Abstract
The antineoplastic properties of gallium are well documented. Owing to their robust accumulation of gallium, melanoma cells should be amenable to gallium-based anticancer drugs. With the aim of improving the disappointingly low activity of inorganic gallium salts, we have developed the orally bioavailable gallium complex KP46 [tris(8-quinolinolato)gallium(III)] that was already successfully studied in a phase I clinical trial. To assess its therapeutic potential in malignant melanoma, its antiproliferative effects were investigated in series of human cell lines and primary explanted melanoma samples by means of the MTT [3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay and the Human Tumor Cloning Assay, respectively. When compared with other cell lines, the majority of melanoma cells rank among the KP46-sensitive cell lines (50% inhibitory concentration values: 0.8–3.7 μmol/l). Clinically achievable concentrations of KP46 proved to be highly effective in melanoma cells from primary explants of cutaneous and lymph node metastases. Colony growth was inhibited in 10 of 10 specimens by 5 lmol/l KP46 (corresponding to the steady-state plasma concentration measured earlier in a study patient) and in four of 10 specimens by 0.5 μmol/l KP46. In-vitro potency of KP46 is higher than that of dacarbazine or fotemustine and comparable with that of cisplatin. The effects induced by KP46 in melanoma cell lines involve cell cycle perturbations (S-phase arrest) and apoptosis (activation of caspase-9, PARP [poly(ADP-ribose) polymerase] cleavage, formation of apoptotic bodies). No effects on DNA secondary structure could be observed in an electrophoretic mobility shift assay using double-stranded plasmid DNA. Thus, further studies on the therapeutic applicability of KP46 in malignant melanoma are warranted.
Keywords: apoptosis, cell cycle arrest, chemotherapy, cytotoxicity, gallium, Human Tumor Cloning Assay
Introduction
Metastatic melanoma is a devastating disease with a rapidly increasing global incidence. No currently available treatment has been shown to consistently achieve a survival benefit for these patients, despite in part considerable systemic toxicity. Therefore, novel drugs with higher antimelanoma activity and improved toxicity profiles are urgently needed.
Gallium compounds may open up new avenues, given the known affinity of this metal to a variety of tumors. Studies on the applicability of 67Ga scintigraphy for the visualization of occult metastatic disease in melanoma patients have shown that these tumors accumulate gallium regularly [1], with an overall sensitivity of 75–82% and a specificity of 90–99% [2,3]. In addition, gallium nitrate has long been investigated as an anticancer drug, and proved to be clinically active in lymphoma [4] and bladder cancer [5]. Its mechanism of cytotoxicity in cancer cells is thought to involve disruption of cellular iron transport and homeostasis, inhibition of ribonucleotide reductase, and blockade of the cell cycle mainly in the S phase [6-9]. Gallium nitrate has also been explored as an antimelanoma drug. However, a phase II trial yielded a disappointing objective response rate of only 3% (only one partial response with nearly complete resolution of metastases for 7 months beside two cases of disease stabilization for 3 or more months in a total of 31 evaluable patients), discouraging further evaluation [10].
Generally, the clinical development of gallium salts faced the problem of adequate delivery. Severe toxicities as observed after bolus infusion could be overcome to a great extent with continuous infusion over several days, whereas attempts at oral administration failed because of insufficient bioavailability [11]. Fortunately, the wide, yet scarcely explored possibilities of preparing biologically active coordination compounds offer the chance of expanding the pharmacological capacity of this metal.
The lipophilic complex KP46, that is, tris(8-quinolinolato)gallium(III), was developed with the rationale of generating a hydrolytically stable gallium-based drug that is sufficiently absorbed when given through the oral route. Improved bioavailability in vivo and uptake into tumor cells in vitro as well as increased cytotoxic potency in vitro and antitumor activity in vivo as compared with gallium salts have been shown earlier [12,13]. A phase I dose-escalation study in patients with advanced solid tumors yielded promising results, showing the high tolerability of the compound and signs of activity in renal cell carcinoma [14]. As no preclinical data in malignant melanoma were available so far, we decided to investigate KP46 for its cytotoxic potency in vitro in a variety of human melanoma cell lines and subsequently in a series of freshly explanted human melanoma specimens. The results reported here confirm the high in-vitro activity of KP46 in clinically achievable concentrations, encouraging further evaluation of this compound in the melanoma setting.
Materials and methods
Drugs
KP46, cisplatin, and dacarbazine (DTIC) were synthesized in high purity at the Institute of Inorganic Chemistry, University of Vienna, Austria, according to the established procedures [15-17]. Excipient-free fotemustine (Muphoran; Servier Pharma, Vienna, Austria) was applied. Gallium nitrate was purchased from Sigma-Aldrich (Vienna, Austria) as a hydrate of the composition Ga(NO3)3·5.8H2O, as determined by thermogravimetric analysis. KP46 was applied from fresh stock solutions in dimethylsulfoxide (DMSO) and diluted in media or buffer as appropriate to a maximum of 0.3% DMSO. All other drugs were dissolved straight in cell culture medium or buffer as described below.
