0.0001; followed by Student’s t test, T > 4, P < 0.001 for all paired comparisons). Intravenous administration of a monoclonal DNRAb, R4A, to female mice on embryonic day (E) 13 of gestation caused a similar skewing of the sex ratio in live births (Fig. 1 C).
Figure 1.
Female to male (F/M) ratio in offspring of lupus mice. (A) PCR of Sry DNA was used to identify male fetuses, with Myogenin acting as control for the integrity of DNA. (B) Gonads were stained with cresyl violet (stains cell bodies) for histological analysis. Bar, 50 µm. (C, left) Graph showing the percentage of male and female offspring born of MP (mice immunized with a peptide mimetope of DNA [DWEYSVWLSN] multimerized on a poly-lysine backbone)-, MC (mice immunized with a poly-lysine backbone alone)-, or MS (mice immunized with a scrambled peptide [WSDYEVWLSN] on a poly-lysine backbone)-immunized dams and WT dams. Number of offspring is indicated in each column. (right) F/M ratio per litter (mean ± SEM). Ratios for offspring of mice born to dams given DNRAb R4A or control IgG2b intravenously on day E13 are also shown. (D, left) The graph shows serum binding to DWEYS and double-stranded DNA (dsDNA) in young (<16 wk) and old (>25 wk) triple congenic Sle1,2,3 mice (a model of SLE; mean ± SEM). (middle) F/M ratio per litter of Sle1,2,3 mice. (right) Graph showing the F/M ratio in offspring of young and old Sle1,2,3 mice (mean ± SEM). (E) Fetuses were obtained on day E12–E15 or E17–E19. The percentage of intact male and female fetuses is shown with actual numbers within each column of the graph. (F) F/M ratios per litter (mean ± SEM). (G) Concentration of IgG in the fetal brain. Cohort sizes, 21–85 fetuses, are indicated in each column. *, P < 0.05; **, P < 0.01; ***, P < 0.001, ANOVA. Data are representative of two independent experiments.
Remarkably, comparable loss of viable female offspring was evident in old (>25 wk) but not young (<16 wk) triple congenic Sle1, Sle2, Sle3 mice, which spontaneously develop a lupus-like disease (Morel et al., 1999) and have elevated titers of DNRAbs starting at ∼20 wk (Fig. 1 D; Student’s t test, P < 0.05; n = 17). Thus, the loss of female fetuses was not restricted to the antigen-induced model of systemic lupus erythematosus (SLE). To ascertain when female embryo loss occurred, we collected embryos at E12–E15 and E17–E19. No difference in the F/M ratios between MP- and MC-immunized dams was observed at the early interval (Fig. 1 E); however, the F/M ratios were significantly reduced at E17–E19 (Fig. 1 F; Student’s t test, P < 0.05; n = 12).
Histological assessment showed no abnormality in the heart, lungs, liver, kidney, and intestines of female MP fetuses (not depicted); therefore, we focused on the fetal brain and asked whether the selective vulnerability of female fetuses occurred from higher transplacental transmission of maternal autoantibody into female fetal brain. We tagged IgG with infrared label (Ir-IgG), administered it to pregnant MP dams (Lee et al., 2009), and quantified Ir-IgG levels in fetal brains. No gender difference was observed (Fig. 1 G), indicating that maternal autoantibodies had equal access to fetal brains of both sexes.
