To be added
Abstract
The mammalian immune system and the nervous system coevolved under the influence of infection and sterile injury. Knowledge of homeostatic mechanisms by which the nervous system controls organ function was originally applied to the cardiovascular, gastrointestinal, musculoskeletal, and other body systems. Development of advanced neurophysiological and immunological techniques recently enabled the study of reflex neural circuits that maintain immunological homeostasis, and are essential for health in mammals. Such reflexes are evolutionarily ancient, dating back to invertebrate nematode worms that possess primitive immune and nervous systems. Failure of these reflex mechanisms in mammals contributes to nonresolving inflammation and disease. It is also possible to target these neural pathways using electrical nerve stimulators and pharmacological agents to hasten the resolution of inflammation and provide therapeutic benefit.
The 1908 Nobel Prize in Physiology or Medicine was shared by Ilya Mechnikov and Paul Ehrlich for identifying the cellular and humoral basis of immunity. Although this represented a landmark celebration of two giants in the nascent immunology field, the shared prize also stoked the ongoing controversy over which of their two theories primarily accounted for protective immunity. During this same period, another fledgling scientific field was entangled in a different, but equally raging intellectual debate. Neuroscientists, freshly armed with the ability to visualize neurons using the staining methods of Cajal and further endowed with new concepts of “synapses” and the motor cortex elucidated by Charles Sherrington, argued whether the fundamental unit of information transfer between neurons was electrical or chemical.
The answer came in 1921 when Otto Loewi placed two beating frog hearts in separate saline baths: one heart he left attached to its vagus nerve and the other he denervated. After observing that electrically stimulating the intact vagus nerve slowed the innervated heart, he then harvested saline from this bath, and transferred it to the denervated system. Reporting that a substance in the conditioned media caused the denervated heart to slow, Loewi named the transferable agent “Vagusstoff.” Henry Dale, who later shared the Nobel Prize with Loewi, identified the active agent as acetylcholine, which he isolated from ox and horse spleens (Dale and Dudley, 1929).
From that point forward, immunology and neuroscience progressed on parallel paths. Physiologists came to understand that neural circuits, operating reflexively, controlled the function of the cardiovascular, gastrointestinal, and musculoskeletal systems, but there was considerable resistance to extending these mechanisms to immune system homeostasis. Immunity was viewed as autonomous to the immune system, mediated by interactions between immune cells in a largely self-regulated system that could be influenced by external factors, humoral mediators, and products of immunocompetent cells. Until very recently, neither field paid much notice to direct evidence that the immune system is functionally and anatomically connected to the nervous system.
The origin of meaningful collaborations between these fields perhaps began with the identification of the antiinflammatory action of neuroendocrine hormones. Early animal studies established that adrenal hormones, produced under the regulation of the hypothalamic pituitary axis and adrenocorticotropic hormone, possessed activities that could be placed into two categories. One influenced fluid homeostasis, and the other exerted beneficial effects against shock and rheumatoid arthritis. Clinical studies demonstrating the therapeutic effectiveness of corticosteroids in rheumatoid arthritis won Philip Hench the Nobel Prize in 1950. This catalyzed investigation into mechanisms by which hormones and other soluble mediators normally balance the immune system.
Work dating back to 1961 demonstrated that electrically stimulating the vagus nerve significantly enhanced the release of acetylcholine from the spleen (Brandon and Rand, 1961; Leaders and Dayrit, 1965). By then, anatomy studies had already revealed the innervation of the spleen, thymus, lymph nodes, and even the bursa of Fabricus. Later advances in electron microscopy and immunohistochemistry, when used to image the principle organs of the immune system, revealed nerve endings in the vicinity of T cells, B cells, and macrophages. The origin of these neurons was traced to nuclei residing in the brain stem, the sympathetic chain, and peripheral ganglion cells. Synapse-like structures formed by nerve endings were found lying within mere nanometers of immune cells that express receptors for acetylcholine, catecholamines, neuropeptides, and other principle neurotransmitters. These incipient neural circuits attracted little notice from the broader field of neuroscience, perhaps because it was not known whether information transfer from the nervous system could meaningfully regulate immune responses or how this communication could be studied. Immunologists also took little notice, perhaps because of the prevailing view that the immune system is autonomous, and that immunity could be fully understood by studying the activities and interactions between its principle cells.
Neuroscience and immunology directly intersected in the 1990s, when Linda Watkins made the seminal discovery that fever in rodents after intraabdominally administered IL-1β required an intact vagus nerve (Watkins et al., 1995a). This meant that action potentials transmitted in sensory nerve fibers to the brainstem report the presence of inflammatory substrates in peripheral tissues. Using sensitive neurophysiological techniques to record nerve fiber activity, Niijima traced the path of action potentials traveling in the vagus nerve from the liver to the brainstem, and showed that these signals activated descending neurotransmission to the spleen, thymus, and other organs (Niijima et al., 1991, 1995, Niijima et al., 1996). This new paradigm established an essential early role for the sensory nervous system in a fundamental mechanism of host defense.
At about this time, we were working with colleagues to understand how a tetravalent guanylhydrazone (CNI1493) significantly blocked cytokine release and attenuated disease in arthritis and other animal models (Bianchi et al., 1996). We surreptitiously observed that an intact vagus nerve was required for CNI1493 to effectively inhibit cytokine release in vivo (Borovikova et al., 2000a). To determine the underlying mechanisms, we electrically stimulated the vagus nerve, and showed that driving action potentials into the reticuloendothelial organs significantly inhibited cytokine production (Borovikova et al., 2000b). Considering these findings together with Watkins and Niijima’s observations, we proposed that afferent and efferent signals transmitted in the vagus nerve are components of an inflammatory reflex, a neural circuit that modulates innate immune responses (Tracey, 2002). This became the founding, prototypical member of an expanding family of reflex neural circuits that maintain immunological homeostasis. These neural mechanisms are essential to understanding immunity.
In the remainder of this review, we discuss illustrative examples of neural circuits that modulate immune responses. The discussion is not encyclopedic, but instead focuses on prototypical neural circuits that interact with immune cells to maintain stable physiological responses and immunological homeostasis. We also discuss recent advances in understanding how neural and immunological information merge.
Immunological homeostasis and nonresolving inflammation
The functional operation of organ systems is maintained within a narrow, optimal range, largely under the control of neural circuits that operate reflexively to adjust organ function relative to an established set point. Reflexes fine-tune the functional output of the cardiovascular, gastrointestinal, respiratory, renal, musculoskeletal, and neuroendocrine systems in response to alterations in the external and internal environment. “Immunological homeostasis” is also essential for health, because insufficient or excessive activity in the immune system causes disease (Tracey, 2007). During sterile injury, ischemia, or infection, the activation of humoral and cellular immune responses protects against invasion, cleans up the detritus of tissue injury, and initiates wound healing and repair mechanisms. These responses are normally short-lived, and the resolution of inflammation is an essential component of immunological homeostasis. Abundant evidence indicates that nonresolution of inflammation drives the pathogenesis of sepsis, atherosclerosis, obesity, cancer, pulmonary disease, inflammatory bowel disease, neurodegeneration, multiple sclerosis, and rheumatoid arthritis (Nathan and Ding, 2010).