Cell lines and culture conditions
A series of 10 human melanoma cell lines described previously [18] was used in this study: VM-1 and VM-10 (superficially spreading melanoma); VM-8, VM-21, VM-22, VM-24, VM-25, and VM-33 (nodular melanoma); VM-18 (acral lentigenous melanoma); and VM-48 (type unknown). In addition, 15 other human cancer cell lines were used: MDA-MB-231, MCF-7 (both adenocarcinoma of the breast); GLC-4 (small-cell lung cancer); SW1573 (non-small-cell lung cancer); CaCo-2, HCT-116, SW480 (all colon carcinoma); Hep 3B, Hep G2 (both hepatoma); KB-3-1 (cervical carcinoma); TC-71, U2-OS (both osteosarcoma); T98-G, U373 (both glioblastoma); and HL-60 (promyelocytic leukemia). Most of these cell lines were kindly donated as published previously [19]. All other cell lines were obtained from the American Type Culture Collection (Rockville, Maryland, USA), with the exception of SW1573, which was kindly donated by H.J. Broxterman (Amsterdam, The Netherlands) [20]. Most cell lines were cultivated in RPMI 1640 medium (Sigma–Aldrich, St. Louis, Missouri, USA), with the exception of HCT-116 (in McCoy’s medium; Invitrogen, Carlsbad, California, USA); both osteosarcoma cell lines (in Iscove’s modified Dulbecco’s medium; Invitrogen); SW1573, SW480, CaCo-2 [in Minimum Essential Medium from Eagle (MEME); Sigma–Aldrich, St. Louis, Missouri, USA]; T98-G (in Dulbecco’s modified Eagle medium; Sigma–Aldrich, St. Louis, Missouri, USA); and U373 (in MEME with non-essential amino acids; Sigma–Aldrich, St. Louis, Missouri, USA). All culture media were supplemented with 10% fetal calf serum (FCS) (PAA, Linz, Austria) and used without antibiotics.
Further five human melanoma cell lines (518A2, 607B, A375, MEL-JUSO, SK-MEL-28) were kindly provided by Christoph Hoeller (Department of Dermatology, Medical University of Vienna, Austria). These cells were grown in 75-cm2 culture flasks as adherent monolayer cultures in complete culture medium, that is, MEM supplemented with 10% heat-inactivated FCS, 1 mmol/l sodium pyruvate, 4 mmol/l l-glutamine, and 1% non-essential amino acids (100×) (all purchased from Sigma–Aldrich, Vienna, Austria) without antibiotics. All cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2 and periodically checked for Mycoplasma contamination.
Cytotoxicity assay
For screening in 25 cell lines, cells were seeded (2 × 104 cells/ml) in 100 μl/well into 96-well plates and allowed to recover for 24 h. Concentrations of KP46 ranging from 0.5 up to 10 μmol/l were added in another 100 μl of growth medium, and cells were exposed for 72 h. The cytostatic/cytotoxic effects of KP46 were determined by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-based vitality assay (EZ4U, Biomedica, Vienna, Austria). In brief, 100 μl of the supernatant was removed and replaced with 100 μl of EZ4U assay solution. After 1–2 h of incubation, absorbance was measured by a microplate reader set at 450 nm with 620 nm as reference wavelength. All experiments were carried out at least twice in triplicate.
For tests in the further five melanoma cell lines, cells were seeded in 100 μl aliquots into 96-well microculture plates in cell densities of 1.5–3.5 × 104 cells/ml as appropriate to ensure exponential growth of untreated controls throughout the experiment. Cells were allowed to settle in drug-free complete culture medium for 24 h, followed by the addition of drug dilutions in 100 μl/well complete culture medium and incubation for 96 h. In experiments with short-term exposure (1 h), medium was replaced after exposure, followed by drug-free incubation for 95 h. At the end of the experiment, all media were replaced by 100 μl/well RPMI 1640 medium (supplemented with 10% heat-inactivated FCS and 4 mmol/l l-glutamine) plus 20 μl/well MTT solution in phosphate-buffered saline (PBS) (5 mg/ml). After incubation for 4 h, medium was removed and the formazan product formed by vital cells was dissolved in DMSO (150 μl/well). Optical densities at 550 nm were measured with a microplate reader (Tecan Spectra Classic, Tecan, Groedig, Austria). Quantities of vital cells were expressed as treated/control values by comparison with untreated controls, and 50% inhibitory concentrations (IC50) were calculated from concentration-effect curves by interpolation. All experiments were carried out independently at least three times in sixtuplicates.