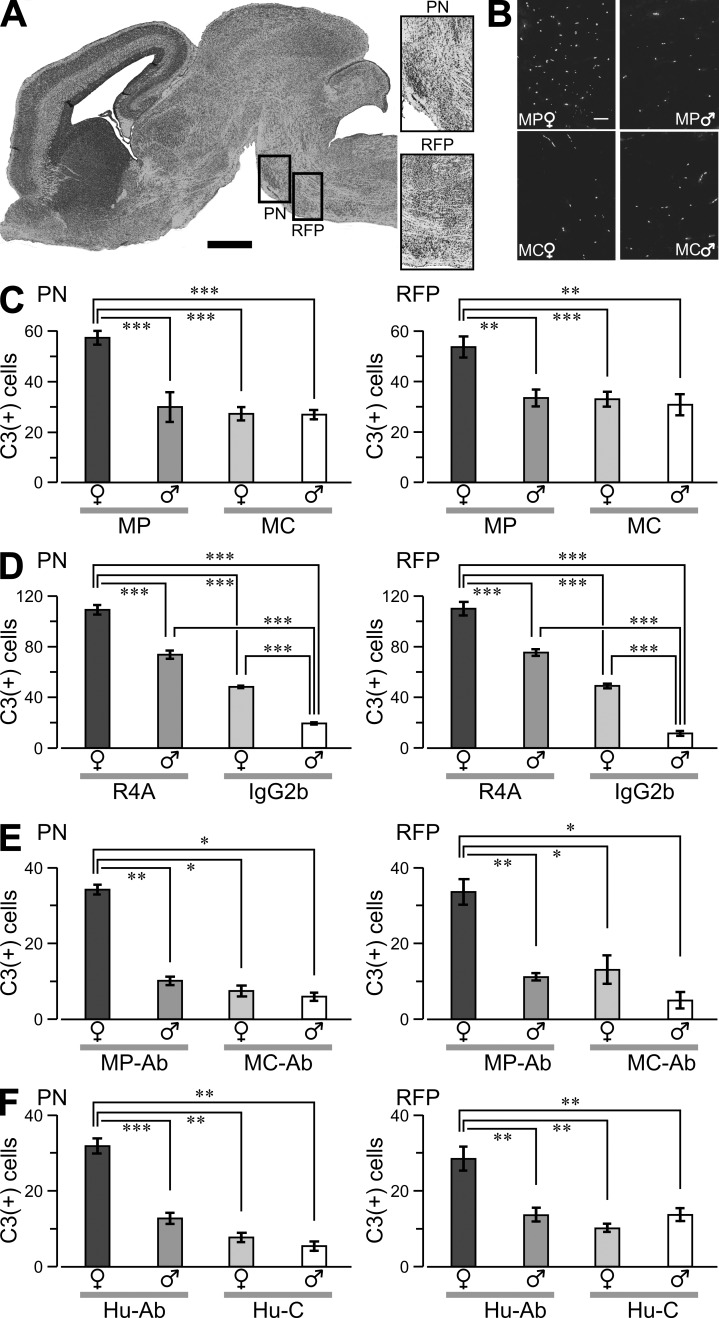
We next explored whether female neurons were selectively vulnerable to the excitotoxic potential of DNRAbs (Faust et al., 2010). E17 fetal brains from MP and MC dams were analyzed for evidence of caspase-3–positive (C3+) cells (DeGiorgio et al., 2001). We focused on the brainstem zones of reticular formation of the pons (RFP) and pontine nucleus (PN; Fig. 2 A), as the newborn brainstem is enriched in glutamatergic pathways and there is precedent for pontine involvement in late fetal demise (Matturri et al., 2002). Female MP fetuses had significantly more C3+ neurons in RFP and PN than male MP fetuses from the same litters (Fig. 2, B and C), whereas other brain areas showed no difference (not depicted). To confirm DNRAb toxicity, organotypic cultures of fetal brainstem were treated with either mouse monoclonal DNRAb, R4A, or isotype control antibody, IgG2b. R4A caused significantly more neuronal apoptosis than IgG2b in female brainstem (Fig. 2 D). Furthermore, R4A caused more neuronal apoptosis in female than male brainstems, indicating increased excitotoxicity in female neurons ex vivo as well as in vivo. DNRAbs isolated from the serum of MP-immunized mice also led to an enhanced toxicity of neurons in female fetal brain (Fig. 2 E), as did anti-DNRAbs isolated from the serum of lupus patients (Fig. 2 F).
Figure 2.
Neuronal death in fetal brains. (A, left) Sagittal whole-brain section (Nissl stained) of female fetus at E17. The boxes highlight the PN and RFP. (right) Representative magnified PN and RFP sections. (B) PN sections labeled for activated caspase-3 (C3+) in fetuses from MP- and MC-immunized dams. Bars: (A) 500 µm; (B) 50 µm. (C–F) Graphs show C3+ cells (mean ± SEM) counted in comparable regions (volume, 1.2 × 106 µm3) of the PN and RFP in the fetal brain. (C) Cell counts from MP and MC fetuses. Data are representative of three experiments. (D) Ex vivo brainstem slices from E17 fetuses were exposed to 100 µg/ml R4A or 100 µg/ml IgG2b for 12 h, fixed, and stained for C3+ reactivity. (E) Slices were treated with 200 µg/ml of purified serum DNARAbs (MP-Ab) or 200 µg/ml of mouse IgG (MC-Ab). (F) Slices were exposed to 400 µg/ml of human DNRAbs (Hu-Ab) or 400 µg/ml of human IgG (Hu-C). Sample sizes, 10–15 sections per column from three to four litters. *, P < 0.05; **, P < 0.01; ***, P < 0.001, ANOVA. Data are representative of two independent experiments.