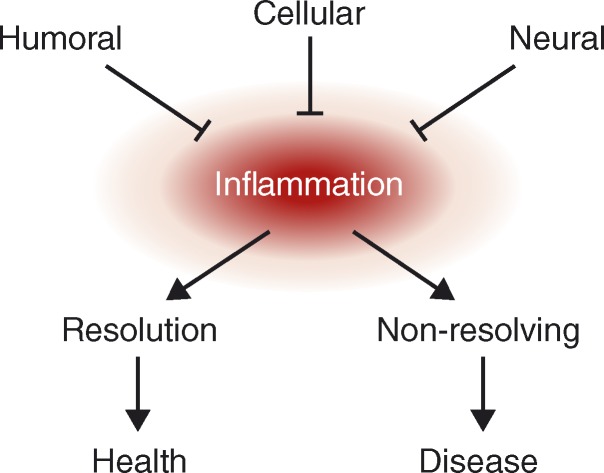
Humoral, cellular, and neural mechanisms prevent nonresolving inflammation (Fig. 1; Nathan and Ding, 2010). Humoral, or soluble, antiinflammatory mediators have been studied since the discovery of the antiinflammatory properties of corticosteroids. Deficiencies in glucocorticoids or the pituitary hormones that normally regulate their production confer exquisite sensitivity to inflammation and infection (Besedovsky and del Rey, 2000; Sternberg, 2006). Glucocorticoid levels rise early with the onset of inflammation or stress, providing important feedback signals to suppress immune responsiveness and protect the host from the toxicity and tissue injury caused by excessive inflammation. The discovery of macrophage-derived TGF-β as an antiinflammatory mediator that deactivates macrophages opened a new field focused on soluble negative feedback mediators produced by the immune system. (Tsunawaki et al., 1988). IL-10 was later implicated as another prototypical inflammation-resolving humoral mediator produced by monocytes (Mosser and Zhang, 2008). The growing list of antiinflammatory mediators includes soluble cytokine receptors, cytokine receptor antagonists, eicosanoids, and oxygenated and nitrated lipids (Nathan, 2002).
Figure 1.
Humoral, cellular, and neural regulation of nonresolving inflammation. Nonresolving inflammation mediates the pathogenesis of many major diseases. Understanding mechanisms that reverse or prevent nonresolving inflammation has important implications for the development of therapeutics. Humoral and cellular antiinflammatory mechanisms are perhaps the most widely studied, and are covered in detail in other reviews. As reviewed here, recent advances in neuroscience and immunology have identified neural circuits that modulate the immune system. Understanding these circuits will reveal mechanisms for the localized and rapid control of immunity with significant therapeutic implications.
Cellular mechanisms also prevent nonresolving inflammation. For example, proinflammatory phagocytes switch to an antiinflammatory phenotype that promotes tissue repair rather than tissue destruction (Nathan and Ding, 2010). TGF-β and IL-4 stimulate macrophages to assume an alternatively activated antiinflammatory phenotype (Nathan, 2002; Mosser and Zhang, 2008). Regulatory T cells and myeloid-derived suppressor cells also limit inflammation.
The nervous system, which receives information from the immune system in the form of soluble mediators and from sensory neurons, also suppresses inflammation (Watkins et al., 1995b; Tracey, 2007). Immune system molecules can cross the blood–brain barrier by exploiting specific saturable transport mechanisms, by transcytosis across brain endothelial cells, or by entering the brain in regions that are devoid of a blood–brain barrier. Inflammatory cells can also cross the blood–brain barrier. The binding of soluble immune signaling molecules to cognate receptors expressed on central nervous system (CNS) neurons, astrocytes, and microglial cells produces cellular responses that change the activity of neural circuits in brain stem and in hypothalamic nuclei, which control the autonomic nervous system and the neuroendocrine system.
As in the aforementioned case of IL-1–mediated fever, afferent information can also arrive in the CNS from peripheral sensory neurons that are activated by immune mediators (Watkins et al., 1995a,b). Sensory neurons propagate action potentials to the brain stem nucleus tractus solatarius and other nuclei that receive afferent information. Afferent neurons express receptors for TNF, IL-1, LPS, and other products of inflammation. Incoming information from the immune system activates neural reflex circuits that underlie the behavioral and physiological responses associated with acute and chronic immune responses, including acute phase protein responses, fever, anorexia, insulin resistance, chronic sickness behavior, depression, and cachexia (Watkins et al., 1995b).
Sensory neurons propagate information very quickly, and from discrete anatomical regions. These communication features enable the CNS to rapidly localize incoming information and transmit outgoing instructions to other organ systems. This is not possible with immune signals sent in the form of diffuse humoral factors. It is helpful to remember that neurotransmission is ideally suited to correctively modulate system functions that deviate acutely from physiological set points. Humoral neuroendocrine mechanisms occupy more important roles in establishing and maintaining chronic set point responses.
The autonomic nervous system primarily regulates organ homeostasis via reflexes that are comprised of afferent and efferent neurons. Specialized afferent neurons initiate the reflex by responding to changes in the environment. Action potentials can be initiated in sensory neurons in response to changes in cytokine levels, pH, oxygen tension, and other molecular and chemical changes in the milieu. Afferent signals travel to the CNS along the vagus, splanchnic, and pelvic nerves; autonomic pain fibers originating in blood vessels may travel with somatic nerves. The efferent or outgoing arc of the basic reflex circuit is comprised of nerve fibers that travel to the organs and use acetylcholine and norepinephrine as principle neurotransmitters. Physiologists, pharmacologists, and anatomists usually characterize autonomic reflexes as either “sympathetic” or “parasympathetic,” but this functional nomenclature is imprecise. Henry Dale, one of fathers of neuroscience, argued strongly against the use of functional monikers to describe neural circuits (Dale, 1954). He called for the labeling of neural circuits by virtue of their neurotransmitters (adrenergic or cholinergic) rather than their function (sympathetic or parasympathetic). Indeed, the limitations are apparent in characterizing the neural circuits that regulate immunity, as they have components that are cholinergic, adrenergic, and peptidergic, and involve T cells and endothelial cells that are neurally controlled to secrete neurotransmitters that ultimately target macrophages and lymphocytes to alter expression of cytokines and cell surface proteins (Andersson and Tracey, 2012). It is not possible to neatly classify these as sympathetic or parasympathetic.
Neural circuits regulate immunity in Caenorhabditis elegans
Direct evidence of neurons regulating immune responses can be found in the nematode worm, C. elegans, an evolutionarily ancient animal that feeds on soil bacteria and is endowed with primitive immune and nervous systems. Physiological homeostasis and motor behavior is regulated by some 302 neurons and 56 glial cells that coordinate the response to the internal and external environment. The animal’s nervous system utilizes signaling molecules closely related to mammalian counterparts including TGF-β, insulin, and the neurotransmitters acetylcholine, γ-aminobutyric acid, and norepinephrine. This early nervous system is sufficient to exert significant control over the activity of the innate immune response to infection (Aballay, 2009; Sun et al., 2011; Tracey, 2011). Neural circuits have therefore coevolved with, and influenced, the evolution of immunity, at least as far back as the invertebrate period.
Inflammatory reflex
In the inflammatory reflex (Fig. 2), a prototypical neural circuit, exogenous and endogenous molecular products of inflammation activate afferent action potentials traveling in the vagus nerve to the nucleus tractus solatarius, which relays the neuronal signals to other brain nuclei located in the hypothalamus and brainstem (Watkins et al., 1995a, 1999; Niijima, 1996; Goehler et al., 1997). Efferent signals travel from the nucleus ambiguus and dorsal motor nucleus back down the vagus nerve, which terminates in the celiac ganglia (Berthoud and Powley, 1996; Borovikova et al., 2000b). Stimulation of the vagus nerve activates adrenergic splenic neurons residing in the celiac ganglion, which travel into the spleen and terminate in synapse-like structures adjacent to T cells in the white pulp (Rosas-Ballina et al., 2011). Norepinephrine released from splenic neurons binds to β2 adrenergic receptor expressed on a subset of T cells that expresses choline acetyltransferase, the rate-limiting enzyme in acetylcholine biosynthesis.
Figure 2.
The inflammatory reflex. The prototypical reflex circuit regulating immunity is comprised of afferent and efferent signals transmitted in the vagus nerve in response to the molecular products of infection and injury, including cytokines, eicosanoids, DAMPs, and PAMPs. The activation of adrenergic neurons in the spleen culminates in the release of norepinephrine in the vicinity of T cells that are capable of secreting acetylcholine. Acetylcholine crosses the marginal zone and enters the red pulp, where it interacts with α7 nAChR expressed on cytokine producing macrophages. α7 nAChR signal transduction suppresses the synthesis and release of TNF, IL-1, IL-18, HMGB1, and other cytokines.