Isolation of melanoma cells from primary explants and Human Tumor Cloning Assay
A total of 10 fresh explants of cutaneous and lymph node metastases of melanoma originated from patients who were surgically treated at the Department of Dermatology, Rudolfstiftung Hospital, Academic Teaching Hospital, Medical University of Vienna, Austria. At the time point of sample acquisition, three patients were chemonaive and seven had been previously treated with chemotherapy (four with dacarbazine, one with fotemustine, and two with both dacarbazine and fotemustine).
Explants were processed for the Human Tumor Cloning Assay (HTCA) based on the method of Dittrich [21] with a few modifications. Samples were transferred at 37°C into McCoy’s 5A medium supplemented with 10% FCS, 50 U/ml penicillin, 50 μg/ml streptomycin, and 1.25 μg/ml amphothericin B (all purchased from Sigma–Aldrich, ienna, Austria). After three washings with this medium, the subcutaneous fat tissue was trimmed off, and the sample was cut with a pair of scissors into approximately 1 mm pieces. All the pieces and liquid were pulled onto a sterile nylon filters (100 μm, Millipore, Bedford, Massachusetts, USA) to remove blood cells as far as possible by reduced pressure using a water-jet pump. Sample pieces were transferred into a 50-ml centrifugation tube and digested in McCoy’s 5A medium containing 0.15% collagenase type IA and 0.015% DNase (Sigma–Aldrich, Vienna, Austria) in a rotating water bath at 37°C for 1 h. After repeated pressing through a 10 ml pipette, the digested product was pushed through a nylon filter (30 μm), and the filtrate was discarded. Residues in the filter were washed by suspending in the same medium and subsequent centrifugation (5 min with 1200 rpm). The pellet was suspended in the McCoy’s 5A medium containing a higher collagenase concentration (0.4%) and 0.015% DNase and digested for 1 h, followed by repeated pressing through a 10 ml pipette and further digestion for 30 min, to obtain a single-cell suspension. The enzyme was eliminated by centrifugation (5 min, 1200 rpm) and washing with medium three times. The isolated single cells were diluted in 10 ml McCoy’s 5A medium, and the concentration of viable cells was determined by trypan blue (0.18%) exclusion.
The obtained single cells were cultured in a double-layer semisolid agar medium, to inhibit the growth of nonmalignant cells. First the under layer, containing McCoy’s 5A medium supplemented with 10% FCS, 5% horse serum, 1% sodium pyruvate, 42 μg/ml l-serine, 2 mmol/l l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 1.25 μg/ml amphothericin B, 10 mmol/l 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 6 mg/ml soybean extract, 79 μg/ml l-asparagine, and 0.5% agar, was prepared. Each Petri dish (35 mm) was filled with 1 ml of the mixture and allowed to solidify. For the preparation of the upper layer, McCoy’s 5A medium was supplemented with 15% horse serum, 2% FCS, 50 U/ml penicillin, 50 μg/ml streptomycin, 1.25 μg/ml amphothericin B, 2 U/ml insulin, 0.3 mmol/l vitamin C, 4 mmol/l l-glutamine, 50 U/ml catalase, 5 μg/ml transferrin, 4 ng/ml hydrocortisone, 1% non-essential amino acids, and 10 mmol/l HEPES (all purchased from Sigma–Aldrich, Missouri). Before addition of 0.3% agar to this mixture, isolated single cells and drugs were added such that a density of 5 × 105 cells/ml and concentrations of 0.5, 5, and 15 μmol/l were obtained in the upper layer medium, respectively. This mixture was layered onto the already solidified under layer in each Petri dish, followed by incubation at 37°C in an atmosphere containing 5% CO2. An agar content of 0.3% in the upper layer was necessary to prevent growth of fibroblasts, which could not be inhibited by 0.25% agar.
Growth progress of the plated melanoma cells was controlled every 2 days, and viable colonies (diameter ≥ 60 μm) were counted after 21 days under the microscope. Counts of vital colonies were expressed in terms of treated/control values by comparison with untreated controls. Each experiment comprised at least two parallel plates per concentration level.
Cell cycle studies
The impact on the cell cycle was studied as described in a previous publication [22]. Briefly, 518A2, SK-MEL-28, and SW480 cells were seeded in aliquots of about 106 cells in 6-well plates (Iwaki/Asahi Technoglass, Gyouda, Japan), allowed to settle for 24 h and then incubated with KP46 for 24 h. Afterwards, cells were collected by trypsinization, washed twice with PBS, transferred into 0.9% NaCl solution, fixed in 70% ice-cold ethanol and stored at −20°C. Before flow cytometry analysis, cells were washed with PBS, diluted in PBS and incubated with RNase A (10 μg/ml) for 30 min at 37°C. Cells were then stained with propidium iodide for 30 min at 4°C, filtered and analyzed for their DNA content by fluorescence-activated cell sorting (FACS) using a FACS Calibur instrument (Becton Dickinson, Palo Alto, California, USA). The resulting DNA histograms were quantified using Cell Quest Pro software (Becton Dickinson and Company, New York, USA).