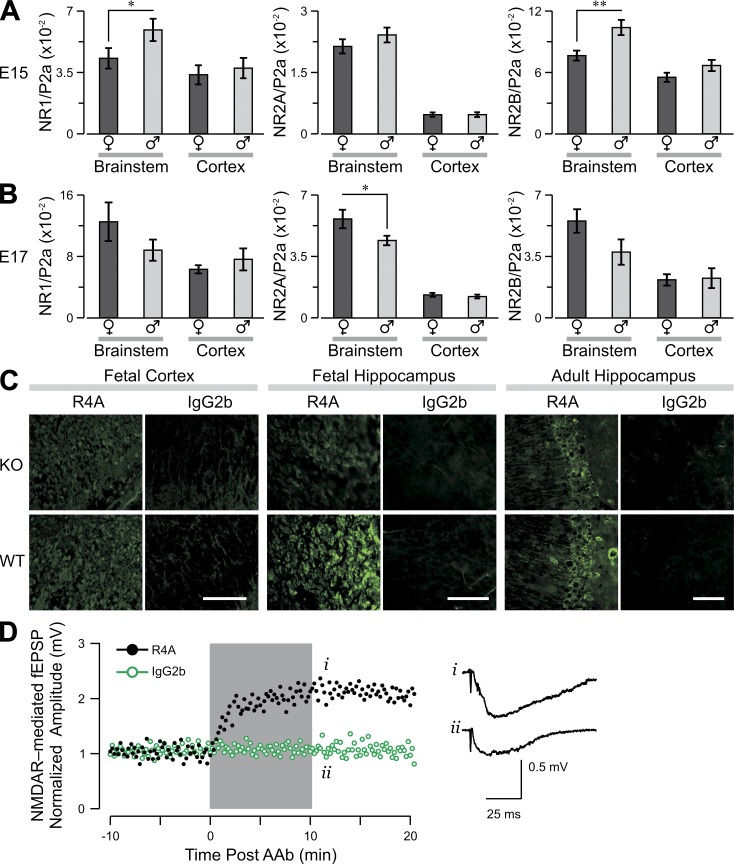
To determine whether the increased vulnerability of neurons from female fetuses was a consequence of increased NMDAR expression, we used quantitative PCR (qPCR) to assess the levels of NR1, NR2A, and NR2B transcripts. NR1 and NR2B levels were significantly greater in male than female brainstems on E15, with no gender difference in NR2A level (Fig. 3 A). By E17, the NR2A level was significantly higher in the female brainstem, and no gender differences in NR1 and NR2B were observed (Fig. 3 B). Levels of all transcripts in other brain regions were similar between genders at both times (data for cortex in Fig. 3, A and B).
Figure 3.
Expression of messenger RNA for NMDAR subunits NR1, NR2A, and NR2B in fetal brain. Graphs show the level of messenger RNA transcript (mean ± SEM) for each subunit, divided by the level of polymerase 2a (P2a). (A and B) Male and female fetuses of WT mice were harvested at E15 (A) or E17 (B), and qPCR was used to assess transcripts of NR1 (left), NR2A (middle), and NR2B (right) in brainstem and cortex. Sample size, 12–15 fetuses per column. *, P < 0.05; **, P < 0.01, Student’s t test. Data show a representative experiment from three independent experiments. (C, left and middle) R4A or control IgG2b staining of WT or NR2A−/− fetal brain. (right) R4A or control IgG2b staining of WT or NR2A−/− adult hippocampus. Bars: (left and middle) 50 µm; (right) 100 µm. (D) R4A triggers an enhancement in the amplitude of excitatory postsynaptic potentials (EPSP), mediated by NMDARs, in NR2A−/− hippocampus. The gray box marks the period of antibody application. (right) Representative traces obtained immediately after treatment with R4A (i) or IgG2b control (ii). Representative data from three independent experiments are shown.