The inflammatory reflex is impaired in nude mice, meaning that vagus nerve stimulation fails to inhibit cytokine release in the absence of T cells (Rosas-Ballina et al., 2011). Strikingly, the passive transfer of T cells expressing choline acetyltransferase, which produce acetylcholine, significantly restores the inflammatory reflex in nude mice. Thus, T cells provide the acetylcholine that acts as the terminal neurotransmitter to complete the inflammatory reflex circuit. Splenic nerve endings in the splenic white pulp form synapse-like structures with T cells that express β2 adrenergic receptors and respond to norepinephrine (Felten and Olschowka, 1987; Rosas-Ballina et al., 2011). These acetylcholine-synthesizing T cells, which express a CD4+CD44hiCD62Llo surface phenotype, represent <3% of the total T cell population and 10% of the total memory T cell population. They produce IL-17A, IL-10, and IFN-γ, but not IL-4 or IL-6, after stimulation with plate-bound anti-CD3. T cell stimulation significantly enhances the capacity of these cells to produce acetylcholine. Acetylcholine-producing T cells are also found in lymph nodes and Peyer’s patches (Rosas-Ballina et al., 2011). The origins and fate of these cells during development and the response to injury and infection is currently being studied.
Acetylcholine released by these splenic T cells binds to α7 nAChR expressed on macrophages in the red pulp and marginal zone (Rosas-Ballina et al., 2011). In macrophages, α7 nAChR signals down-regulate cytokine synthesis by suppressing nuclear translocation of NF-κB (Wang et al., 2003, 2004). α7 nAChR-deficient mice are exquisitely sensitive to nonresolving inflammation induced by endotoxemia or adjuvant arthritis (Wang et al., 2003; van Maanen et al., 2010). However, the inflammatory reflex in these mice is significantly restored by passive transfer of WT bone marrow, indicating that that bone marrow–derived cells, and not neurons (which can also express α7 nAChR), are required for the integrity of the inflammation-resolving circuit (Olofsson et al., 2012).
Anatomical, functional, and molecular lesions in the vagus nerve enhance cytokine production associated with nonresolving inflammation (Tracey, 2007). Under basal conditions, the vagus nerve transmits tonic inhibitory activity that dampens the activity of the innate immune response to pathogen associated molecular products (Rosas-Ballina et al., 2009). The inhibitory activity of the inflammatory reflex can be enhanced by methods that increase the generation of adrenergic signals in the splenic nerve. This has been accomplished experimentally by electrically stimulating the vagus nerve, by electrically stimulating the splenic nerve directly, or by pharmacologically activating the adrenergic splenic neurons using cholinergic agonists (Borovikova et al., 2000b; Bernik et al., 2002; Wang et al., 2003,2004; Metz and Tracey, 2005; Vida et al., 2011) The activity of the splenic adrenergic neurons can be functionally modified by either preganglionic neurons arising on the sympathetic chain, or by signals arriving there from the vagus nerve that terminate on interneurons residing in the celiac ganglion that can modulate the signals arising from the sympathetic chain.
Detailed functional mapping of this pathway has also been aided by studies in which the vagus nerve was transected and electrical stimulation was applied to the distal segment. This efferent signal significantly enhances spleen norepinephrine and acetylcholine release, and suppresses cytokine production (Borovikova et al., 2000b; Bernik et al., 2002; Vida et al., 2011). Moreover, after vagus nerve transection, the adrenergic splenic nerve can also be activated by cholinergic agonists that target the celiac ganglion. In addition, adrenergic neurons in the spleen are modified by the onset of inflammation (Straub et al., 2008). Splenic neurons degenerate during the onset of adjuvant arthritis in rodents, and maximal loss of splenic neurons occurrs just before the onset of maximal inflammation. The functional outcome of this denervation is an absence of the tonic inhibitory influence of the inflammatory reflex and an exacerbation of inflammatory responses similar to that seen after transection or chemical ablation of the splenic nerve (van Maanen et al., 2009a,b, 2010).
Experimental tools that have been used to map these neural circuits to the immune system include electrodes, which directly stimulate the neural components of the inflammatory reflex (vagus and splenic nerves), pharmacological agonists that target α7 nAChR to deactivate macrophages, and pharmacological agonists that target muscarinic acetylcholine (M1) receptors in the brain to up-regulate efferent vagus nerve activity (Pavlov et al., 2006, 2009). CNI-1493 is an M1 agonist, a fact that explains the original unexpected discovery that CNI-1493 activated the vagus nerve to suppress inflammation in vivo (Bianchi et al., 1996; Borovikova et al., 2000a). M1 brain cholinergic networks can also be activated by administration of ghrelin, oxytocin, or centrally acting acetylcholinesterases. Each of these agents significantly stimulates vagus nerve signaling and reduces cytokine release.
Patients with autoimmune disease and nonresolving inflammation develop significantly impaired vagus nerve signaling, which accelerates the progression of inflammation and prevents resolution (Tracey, 2007). Vagus nerve signaling to the spleen “reprograms” leukocytes and attenuates their migration to joints affected with synovitis (Saeed et al., 2005). Studies of collagen-induced arthritis in α7 nAChR-deficient mice confirm that synovial inflammation is markedly increased compared with WT controls (van Maanen et al., 2009a,b). Furthermore, in WT mice with collagen-induced arthritis, treating with α7 nAChR agonists or electrical vagus nerve stimulation significantly ameliorates arthritis activity and tissue destruction.
The measurement of deficient basal levels of vagus nerve activity in human disease has become an area of great potential interest. By analogy to the tonic inhibitory influence of vagus nerve activity on heart rate, depressed vagus nerve activity causes increased heart rate. Heart rate variability (HRV), one method used to quantify the strength of vagus nerve signaling, has been standardized and applied in numerous studies (Sloan et al., 2007a). These clinical results reveal a significant correlation between depressed vagus nerve activity and increased morbidity and mortality in nonresolving inflammatory diseases. For example, mortality rate in severe sepsis was 60% in those patients with decreased vagus nerve activity at admission to hospital, as compared with 0% mortality in subjects with higher vagus nerve activity (Pontet et al., 2003). Trauma patients with vagotomy had significantly increased mortality and septicemia as compared with a cohort of injured control patients matched for age, sex, co-morbidities, concurrent splenectomy, and other factors (Peterson et al., 2012).
Numerous studies of HRV in rheumatoid arthritis revealed significantly reduced vagus nerve activity in patients as compared with controls (Evrengül et al., 2004; Goldstein et al., 2007). These clinical studies have not established a causal relationship between impaired vagus nerve signaling and disease, but this hypothesis has been proven in numerous preclinical animal studies. In the future it should be possible to address causality in prospective studies of vagus nerve modulation in humans (Table 1). An intriguing possibility is that vagus nerve deficiencies might be reversed by vagus nerve stimulation, by administration of α7 nAChR agonists, or by physiological methods that enhance vagus nerve activity, including aerobic exercise, acupuncture, meditation, music therapy, and biofeedback training (Table 1). Clinical trials of an implanted vagus nerve stimulator in rheumatoid arthritis are in progress, and other indications may be studied in the future.
Table 1.
Correlation between human activities/conditions and vagus nerve function.
| Activity/disease | Vagus nerve function | Reference |
| Aerobic exercise | Increase | Sandercock et al., 2005; Jae et al., 2009; Earnest et al., 2012 |
| Meditation | Increase | Peng et al., 2004 |
| Acupuncture | Increase | Haker et al., 2000; Lee et al., 2011; |
| Biofeedback training | Increase | Cowan et al., 1990; Nolan et al., 2005 |
| Relaxation training | Increase | Sakakibara et al., 1994; Terathongkum and Pickler, 2004 |
| Diet-supplemented fish oil or olive oil | Increase | Christensen et al., 1999; Holguin et al., 2005; Berbert et al., 2005 |
| Sepsis | Depressed function coupled to impaired survival | Pontet et al., 2003; Barnaby et al., 2002; Kessler et al., 2006 |
| Acute myocardial infarction | Depressed function coupled to impaired survival | Lanza et al., 1998, 2006 |
| Cardiovascular disease | Decrease | Villareal et al., 2002 |
| Rheumatoid arthritis, inflammatory bowel disease, SLE, sarcoidosis | Decrease | Lindgren et al., 1993; Stein et al., 1996; Laversuch et al., 1997; Evrengül et al., 2004 |
| Head trauma | Decrease | Biswas et al., 2000; Su et al., 2005 |
| Depression | Decrease | Kemp et al., 2010; |
| Obesity | Decrease | Minami et al., 1999; Akehi et al., 2001; Facchini et al., 2003 |
| Aging | Decrease | Zulfiqar et al., 2010 |
Exercise gateway reflex
Neural circuits modulate adaptive immune responses. For example, during the earliest stages of experimental allergic encephalomyelitis, the murine model of human multiple sclerosis (MS), CD4+ T cells primed to attack myelinated neurons cross the blood–brain barrier in a specific anatomical location, entering the spinal cord at the level of the fifth lumbar vertebrae (Fig. 3; Arima et al., 2012). The dorsal blood vessels in this region express CCL20, which is up-regulated by neural signals in response to sensory neuron activity from the soleus muscles. Thus, suspending the mice from their tail to eliminate gravity-dependent activation of the sensory neurons significantly reduced chemokine expression at that specific lumbar level and prevented the entry of pathogenic T cells into the spinal column (Arima et al., 2012). Contraction of other muscle groups was associated with up-regulation of CCL20 at other anatomical locations, a finding that is consistent with the somatotopic organization and development of neural circuitry in the nervous system.
Figure 3.
Gateway reflex. Neural signals arising from soleus muscle contractions travel to the brain stem, and then descend into the sympathetic chain to the lumbar 5 level. This regulates the activity of adrenergic neurons that modulate the expression of CCL20 by endothelial cells, providing a crucial control mechanism that gates the entry of pathogenic T cells into the CNS.
The muscle contraction–dependent reflex circuit that gates the entry of pathogenic T cells has several components, including the sensory neuron that relays afferent signals to the brain stem, cholinergic efferent neurons that descend from the brain stem in the sympathetic chain, and adrenergic neurons that deliver the terminal signal to the endothelial cell (Tracey, 2012). A similar circuit, termed the “exercise pressor reflex,” is activated during exercise by stimulation of mechanoreceptors and metabolically sensitive chemo-receptors in skeletal muscle (Kaufman and Hayes, 2002). Arrival at the brain stem of sensory input from muscles increases the activity of cholinergic neurons descending in the sympathetic chain.
Because adrenergic neurons project to endothelial cells in numerous anatomical regions that can become inflamed, additional neural reflexes may modulate the onset of immunity and inflammation resolution at these other locations. For example, chronic cardiovascular fitness is associated with enhanced vagus nerve activity and decreases in TNF, IL-6, and C reactive protein, in agreement with the reduced metainflammation underlying atherogenesis in blood vessels. The paradigm that exercise can influence trafficking of inflammatory cells to discrete anatomical regions and reduce inflammatory burden in vasculature is particularly compelling (Sloan et al., 2007b; Thayer and Fischer, 2009; Wilund et al., 2009).
Neural circuits in antibody responses
Recent evidence revealed neural mechanisms that regulate B cell trafficking during maturation into antibody-secreting cells. The antibody response to Streptococcus pneumonia, which is T cell independent, is initiated by the appearance of phosphorylcholine in the spleen and culminates in the accumulation of antibody-secreting cells in the red pulp. These cells release antibodies into the splenic venous circulation. Electrical stimulation of the vagus nerve, or administration of nicotine, significantly impaired the migration of B cells, such that they accumulated in the marginal zone (Mina-Osorio et al., 2012). Moreover, electrical stimulation of the vagus nerve caused B cells, neutrophils, monocytes, and dendritic cells to accumulate in the marginal zone, rather than travel to the red pulp, as compared with unstimulated controls (Fig. 4). The net effect of this neural regulation is that antibody titers to specific antigenic challenge were significantly reduced. These findings have important implications, because prior studies of neural interactions with spleen lymphocytes in vivo have not used nerve stimulation to address mechanisms of immunity. It should now be possible to study these mechanisms in the context of specific reflex circuits that are activated by antigens and the innate immune response to invasion. The resultant efferent reflex signals regulate B cell trafficking and influence the nature of the adaptive immune response.
Figure 4.
Neural influence on B cell trafficking and antibody secretion. (A) Stimulation of vagus nerve signals stimulates the adrenergic splenic nerve. This leads to accumulation of CD11+ B cells in the marginal zone and decreased antibody production. (B) In the setting of diminished signaling from the vagus nerve to splenic nerve, antibody-secreting CD11b+ cells traverse the marginal zone and enter the red pulp, where they release antibodies into the circulation.
Dietary antiinflammatory reflex
The gut is richly innervated by cholinergic and adrenergic efferent neurons, and its sensory neurons deliver nearly continuous information to the nervous system. Mechanoreceptors are activated by bowel distention, and chemosensitive receptors are activated by bowel contents. Neither the commensal flora of the bowel nor dietary contents elicit significant activation of immune responses. Recent evidence indicates that neural signals activated by the consumption of dietary fat activate the inflammatory reflex. Specifically, dietary fat ingestion stimulates signaling through cholecystokinin (CCK) receptors, and these signals are relayed to the CNS (Luyer et al., 2005). The arrival of these incoming signals activates efferent vagus nerve signals, which inhibit the production of TNF and IL-6 in response to endotoxin or hemorrhagic shock. Administration of pharmacological antagonists of α7 nAChR or of CCK, or transection of the vagus nerve, abrogated the protective effect of high-fat nutrition. The potential implications of this nutrition-activated pathway may extend to conserved hyporesponsiveness to dietary antigens, and to the well known influence of nutritional content on innate immune responses (Tracey, 2005). Clinical studies of vagus nerve signaling indicate that dietary consumption of fish oil significantly enhances the basal vagus nerve activity, which has been proposed as an antiinflammatory mechanism to enhance resolution of inflammation (Christensen et al., 1999; Berbert et al., 2005; Holguin et al., 2005).
Immunosuppressive stroke reflex
Pneumonia and other infections are a major cause of morbidity and mortality after cerebral infarction (also called stroke). It has been suggested that stroke survivors are “immunosupppressed,” but until recently the underlying mechanisms were unknown. Two studies shed light on the neural circuits that contribute to immunosuppression after stroke, with important therapeutic possibilities.
In an animal model of stroke, surviving mice exhibit significantly increased susceptibility to spontaneous bacterial sepsis and pneumonia (Prass et al., 2003). This occurred in association with extensive lymphocyte apoptosis, and a distinct shift in cytokine production from a T helper cell type 1 (Th1) to Th2 profile. The risk of bacterial infection was significantly reduced by transfer of WT, but not IFN-γ-deficient, T and natural killer cells on the first day after stroke, or by administration of propranolol to inhibit adrenergic signaling (Prass et al., 2003). Thus, an adrenergic-mediated defect in lymphocyte activation induces the immunosuppression that increases susceptibility to bacterial infection after stroke.
Quite recently, adrenergic neurons projecting into the liver, which regulate hepatic invariant natural killer T (iNKT) cells, were implicated in promoting systemic immunosuppression after stroke (Wong et al., 2011). The mechanism was revealed by depleting hepatic adrenergic nerve terminals, and pharmacologically blockading β-adrenergic receptors with propranolol; both interventions significantly modulated iNKT cytokine production and reduced immunosuppression, bacterial infection rates, and mortality. These measures failed to protect mice that were deficient in iNKT cells, and direct administration of norepinephrine into the liver of WT mice worsened the immunosuppression and bacterial infection rates. Thus, it appears that iNKT mediated immunosuppression after stroke is regulated by the adrenergic neural circuit and shifts cytokine production from a Th1 to Th2 profile. It is plausible to consider whether neuromodulation to diminish signals to the liver, or pharmacological agents that act on these pathways, can prevent bacterial infection after stroke. The implications of these results may well extend beyond the phenomenon of cerebral infarction, to other CNS lesions associated with immunosuppression, including head trauma, Alzheimer’s and other neurodegenerative diseases, and severe sepsis survivors.