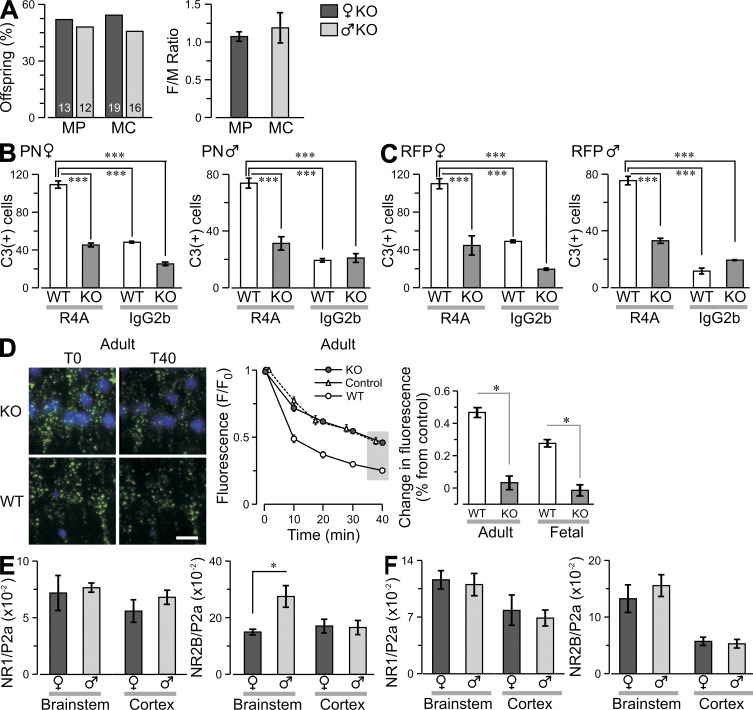
Given the increased expression of NR2A at E17 in the vulnerable female brainstem, we asked whether NR2A-containing NMDARs were responsible for autoantibody-induced toxicity. We demonstrated that R4A reacted with NR2B-containing NMDARs in vivo, as R4A bound NR2A−/− fetal brain as well as adult brain (Fig. 3 C) and functioned to enhance excitatory postsynaptic potentials in adult NR2A−/− brain (Fig. 3 D). NR2A−/− mice were mated to C57BL/6 H-2d/d animals to generate NR2A−/− H-2d/d mice, as the MP response is Ed restricted. NR2A−/− and NR2A+/+ (WT) animals were immunized with MP or MC; both MP-immunized strains mounted a robust antibody response (not depicted). No gender disparity was observed in live offspring of NR2A−/− mice, demonstrating normal viability of female fetuses that lacked NR2A expression (Fig. 4 A). This was not related to maternal genotype as MP-immunized NR2A+/− females mated to NR2A−/− males produced a low F/M ratio of NR2A+/− offspring (0.028) but not NR2A−/− offspring (1.1). To confirm that the brainstem of NR2A−/− female fetuses was protected from R4A-triggered apoptosis, we exposed NR2A−/− and WT brainstem cultures to either R4A or IgG2b; R4A caused significantly less apoptosis in NR2A−/− than WT female brainstems (Fig. 4, B and C). Moreover, R4A caused less mitochondrial stress in NR2A−/− brain slices from fetuses and adults (Fig. 4 D), demonstrating that NR2A-containing NMDARs were responsible for autoantibody-induced neuronal damage in adult as well as fetal brains (Fig. 4 D). The NR2A−/− mice displayed somewhat increased expression of NR2B in both male and female fetuses (Fig. 4, E and F). We cannot know whether the increased NR2A in female fetal brainstem on E17 is responsible for the preferential death of neurons in female fetal brainstem and the death of female fetuses. It is possible that neurons in females are more susceptible to death through NR2A-containing NMDARs because of hormonal influences or nonhormonal gender-specific differences.
Figure 4.
Lack of fetal death in NR2A−/− mice. (A, left) Graph shows the percentage of male and female offspring from MP- or MC-immunized NR2A−/− (KO) dams and offspring of NR2A+/− dams and NR2A−/− males, with offspring numbers indicated in each column. (right) F/M ratios per litter (mean ± SEM) in MP- and MC-immunized mice. (B and C) Ex vivo brainstem slices (E17) were prepared from KO and WT fetuses, exposed to 100 µg/ml R4A or 100 µg/ml IgG2b for 12 h, fixed, and labeled for activated caspase-3 (C3+). The graphs show C3+ cells (mean ± SEM) in the PN (B) and RFP (C; volume, 1.2 × 106 µm3) for female (left) and male (right) fetuses. Sample sizes, 10 sections per column from two to four litters. ***, P < 0.001, ANOVA. Data are representative of three experiments. (D, left) Representative fluorescent micrographs of neurons in the hippocampus in acute slices from KO and WT mice, before (T0) and after (T4) treatment with NMDA and R4A; T0, onset of insult, T40, 40 min after insult (green, calcein; blue, DAPI). Loss of calcein fluorescence indicates increased mitochondrial stress. Bar, 10 µm. (middle) Profile of fluorescence decay in controls and R4A-treated groups (KO and WT). The gray box at T40 highlights the comparison shown at the right where fluorescence levels at T40 are expressed as percent change from control for adult and fetal slices (mean ± SEM). *, P < 0.05, Student’s t test. (E and F) Graphs show the level of messenger RNA transcript (mean ± SEM) for NR1 and NR2B subunits, divided by the level of polymerase 2a (P2a), from KO mice. Male and female fetuses were harvested at E15 (E) and E17 (F), and qPCR was used to assess transcripts of NR1, NR2A, and NR2B in brainstem and cortex. The level of NR2A transcript was zero in both age groups. *, P < 0.05. Data are representative of four experiments.
We have demonstrated that DNRAbs cause sex ratio skewing in mice, with a significant loss of female fetuses. We did not observe gender differences in the transport of maternal antibody into the fetal brain. Rather, brainstem neurons in fetal female brains showed clear increases in vulnerability to antibody-mediated death. Differences in sex hormones in male and female fetuses can be detected by E17, and estrogen has been shown to modulate NMDAR subunit expression in an age- and region-dependent fashion (Woolley et al., 1997; Hsu et al., 1999; Cyr et al., 2001). Thus, increased estrogen activity may contribute to the sex-specific differences in subunit expression in the developing brainstem or may alter downstream signaling so as to increase vulnerability to NMDAR-mediated apoptosis.
The timing of expression of NR2A during fetal development is controversial. An in situ hybridization study suggests that NR2A is not expressed until birth (Monyer et al., 1992), whereas immunohistochemistry with subunit-specific antibody shows the presence of NR2A protein in hindbrain late in fetal development (Portera-Cailliau et al., 1996). Using the highly sensitive technique of qPCR, we observed NR2A expression increasing in the third trimester of pregnancy, with a greater increase in female fetal brain in the brainstem. The relative importance of NR2A and NR2B in excitotoxicity is also disputed (Liu et al., 2007; von Engelhardt et al., 2007; Hardingham and Bading, 2010). Our data, from both in vivo and ex vivo experiments, demonstrate that NR2A-containing NMDARs are critical for neuronal death by DNRAbs in both fetuses and adults. This toxicity does not arise from a preferential interaction of NR2A with DNRAbs, which bind equally well to NR2A and NR2B extracellular domains by ELISA and can signal through NR2B as well as NR2A (DeGiorgio et al., 2001; Kowal et al., 2006; Faust et al., 2010) and bind strongly to NR2B in vivo. NMDARs containing both NR2A and NR2B are commonly expressed in adult neurons, but the functional significance of these molecules during gestation and whether they can mediate excitotoxicity are unknown. Although little is known about gender-specific differences in fetal brain development, there is precedent for gender-specific sensitivity to altered neurodevelopment; the neural tube defect observed in p53-null mice appears more commonly in female offspring, demonstrating another gender-specific brain developmental abnormality (Chen et al., 2008). In this model, it remains to be determined whether small changes in NR2A expression are responsible for increased sensitivity to maternal autoantibodies in female fetuses or whether differences downstream of the receptor mediate female vulnerability.
The literature on the sex ratio of offspring of SLE mothers is scant; however, meta-analysis suggests a high male to female sex ratio (Novack et al., 2006; James, 2007). Lupus pregnancies can result in fetal loss as the result of anticardiolipin antibodies causing placental insufficiency; however, there is no reported gender bias for this outcome (Salmon et al., 2007). A study of >1,000 consecutive SLE pregnancies demonstrated increased fetal loss in mothers with anti-DNA antibodies (Iijima et al., 1997). Although this study showed no sex ratio skewing, it was not reported whether mothers carrying anti-DNA antibodies or DNRAbs showed greater loss of female fetuses.
Clearly, activation of the maternal immune system can affect fetal outcome. Maternal influenza infection in mouse models leads to cognitive deficits in the offspring (Shi et al., 2005; Meyer et al., 2009); this is replicated by elevated IL-6 in gestating dams. An epidemiological study (Oster, 2005) showed that maternal hepatitis B infection dramatically skewed the sex ratio toward much higher frequencies of male births, which led to the calculation that hepatitis B may account for as many as one third of the “lost” girl babies in Asia and North Africa (Sen, 1992). The mechanism by which maternal hepatitis B infection mediates this skewing is unknown; viral fetal infection, cytokine response to infection, or antibody may all be implicated.