Neural circuits in cytokine-mediated muscle wasting
Sensory neurons express cytokine receptors (e.g., type I and type II TNF receptors and IL-1 receptors) and Toll like receptors. Activation of IL-1 receptors in the brain is sufficient to accelerate catabolism in skeletal muscle (Braun et al., 2011). Adrenalectomy prevented the expression of a distinct gene set in skeletal muscle of animals with increased brain IL-1 levels, indicating that signaling pathways initiated by inflammatory mediators in the brain contribute to peripheral muscle wasting. Evidence that elevated levels of TNF in brain accelerate skeletal muscle breakdown was obtained in mice that received brain tumors genetically engineered to secrete TNF (Tracey et al., 1990). Compared with animals bearing leg tumors with comparable serum TNF levels, the rate of skeletal muscle protein loss was significantly higher in animals with brain tumors expressing significantly higher levels of intracerebral TNF. Together, these results raise the intriguing possibility that neural circuits, in part by signaling through the hypothalamic-pituitary axis, occupy a critical role in the development of muscle wasting that is ubiquitous in chronic inflammatory diseases.
TNF-mediated neural circuits in arthritis
Cytokine mediated neural circuits that drive inflammation have been described in adjuvant arthritis. Unilateral antigen-induced arthritis in the rat knee induced macrophage infiltration in the lumbar, but not thoracic dorsal root ganglions, and enhanced expression of vascular cell adhesion molecule-1 (VCAM-1) in vessels of the lumbar ganglions (Segond von Banchet et al., 2009). Administration of anti-TNF antibodies reduced both VCAM-1 expression and macrophage infiltration, and significantly reduced the pain threshold (measured by mechanical hyperalgesia) in the affected joint and the unaffected contralateral knee. Intrathecal administration of anti-TNF significantly attenuated the severity of arthritis and reduced the signaling through adrenergic neurons (Boettger et al., 2008). Thus, TNF can drive neural signals that modulate inflammation in a specific anatomical region.
Clinical experience in treating rheumatoid arthritis patients with anti-TNF reveals that patients frequently receive significant pain relief within a few hours or days after first dosing, a time frame too early to be explained by inflammation resolution. Functional MRI focusing on blood oxygen level-dependent (BOLD) signals in brain regions corresponding to metacarpal phalangeal joints, pain perception, and the processing of body sensations and painful emotions (Hess et al., 2011) revealed that activity in these regions was significantly reduced within 24 h after the administration of anti-TNF, even though composite inflammation scores that included levels of C-reactive protein did not improve for at least 24 h. Strikingly, similar findings were obtained in TNF transgenic mice with arthritis, in which anti-TNF mediated a rapid improvement of mechanical hyperalgesia in a von Frey filament test; this was associated with significantly attenuated spreading of BOLD signals in the somatosensory cortex (Hess et al., 2011). It therefore appears that TNF activates neural signals to the CNS, which converge on pain circuit neurons, and that these circuits are interdependent or perhaps overlapping with neural circuits that influence inflammation resolution in the affected joints (Diamond and Tracey, 2011).
Neural development
Neural circuits that regulate immunity evolved in response to products of immunity to infection and injury, and are directly relevant to the developing fetal brain. Autoantibodies against double-stranded DNA, found in a significant percentage of women with autoimmune diseases, also bind the N-methyl-d-aspartate (NMDA) receptor with functional consequence (DeGiorgio et al., 2001). In a murine model of systemic lupus erythematosus, high titers of anti-NMDA receptor autoantibodies in pregnant dams entered the fetal brain resulting in abnormal development of neural circuitry and offspring that were cognitively impaired (Faust et al., 2010). These autoantibodies were functional, because they exerted agonist activity by specifically binding to the extracellular domains of the NR2A and NR2B subunits of the glutamatergic NMDA receptor. Direct administration of these antibodies into mouse hippocampus caused significant neuronal death. Moreover, expression of these antibodies in pregnant dams caused apoptosis of NR2A-expressing neurons within the fetal brainstem and loss of viability of female pups (Wang et al., 2012). This maternal antibody-dependent neural mechanism of fetal death is consistent with the observed live birth sex ratio in SLE pregnancy. The implications of this work provide a direct link through which antibodies sculpt neuronal circuitry in the developing brain (DeGiorgio et al., 2001; Kowal et al., 2004; Lapteva et al., 2006; Faust et al., 2010; Wang et al., 2012).
Microbial colonization of the gut may also influence neural development. Germ-free animals show increased motor activity and reduced anxiety compared with mice harboring a normal gut microbiota (Heijtz et al., 2011). These findings correlate with gene expression patterns in brain regions that regulate motor function and anxiety. Reconstituting the gut microbiome in germ-free mice reversed the behavioral and gene expression patterns, revealing that microbial colonization mediates signals to the CNS that influence the development and function of neuronal circuits. It is likely that humoral and neural signaling arising from the immune system in response to gut microbiota influences brain development (Diamond et al., 2011).
Brain development and neuroplasticity continue after birth, as experience molds the efficiency of signals through basic CNS circuits formed by extensive networks of synaptic connections. During early life there is a degree of plasticity that shapes these pathways underlying behavior and homeostatic reflex responses. Cytokine levels can increase significantly in the brain in response to infection or injury, or by entering the brain during periods of stress-induced opening of the blood–brain barrier. Elevated brain TNF levels occur during brain inflammation, ischemia, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and multiple sclerosis. In addition to the behavioral effects on physiological and metabolic homeostasis noted above, increased brain levels of TNF and IL-1 can significantly modify neuronal activity underlying the function of brain networks. TNF modulates the neuronal expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and NMDA receptors. TNF also modulates homeostatic synaptic scaling, a neurophysiological mechanism that maintains optimal neural network function and stability (Stellwagen and Malinka, 2006). By modulating NMDA and AMPA receptor expression, TNF adjusts the strength of neuronal synapses in response to long-lived changes in electrical activity. IL-1β and HMGB1 are also increased during brain inflammation or injury, and have been implicated in modulating NMDA receptor activity during neurodegeneration, ischemia, and seizure disorders.
Future directions
Neuroscience and immunology are now armed with the new paradigm that specific steps in immunity are modulated by discrete neural circuits that signal through defined molecular mechanisms. Accordingly, it is reasonable to propose that additional neural pathways will be identified and mapped. This can be accomplished from either a top-down or a bottom-up approach, because reflexes require both afferent signals from the interaction of the immune system with the nervous system, and efferent messages to the immune system from the nervous system. Mapping such circuits anatomically and functionally is likely to reveal important information about the network of information sharing between two elaborate, highly coevolved systems of defense and memory.