Our study clearly shows that antibodies play a role in this arena. How many maternal antibody specificities may affect brain development or differentially affect male and female fetuses is unknown, but an increased awareness that fetal loss may be both antibody mediated and gender specific will lead to a greater appreciation of the role of the immune system in maternal–fetal health.
MATERIALS AND METHODS
Animals and immunization.
BALB/cJ female mice (6–8 wk old) were obtained from the Jackson Laboratory. Triple congenic Sle1, Sle2, Sle3 mice were a gift from E.K. Wakeland (University of Texas Southwestern Medical Center, Dallas, TX). NR2A−/− C57BL/6 mice backcrossed for >10 generations were a gift from H. Monyer (Heidelberg University, Heidelberg, Germany); they were backcrossed onto the C57BL/6 H-2d/d background. Animal use was in accordance with institutional guidelines of the Feinstein Institute for Medical Research. Mice were immunized with multi-antigenic peptide coupled to 100 µg DWEYSVWLSN (MP), 100 µg of scrambled peptide WSDYEVWLSN (MS), and 100 µg of control poly-lysine core (MC; AnaSpec) as previously described (Kowal et al., 2006). Time-pregnant immunized mice were killed by cervical dislocation, and embryos were harvested between E12 and E19 and processed for sex identification and fetal brain culture or pathology.
ELISA.
Antibody titers were determined as previously described (Kowal et al., 2006).
Maternal antibody transfer.
Mouse monoclonal DNRAb, R4A, was infrared labeled (Ir-R4A) using infrared labeling chelate IRDye 800CW (LI-COR Biosciences) according to the manufacturer’s protocol. 200 µg Ir-R4A was intravenously administered to E15 gestating mice, and the fetal brains were obtained 24 h later. Each fetal brain was weighed, and Ir-R4A counts (cpm) in each fetal brain were measured using a 1420 Victor Multilabel Counter (PerkinElmer).
Fetal sex determination.
Fetal sex was determined by PCR assay for detection of the male-specific gene, Sry, with the autosomal gene, myogenin, serving as a positive control. The following conditions were used for PCR: 1 cycle 94°C 4 min; 30 cycles 94°C 45 s, 61°C 45 s, 72°C 45 s; and 1 cycle 72°C 5 min. PCR reaction generated a male-specific Sry band of 441 bp and a control myogenin band of 245 bp. Primers for Sry were 5′-TCATGAGACTGCCAACCACAG-3′ and 5′-CATGACCACCACCACCACCAA-3′ and for myogenin were 5′-TTACGTCCATCGTGGACAGC-3′ and 5′-TGGGCTGGGTGTTAGTCTTA-3′.
NMDAR subunit quantification.
RNA was extracted from the cortex and brainstem of fetal brain at defined times using the RNeasy Lipid Tissue Mini kit (QIAGEN) according to the manufacturer’s protocol. RT-PCR was performed on 15 µl RNA using iScriptcDNA Synthesis kit (Bio-Rad Laboratories) in a final volume of 20 µl; qPCR was performed on a LightCycler480 Real-Time PCR System (Roche) and analyzed using LightCycler480 Software (Roche). TaqMan Gene Expression Assays sets (Applied Biosystems) were used; reactions were performed using LightCycler480 Probes Master Mix (Roche) in a final volume of 10 µl. All primer sets spanned an intron/exon border. Applied Biosystems primer IDs are as follows: NR1, Mm00433800_m1; NR2A, Mm00433802_m1; NR2B, Mm00433820_m1; and polr2a, Mm00839493_m1.
Fetal organotypic brain cultures.
Pregnant dams were killed by cervical dislocation, and fetuses were dissected from the uterine horns on 1 mg/ml of ice-cold PBS/glucose. Tail DNA was used for genotyping. Brains were isolated in ice-cold PBS/glucose, divided into two equal parts by fine forceps, and stripped of meninges. Each half brain was transferred to a 48-well plate containing neurobasal medium with R4A or IgG2b and incubated at 37°C for 12 h. The cultured brains were immersion fixed in 4% paraformaldehyde/PBS overnight at 4°C, followed by equilibration in 30% sucrose/PBS for a minimum of 24 h. DNRAbs from MP-immunized mice and human SLE sera were purified on a peptide affinity column as previously described (DeGiorgio et al., 2001). Mouse DNRAbs and 200 µg/ml of control IgG or human DNRAbs and 400 µg/ml of control IgG were incubated in parallel.
Histology and immunohistochemistry.
Pregnant dams were killed, and uteri were removed and placed on ice. Fetal brains were removed and immersion fixed in 4% paraformaldehyde/PBS overnight at 4°C followed by equilibration in 30% sucrose/PBS. They were embedded in OCT, and 10-µm sections were obtained by cryostat. Sections were frozen until immunohistochemistry was completed. For all experiments, a minimum of three litters was used, and littermates were compared. Sections were treated with citrate buffer for 10 min at 95°C and then cooled to room temperature. Slides were then rinsed three times in PBS and blocked in 10% normal goat serum/PBS containing 0.1% Tween for 45 min at room temperature. Sections from fetuses from each group of dams or sections of ex vivo brain cultures were stained for DAPI and monoclonal antibody targeting activated caspase-3 (G7481; Promega). The histological images were acquired using AxioVision software (Carl Zeiss). Comparable regions of identical area from the PN, RFP, cortex, thalamus, and hippocampus were imaged (Axio-Imager z1; Carl Zeiss), and signals from cells labeled by anti–active caspase-3 antibody and Alexa Fluor 594 were captured and automatically counted (AxioVision). Targets were counted in six optical dissectors (30,000 µm2) randomly placed within the larger region of anatomical interest; thus, counts represent the number of targets per constant volume. There were three sections per embryo, and each group had at least three animals.
Mitochondrial stress in fetal and adult slices.
Acute slices (350-µm thick) from the hippocampus were prepared from female adult mice (3–6 mo of age) or from fetal brain (E17). Slices were incubated, loaded with mitochondrial indicator, and treated with 200 µg/ml R4A or 200 µg/ml IgG2b, as previously described (Faust et al., 2010).
Acknowledgments
We thank Matthew Scharff and Kevin Tracey for their thoughtful comments.
This work was supported by National Institutes of Health Program Grant 5P01AI073693 (to B. Diamond, P.T. Huerta, and B.T. Volpe).
The authors have no conflicting financial interests to declare.
Footnotes
Abbreviations used:
- ANOVA
- analysis of variance
- DNRAb
- DNA/NMDAR cross-reactive antibody
- NMDAR
- N-methyl-d-aspartate receptor
- PN
- pontine nucleus
- qPCR
- quantitative PCR
- RFP
- reticular formation of the pons
- SLE
- systemic lupus erythematosus
References
- Chen X., Watkins R., Delot E., Reliene R., Schiestl R.H., Burgoyne P.S., Arnold A.P. 2008. Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev. Neurobiol. 68:265–273 10.1002/dneu.20581 [DOI] [PubMed] [Google Scholar]
- Cyr M., Thibault C., Morissette M., Landry M., Di Paolo T. 2001. Estrogen-like activity of tamoxifen and raloxifene on NMDA receptor binding and expression of its subunits in rat brain. Neuropsychopharmacology. 25:242–257 10.1016/S0893-133X(01)00233-0 [DOI] [PubMed] [Google Scholar]
- DeGiorgio L.A., Konstantinov K.N., Lee S.C., Hardin J.A., Volpe B.T., Diamond B. 2001. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 7:1189–1193 10.1038/nm1101-1189 [DOI] [PubMed] [Google Scholar]
- Faust T.W., Chang E.H., Kowal C., Berlin R., Gazaryan I.G., Bertini E., Zhang J., Sanchez-Guerrero J., Fragoso-Loyo H.E., Volpe B.T., et al. 2010. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proc. Natl. Acad. Sci. USA. 107:18569–18574 10.1073/pnas.1006980107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham G.E., Bading H. 2010. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 11:682–696 10.1038/nrn2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C., Hsieh Y.L., Lue S.I., Hsu H.K. 1999. Sex-specific expression of N-methyl D-aspartate receptor (NMDAR) in the preoptic area of neonatal rats. Neurosci. Lett. 262:85–88 10.1016/S0304-3940(99)00038-5 [DOI] [PubMed] [Google Scholar]
- Iijima T., Tada H., Hidaka Y., Mitsuda N., Murata Y., Amino N. 1997. Effects of autoantibodies on the course of pregnancy and fetal growth. Obstet. Gynecol. 90:364–369 10.1016/S0029-7844(97)00283-4 [DOI] [PubMed] [Google Scholar]