References
- Aballay A. 2009. Neural regulation of immunity: role of NPR-1 in pathogen avoidance and regulation of innate immunity. Cell Cycle. 8:966–969 10.4161/cc.8.7.8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akehi Y., Yoshimatsu H., Kurokawa M., Sakata T., Eto H., Ito S., Ono J. 2001. VLCD-induced weight loss improves heart rate variability in moderately obese Japanese. Exp. Biol. Med. 226:440–445 [DOI] [PubMed] [Google Scholar]
- Andersson U., Tracey K.J. 2012. Reflex principles of immunological homeostasis. Annu. Rev. Immunol. 30:313–335 10.1146/annurev-immunol-020711-075015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima Y., Harada M., Kamimura D., Park J.H., Kawano F., Yull F.E., Kawamoto T., Iwakura Y., Betz U.A., Márquez G., et al. 2012. Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell. 148:447–457 10.1016/j.cell.2012.01.022 [DOI] [PubMed] [Google Scholar]
- Barnaby D., Ferrick K., Kaplan D.T., Shah S., Bijur P., Gallagher E.J. 2002. Heart rate variability in emergency department patients with sepsis. Acad. Emerg. Med. 9:661–670 10.1111/j.1553-2712.2002.tb02143.x [DOI] [PubMed] [Google Scholar]
- Berbert A.A., Kondo C.R., Almendra C.L., Matsuo T., Dichi I. 2005. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 21:131–136 10.1016/j.nut.2004.03.023 [DOI] [PubMed] [Google Scholar]
- Bernik T.R., Friedman S.G., Ochani M., DiRaimo R., Ulloa L., Yang H., Sudan S., Czura C.J., Ivanova S.M., Tracey K.J. 2002. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J. Exp. Med. 195:781–788 10.1084/jem.20011714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H.R., Powley T.L. 1996. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc. Res. Tech. 35:80–86 [DOI] [PubMed] [Google Scholar]
- Besedovsky H.O., del Rey A. 2000. The cytokine-HPA axis feed-back circuit. Z. Rheumatol. 59:26–30 10.1007/s003930070014 [DOI] [PubMed] [Google Scholar]
- Bianchi M., Bloom O., Raabe T., Cohen P.S., Chesney J., Sherry B., Schmidtmayerova H., Calandra T., Zhang X., Bukrinsky M., et al. 1996. Suppression of proinflammatory cytokines in monocytes by a tetravalent guanylhydrazone. J. Exp. Med. 183:927–936 10.1084/jem.183.3.927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas A.K., Scott W.A., Sommerauer J.F., Luckett P.M. 2000. Heart rate variability after acute traumatic brain injury in children. Crit. Care Med. 28:3907–3912 10.1097/00003246-200012000-00030 [DOI] [PubMed] [Google Scholar]
- Boettger M.K., Hensellek S., Richter F., Gajda M., Stöckigt R., von Banchet G.S., Bräuer R., Schaible H.G. 2008. Antinociceptive effects of tumor necrosis factor alpha neutralization in a rat model of antigen-induced arthritis: evidence of a neuronal target. Arthritis Rheum. 58:2368–2378 10.1002/art.23608 [DOI] [PubMed] [Google Scholar]
- Borovikova L.V., Ivanova S., Nardi D., Zhang M., Yang H., Ombrellino M., Tracey K.J. 2000a. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton. Neurosci. 85:141–147 10.1016/S1566-0702(00)00233-2 [DOI] [PubMed] [Google Scholar]
- Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. 2000b. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 405:458–462 10.1038/35013070 [DOI] [PubMed] [Google Scholar]
- Brandon K.W., Rand M.J. 1961. Acetylcholine and the sympathetic innervation of the spleen. J. Physiol. 157:18–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T.P., Zhu X., Szumowski M., Scott G.D., Grossberg A.J., Levasseur P.R., Graham K., Khan S., Damaraju S., Colmers W.F., et al. 2011. Central nervous system inflammation induces muscle atrophy via activation of the hypothalamic–pituitary–adrenal axis. J. Exp. Med. 208:2449–2463 10.1084/jem.20111020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J.H., Christensen M.S., Dyerberg J., Schmidt E.B. 1999. Heart rate variability and fatty acid content of blood cell membranes: a dose-response study with n-3 fatty acids. Am. J. Clin. Nutr. 70:331–337 [DOI] [PubMed] [Google Scholar]
- Cowan M.J., Kogan H., Burr R., Hendershot S., Buchanan L. 1990. Power spectral analysis of heart rate variability after biofeedback training. J. Electrocardiol. 23:85–94 10.1016/0022-0736(90)90081-C [DOI] [PubMed] [Google Scholar]
- Dale H.H. 1954. The beginnings and the prospects of neurohumoral transmission. Pharmacol. Rev. 6:7–13 [PubMed] [Google Scholar]
- Dale H.H., Dudley H.W. 1929. The presence of histamine and acetylcholine in the spleen of the ox and the horse. J. Physiol. 68:97–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgio L.A., Konstantinov K.N., Lee S.C., Hardin J.A., Volpe B.T., Diamond B. 2001. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 7:1189–1193 10.1038/nm1101-1189 [DOI] [PubMed] [Google Scholar]
- Diamond B., Tracey K.J. 2011. Mapping the immunological homunculus. Proc. Natl. Acad. Sci. USA. 108:3461–3462 10.1073/pnas.1100329108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond B., Huerta P.T., Tracey K., Volpe B.T. 2011. It takes guts to grow a brain: Increasing evidence of the important role of the intestinal microflora in neuro- and immune-modulatory functions during development and adulthood. Bioessays. 33:588–591 10.1002/bies.201100042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest C.P., Blair S.N., Church T.S. 2012. Heart rate variability and exercise in aging women. J. Womens Health (Larchmt). 21:334–339 10.1089/jwh.2011.2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrengül H., Dursunoglu D., Cobankara V., Polat B., Seleci D., Kabukçu S., Kaftan A., Semiz E., Kilic M. 2004. Heart rate variability in patients with rheumatoid arthritis. Rheumatol. Int. 24:198–202 10.1007/s00296-003-0357-5 [DOI] [PubMed] [Google Scholar]
- Facchini M., Malfatto G., Sala L., Silvestri G., Fontana P., Lafortuna C., Sartorio A. 2003. Changes of autonomic cardiac profile after a 3-week integrated body weight reduction program in severely obese patients. J. Endocrinol. Invest. 26:138–142 [DOI] [PubMed] [Google Scholar]
- Faust T.W., Chang E.H., Kowal C., Berlin R., Gazaryan I.G., Bertini E., Zhang J., Sanchez-Guerrero J., Fragoso-Loyo H.E., Volpe B.T., et al. 2010. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proc. Natl. Acad. Sci. USA. 107:18569–18574 10.1073/pnas.1006980107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felten S.Y., Olschowka J. 1987. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J. Neurosci. Res. 18:37–48 10.1002/jnr.490180108 [DOI] [PubMed] [Google Scholar]
- Goehler L.E., Relton J.K., Dripps D., Kiechle R., Tartaglia N., Maier S.F., Watkins L.R. 1997. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res. Bull. 43:357–364 10.1016/S0361-9230(97)00020-8 [DOI] [PubMed] [Google Scholar]
- Goldstein R.S., Bruchfeld A., Yang L., Qureshi A.R., Gallowitsch-Puerta M., Patel N.B., Huston B.J., Chavan S., Rosas-Ballina M., Gregersen P.K., et al. 2007. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol. Med. 13:210–215 10.2119/2006-00108.Goldstein [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haker E., Egekvist H., Bjerring P. 2000. Effect of sensory stimulation (acupuncture) on sympathetic and parasympathetic activities in healthy subjects. J. Auton. Nerv. Syst. 79:52–59 10.1016/S0165-1838(99)00090-9 [DOI] [PubMed] [Google Scholar]
- Heijtz R.D., Wang S., Anuar F., Qian Y., Björkholm B., Samuelsson A., Hibberd M.L., Forssberg H., Pettersson S. 2011. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA. 108:3047–3052 10.1073/pnas.1010529108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A., Axmann R., Rech J., Finzel S., Heindl C., Kreitz S., Sergeeva M., Saake M., Garcia M., Kollias G., et al. 2011. Blockade of TNF-α rapidly inhibits pain responses in the central nervous system. Proc. Natl. Acad. Sci. USA. 108:3731–3736 10.1073/pnas.1011774108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin F., Téllez-Rojo M.M., Lazo M., Mannino D., Schwartz J., Hernández M., Romieu I. 2005. Cardiac autonomic changes associated with fish oil vs soy oil supplementation in the elderly. Chest. 127:1102–1107 10.1378/chest.127.4.1102 [DOI] [PubMed] [Google Scholar]
- Jae S.Y., Heffernan K.S., Yoon E.S., Lee M.K., Fernhall B., Park W.H. 2009. The inverse association between cardiorespiratory fitness and C-reactive protein is mediated by autonomic function: a possible role of the cholinergic antiinflammatory pathway. Mol. Med. 15:291–296 10.2119/molmed.2009.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman M.P., Hayes S.G. 2002. The exercise pressor reflex. Clin. Auton. Res. 12:429–439 10.1007/s10286-002-0059-1 [DOI] [PubMed] [Google Scholar]
- Kemp A.H., Quintana D.S., Gray M.A., Felmingham K.L., Brown K., Gatt J.M. 2010. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol. Psychiatry. 67:1067–1074 10.1016/j.biopsych.2009.12.012 [DOI] [PubMed] [Google Scholar]
- Kessler W., Traeger T., Westerholt A., Neher F., Mikulcak M., Müller A., Maier S., Heidecke C.D. 2006. The vagal nerve as a link between the nervous and immune system in the instance of polymicrobial sepsis. Langenbecks Arch. Surg. 391:83–87 10.1007/s00423-006-0031-y [DOI] [PubMed] [Google Scholar]
- Kowal C., DeGiorgio L.A., Nakaoka T., Hetherington H., Huerta P.T., Diamond B., Volpe B.T. 2004. Cognition and immunity; antibody impairs memory. Immunity. 21:179–188 10.1016/j.immuni.2004.07.011 [DOI] [PubMed] [Google Scholar]
- Lanza G.A., Guido V., Galeazzi M.M., Mustilli M., Natali R., Ierardi C., Milici C., Burzotta F., Pasceri V., Tomassini F., et al. 1998. Prognostic role of heart rate variability in patients with a recent acute myocardial infarction. Am. J. Cardiol. 82:1323–1328 10.1016/S0002-9149(98)00635-3 [DOI] [PubMed] [Google Scholar]
- Lanza G.A., Sgueglia G.A., Cianflone D., Rebuzzi A.G., Angeloni G., Sestito A., Infusino F., Crea F., Maseri A.; SPAI (Stratificazione Prognostica dell’Angina Instabile) Investigators 2006. Relation of heart rate variability to serum levels of C-reactive protein in patients with unstable angina pectoris. Am. J. Cardiol. 97:1702–1706 10.1016/j.amjcard.2006.01.029 [DOI] [PubMed] [Google Scholar]
- Lapteva L., Nowak M., Yarboro C.H., Takada K., Roebuck-Spencer T., Weickert T., Bleiberg J., Rosenstein D., Pao M., Patronas N., et al. 2006. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 54:2505–2514 10.1002/art.22031 [DOI] [PubMed] [Google Scholar]
- Laversuch C.J., Seo H., Modarres H., Collins D.A., McKenna W., Bourke B.E. 1997. Reduction in heart rate variability in patients with systemic lupus erythematosus. J. Rheumatol. 24:1540–1544 [PubMed] [Google Scholar]
- Leaders F.E., Dayrit C. 1965. The cholinergic component in the sympathetic innervation to the spleen. J. Pharmacol. Exp. Ther. 147:145–152 [PubMed] [Google Scholar]
- Lee J.H., Kim K.H., Hong J.W., Lee W.C., Koo S. 2011. Comparison of electroacupuncture frequency-related effects on heart rate variability in healthy volunteers: a randomized clinical trial. J. Acupunct. Meridian. Stud. (Roma). 4:107–115 10.1016/S2005-2901(11)60016-2 [DOI] [PubMed] [Google Scholar]
- Lindgren S., Stewenius J., Sjölund K., Lilja B., Sundkvist G. 1993. Autonomic vagal nerve dysfunction in patients with ulcerative colitis. Scand. J. Gastroenterol. 28:638–642 10.3109/00365529309096103 [DOI] [PubMed] [Google Scholar]
- Luyer M.D., Greve J.W., Hadfoune M., Jacobs J.A., Dejong C.H., Buurman W.A. 2005. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J. Exp. Med. 202:1023–1029 10.1084/jem.20042397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz C.N., Tracey K.J. 2005. It takes nerve to dampen inflammation. Nat. Immunol. 6:756–757 10.1038/ni0805-756 [DOI] [PubMed] [Google Scholar]
- Mina-Osorio P., Rosas-Ballina M., Valdes-Ferrer S.I., Al-Abed Y., Tracey K.J., Diamond B. 2012. Neural signaling in the spleen controls B cell responses to blood-borne antigen. Mol. Med. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami J., Kawano Y., Ishimitsu T., Matsuoka H., Takishita S. 1999. Acute and chronic effects of a hypocaloric diet on 24-hour blood pressure, heart rate and heart-rate variability in mildly-to-moderately obese patients with essential hypertension. Clin. Exp. Hypertens. 21:1413–1427 10.3109/10641969909070857 [DOI] [PubMed] [Google Scholar]
- Mosser D.M., Zhang X. 2008. Interleukin-10: new perspectives on an old cytokine. Immunol. Rev. 226:205–218 10.1111/j.1600-065X.2008.00706.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. 2002. Points of control in inflammation. Nature. 420:846–852 10.1038/nature01320 [DOI] [PubMed] [Google Scholar]
- Nathan C., Ding A. 2010. Nonresolving inflammation. Cell. 140:871–882 10.1016/j.cell.2010.02.029 [DOI] [PubMed] [Google Scholar]
- Niijima A. 1996. The afferent discharges from sensors for interleukin 1 beta in the hepatoportal system in the anesthetized rat. J. Auton. Nerv. Syst. 61:287–291 10.1016/S0165-1838(96)00098-7 [DOI] [PubMed] [Google Scholar]
- Niijima A., Hori T., Aou S., Oomura Y. 1991. The effects of interleukin-1 beta on the activity of adrenal, splenic and renal sympathetic nerves in the rat. J. Auton. Nerv. Syst. 36:183–192 10.1016/0165-1838(91)90042-2 [DOI] [PubMed] [Google Scholar]
- Niijima A., Hori T., Katafuchi T., Ichijo T. 1995. The effect of interleukin-1 beta on the efferent activity of the vagus nerve to the thymus. J. Auton. Nerv. Syst. 54:137–144 10.1016/0165-1838(95)00003-G [DOI] [PubMed] [Google Scholar]
- Nolan R.P., Kamath M.V., Floras J.S., Stanley J., Pang C., Picton P., Young Q.R. 2005. Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. Am. Heart J. 149:1137 10.1016/j.ahj.2005.03.015 [DOI] [PubMed] [Google Scholar]
- Olofsson P., Katz D., Rosas-Ballina M., Levine Y., Ochani M., Valdes-Ferrer S., Pavlov V., Tracey K., Chavan S. 2012. alpha7nAChR Expression in bone-marrow derived non T Cells is required for the inflammatory reflex. Mol. Med. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V.A., Ochani M., Gallowitsch-Puerta M., Ochani K., Huston J.M., Czura C.J., Al-Abed Y., Tracey K.J. 2006. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl. Acad. Sci. USA. 103:5219–5223 10.1073/pnas.0600506103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V.A., Parrish W.R., Rosas-Ballina M., Ochani M., Puerta M., Ochani K., Chavan S., Al-Abed Y., Tracey K.J. 2009. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 23:41–45 10.1016/j.bbi.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C.K., Henry I.C., Mietus J.E., Hausdorff J.M., Khalsa G., Benson H., Goldberger A.L. 2004. Heart rate dynamics during three forms of meditation. Int. J. Cardiol. 95:19–27 10.1016/j.ijcard.2003.02.006 [DOI] [PubMed] [Google Scholar]
- Peterson C.Y., Krzyzaniak M., Coimbra R., Chang D.C. 2012. Vagus nerve and postinjury inflammatory response. Arch. Surg. 147:76–80 10.1001/archsurg.2011.237 [DOI] [PubMed] [Google Scholar]
- Pontet J., Contreras P., Curbelo A., Medina J., Noveri S., Bentancourt S., Migliaro E.R. 2003. Heart rate variability as early marker of multiple organ dysfunction syndrome in septic patients. J. Crit. Care. 18:156–163 10.1016/j.jcrc.2003.08.005 [DOI] [PubMed] [Google Scholar]
- Prass K., Meisel C., Höflich C., Braun J., Halle E., Wolf T., Ruscher K., Victorov I.V., Priller J., Dirnagl U., et al. 2003. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1–like immunostimulation. J. Exp. Med. 198:725–736 10.1084/jem.20021098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M., Goldstein R.S., Gallowitsch-Puerta M., Yang L., Valdés-Ferrer S.I., Patel N.B., Chavan S., Al-Abed Y., Yang H., Tracey K.J. 2009. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol. Med. 15:195–202 10.2119/molmed.2009.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M., Olofsson P.S., Ochani M., Valdés-Ferrer S.I., Levine Y.A., Reardon C., Tusche M.W., Pavlov V.A., Andersson U., Chavan S., et al. 2011. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 334:98–101 10.1126/science.1209985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed R.W., Varma S., Peng-Nemeroff T., Sherry B., Balakhaneh D., Huston J., Tracey K.J., Al-Abed Y., Metz C.N. 2005. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J. Exp. Med. 201:1113–1123 10.1084/jem.20040463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara M., Takeuchi S., Hayano J. 1994. Effect of relaxation training on cardiac parasympathetic tone. Psychophysiology. 31:223–228 10.1111/j.1469-8986.1994.tb02210.x [DOI] [PubMed] [Google Scholar]