- James W.H. 2007. The sex ratio of offspring of patients with systemic lupus erythematosus. Lupus. 16:65–66 10.1177/0961203306073365 [DOI] [PubMed] [Google Scholar]
- Khalil M., Inaba K., Steinman R., Ravetch J., Diamond B. 2001. T cell studies in a peptide-induced model of systemic lupus erythematosus. J. Immunol. 166:1667–1674 [DOI] [PubMed] [Google Scholar]
- Koopman P., Münsterberg A., Capel B., Vivian N., Lovell-Badge R. 1990. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 348:450–452 10.1038/348450a0 [DOI] [PubMed] [Google Scholar]
- Kowal C., Degiorgio L.A., Lee J.Y., Edgar M.A., Huerta P.T., Volpe B.T., Diamond B. 2006. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl. Acad. Sci. USA. 103:19854–19859 10.1073/pnas.0608397104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y., Huerta P.T., Zhang J., Kowal C., Bertini E., Volpe B.T., Diamond B. 2009. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat. Med. 15:91–96 10.1038/nm.1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wong T.P., Aarts M., Rooyakkers A., Liu L., Lai T.W., Wu D.C., Lu J., Tymianski M., Craig A.M., Wang Y.T. 2007. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 27:2846–2857 10.1523/JNEUROSCI.0116-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matturri L., Minoli I., Lavezzi A.M., Cappellini A., Ramos S., Rossi L. 2002. Hypoplasia of medullary arcuate nucleus in unexpected late fetal death (stillborn infants): a pathologic study. Pediatrics. 109:E43 10.1542/peds.109.3.e43 [DOI] [PubMed] [Google Scholar]
- Meyer U., Feldon J., Fatemi S.H. 2009. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci. Biobehav. Rev. 33:1061–1079 10.1016/j.neubiorev.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P.H. 1992. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 256:1217–1221 10.1126/science.256.5060.1217 [DOI] [PubMed] [Google Scholar]
- Morel L., Tian X.H., Croker B.P., Wakeland E.K. 1999. Epistatic modifiers of autoimmunity in a murine model of lupus nephritis. Immunity. 11:131–139 10.1016/S1074-7613(00)80088-6 [DOI] [PubMed] [Google Scholar]
- Novack V., Erez O., Novack L., Jotkowitz A., Meir A., Mazor M. 2006. Sex distribution of newborns to mothers with systemic lupus erythematosus. Epidemiology. 17:341–342 10.1097/01.ede.0000209417.14819.44 [DOI] [PubMed] [Google Scholar]
- Oster E. 2005. Hepatitis B and the case of the missing women. J. Polit. Econ. 113:1163–1216 10.1086/498588 [DOI] [Google Scholar]
- Portera-Cailliau C., Price D.L., Martin L.J. 1996. N-methyl-D-aspartate receptor proteins NR2A and NR2B are differentially distributed in the developing rat central nervous system as revealed by subunit-specific antibodies. J. Neurochem. 66:692–700 10.1046/j.1471-4159.1996.66020692.x [DOI] [PubMed] [Google Scholar]
- Putterman C., Diamond B. 1998. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J. Exp. Med. 188:29–38 10.1084/jem.188.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon J.E., Girardi G., Lockshin M.D. 2007. The antiphospholipid syndrome as a disorder initiated by inflammation: implications for the therapy of pregnant patients. Nat. Clin. Pract. Rheumatol. 3:140–147, quiz :1: 187 10.1038/ncprheum0432 [DOI] [PubMed] [Google Scholar]
- Sen A. 1992. Missing women. BMJ. 304:587–588 10.1136/bmj.304.6827.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Tu N., Patterson P.H. 2005. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int. J. Dev. Neurosci. 23:299–305 10.1016/j.ijdevneu.2004.05.005 [DOI] [PubMed] [Google Scholar]
- von Engelhardt J., Coserea I., Pawlak V., Fuchs E.C., Köhr G., Seeburg P.H., Monyer H. 2007. Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology. 53:10–17 10.1016/j.neuropharm.2007.04.015 [DOI] [PubMed] [Google Scholar]
- Woolley C.S., Weiland N.G., McEwen B.S., Schwartzkroin P.A. 1997. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J. Neurosci. 17:1848–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]