- Sandercock G.R., Bromley P.D., Brodie D.A. 2005. Effects of exercise on heart rate variability: inferences from meta-analysis. Med. Sci. Sports Exerc. 37:433–439 10.1249/01.MSS.0000155388.39002.9D [DOI] [PubMed] [Google Scholar]
- Segond von Banchet G., Boettger M.K., Fischer N., Gajda M., Bräuer R., Schaible H.G. 2009. Experimental arthritis causes tumor necrosis factor-alpha-dependent infiltration of macrophages into rat dorsal root ganglia which correlates with pain-related behavior. Pain. 145:151–159 10.1016/j.pain.2009.06.002 [DOI] [PubMed] [Google Scholar]
- Sloan R.P., McCreath H., Tracey K.J., Sidney S., Liu K., Seeman T. 2007a. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol. Med. 13:178–184 10.2119/2006-00112.Sloan [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan R.P., Shapiro P.A., Demeersman R.E., McKinley P.S., Tracey K.J., Slavov I., Fang Y., Flood P.D. 2007b. Aerobic exercise attenuates inducible TNF production in humans. J. Appl. Physiol. 103:1007–1011 10.1152/japplphysiol.00147.2007 [DOI] [PubMed] [Google Scholar]
- Stein K.S., McFarlane I.C., Goldberg N., Ginzler E.M. 1996. Heart rate variability in patients with systemic lupus erythematosus. Lupus. 5:44–48 [DOI] [PubMed] [Google Scholar]
- Stellwagen D., Malenka R.C. 2006. Synaptic scaling mediated by glial TNF-alpha. Nature. 440:1054–1059 10.1038/nature04671 [DOI] [PubMed] [Google Scholar]
- Sternberg E.M. 2006. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 6:318–328 10.1038/nri1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub R.H., Rauch L., Fassold A., Lowin T., Pongratz G. 2008. Neuronally released sympathetic neurotransmitters stimulate splenic interferon-gamma secretion from T cells in early type II collagen-induced arthritis. Arthritis Rheum. 58:3450–3460 10.1002/art.24030 [DOI] [PubMed] [Google Scholar]
- Su C.F., Kuo T.B., Kuo J.S., Lai H.Y., Chen H.I. 2005. Sympathetic and parasympathetic activities evaluated by heart-rate variability in head injury of various severities. Clin. Neurophysiol. 116:1273–1279 10.1016/j.clinph.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Sun J., Singh V., Kajino-Sakamoto R., Aballay A. 2011. Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science. 332:729–732 10.1126/science.1203411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terathongkum S., Pickler R.H. 2004. Relationships among heart rate variability, hypertension, and relaxation techniques. J. Vasc. Nurs. 22:78–82 10.1016/j.jvn.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Fischer J.E. 2009. Heart rate variability, overnight urinary norepinephrine and C-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults. J. Intern. Med. 265:439–447 10.1111/j.1365-2796.2008.02023.x [DOI] [PubMed] [Google Scholar]
- Tracey K.J. 2002. The inflammatory reflex. Nature. 420:853–859 10.1038/nature01321 [DOI] [PubMed] [Google Scholar]
- Tracey K.J. 2005. Fat meets the cholinergic antiinflammatory pathway. J. Exp. Med. 202:1017–1021 10.1084/jem.20051760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K.J. 2007. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 117:289–296 10.1172/JCI30555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K.J. 2011. Cell biology. Ancient neurons regulate immunity. Science. 332:673–674 10.1126/science.1206353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K.J. 2012. Immune cells exploit a neural circuit to enter the CNS. Cell. 148:392–394 10.1016/j.cell.2012.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K.J., Morgello S., Koplin B., Fahey T.J., III, Fox J., Aledo A., Manogue K.R., Cerami A. 1990. Metabolic effects of cachectin/tumor necrosis factor are modified by site of production. Cachectin/tumor necrosis factor-secreting tumor in skeletal muscle induces chronic cachexia, while implantation in brain induces predominantly acute anorexia. J. Clin. Invest. 86:2014–2024 10.1172/JCI114937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunawaki S., Sporn M., Ding A., Nathan C. 1988. Deactivation of macrophages by transforming growth factor-beta. Nature. 334:260–262 10.1038/334260a0 [DOI] [PubMed] [Google Scholar]
- van Maanen M.A., Lebre M.C., van der Poll T., LaRosa G.J., Elbaum D., Vervoordeldonk M.J., Tak P.P. 2009a. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 60:114–122 10.1002/art.24177 [DOI] [PubMed] [Google Scholar]
- van Maanen M.A., Vervoordeldonk M.J., Tak P.P. 2009b. The cholinergic anti-inflammatory pathway: towards innovative treatment of rheumatoid arthritis. Nat Rev Rheumatol. 5:229–232 10.1038/nrrheum.2009.31 [DOI] [PubMed] [Google Scholar]
- van Maanen M.A., Stoof S.P., Larosa G.J., Vervoordeldonk M.J., Tak P.P. 2010. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor α7 subunit gene knockout mice. Ann. Rheum. Dis. 69:1717–1723 10.1136/ard.2009.118554 [DOI] [PubMed] [Google Scholar]
- Vida G., Peña G., Deitch E.A., Ulloa L. 2011. α7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J. Immunol. 186:4340–4346 10.4049/jimmunol.1003722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal R.P., Liu B.C., Massumi A. 2002. Heart rate variability and cardiovascular mortality. Curr. Atheroscler. Rep. 4:120–127 10.1007/s11883-002-0035-1 [DOI] [PubMed] [Google Scholar]
- Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L., et al. 2003. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 421:384–388 10.1038/nature01339 [DOI] [PubMed] [Google Scholar]
- Wang H., Liao H., Ochani M., Justiniani M., Lin X., Yang L., Al-Abed Y., Wang H., Metz C., Miller E.J., et al. 2004. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 10:1216–1221 10.1038/nm1124 [DOI] [PubMed] [Google Scholar]
- Wang L., Zhou D., Lee J., Niu H., Faust T.W., Frattini S., Kowal C., Huerta P.T., Volpe B.T., Diamond B. 2012. Female mouse fetal loss mediated by maternal autoantibody. J. Exp. Med. 209:987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins L.R., Goehler L.E., Relton J.K., Tartaglia N., Silbert L., Martin D., Maier S.F. 1995a. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci. Lett. 183:27–31 10.1016/0304-3940(94)11105-R [DOI] [PubMed] [Google Scholar]
- Watkins L.R., Maier S.F., Goehler L.E. 1995b. Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci. 57:1011–1026 10.1016/0024-3205(95)02047-M [DOI] [PubMed] [Google Scholar]
- Watkins L.R., Maier S.F., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L., et al. 1999. Implications of immune-to-brain communication for sickness and pain. Proc. Natl. Acad. Sci. USA. 96:7710–7713 10.1073/pnas.96.14.7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilund K.R., Rosenblat M., Chung H.R., Volkova N., Kaplan M., Woods J.A., Aviram M. 2009. Macrophages from alpha 7 nicotinic acetylcholine receptor knockout mice demonstrate increased cholesterol accumulation and decreased cellular paraoxonase expression: a possible link between the nervous system and atherosclerosis development. Biochem. Biophys. Res. Commun. 390:148–154 10.1016/j.bbrc.2009.09.088 [DOI] [PubMed] [Google Scholar]
- Wong C.H., Jenne C.N., Lee W.Y., Léger C., Kubes P. 2011. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 334:101–105 10.1126/science.1210301 [DOI] [PubMed] [Google Scholar]
- Zulfiqar U., Jurivich D.A., Gao W., Singer D.H. 2010. Relation of high heart rate variability to healthy longevity. Am. J. Cardiol. 105:1181–1185 10.1016/j.amjcard.2009.12.022 [DOI] [PubMed] [Google Scholar]