Effects on secondary structure of DNA
The effects of KP46 and cisplatin on the secondary structure of double-stranded plasmid DNA were studied as described in a previous publication [23]. For this purpose, plasmid pTZ18u (2860 bp) from Bio-Rad Laboratories (Munich, Germany) was transformed in competent TOP10F′ cells, isolated and purified according to the standard procedures (Promega, Pure Yield Plasmid, Madison, Wisconsin, USA) and dissolved in double-distilled water. A stock solution of KP46 was prepared in DMSO and diluted to 0.3% DMSO in water, and cisplatin was dissolved in water. Both solutions were immediately used for incubation with 1 μg plasmid in 1% TE buffer (1 mmol/l Tris–HCl, pH 7.5, and 0.1 mmol/l EDTA) for different incubation times at 37°C. The reaction products after incubation were separated in a 1% agarose gel at 80 V for 1 h, and the electrophoretic mobility of DNA was visualized under ultraviolet light after staining with ethidium bromide.
Detection of apoptosis
Nuclear morphology was visualized by histochemical labeling with 1 μg/ml DAPI (4′,6-diamidine-2′-phenylindole) dihydrochloride (Sigma–Aldrich, Vienna, Austria) for 30 min at room temperature. SK-MEL-28 cells grown to about 60% confluence on sterile coverslips were exposed to KP46 for 48 h and then washed with PBS. Cells were fixed with 4% paraformaldehyde in PBS (30 min, room temperature), washed with PBS and then incubated in 70% ethanol at −20°C for at least 2 h to permeabilize nuclei. After removal of fixative solution and washing, cells were incubated with DAPI dihydrochloride (as described above), washed thrice with PBS and examined with a fluorescence microscope.
In addition, activation of caspase-9 and PARP [poly(ADP-ribose) polymerase] cleavage were detected by western blotting to verify induction of apoptosis. For this purpose, 518A2 cells were treated with KP46 for 48 h, and whole cell lysates were diluted in 2X sample buffer (125 mmol/l Tris pH 6.8, 4% SDS, 10% glycerol, 0.006% bromophenol blue, 1.8% β-mercaptoethanol). Denatured cellular extracts were resolved by SDS–polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes (Amersham Hybond ECL, GE Healthcare, Little Chalfont, UK), blocked in FCS, and incubated with appropriate antibodies. Primary antibodies for active/cleaved caspase-9 and PARP (Cell Signaling Technology, Danvers, Massachusetts, USA) were used in dilutions of 1: 1000. Horseradish peroxidase conjugated goat anti-rabbit secondary antibodies (Cell Signaling Technology) were used. For visualization of the proteins, western blotting luminal reagent (Santa Cruz Biotechnology, Santa Cruz, California, USA) was used according to the manufacturer instructions, and X-ray film was exposed to the blots.
Results
Cytotoxicity in melanoma cell lines
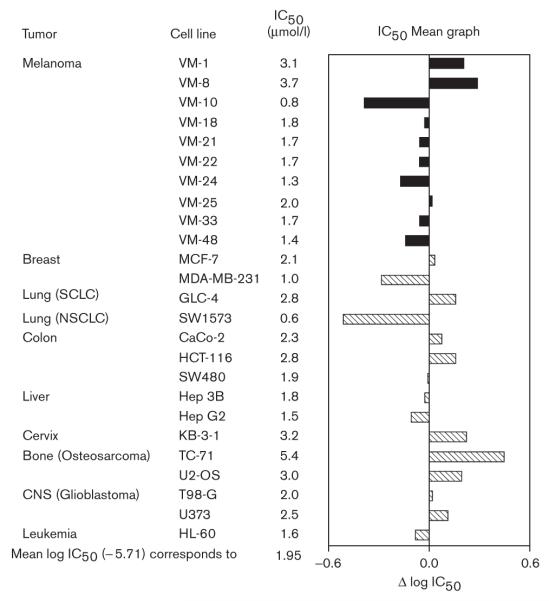
The IC50 values obtained with KP46 in 10 human melanoma cell lines are in a relatively narrow range of low micromolar concentrations (0.8–3.7 μmol/l). An IC50 mean graph displaying deviations from the mean log IC50 calculated from 25 cell lines originating from various tumor entities is depicted in Fig. 1. This graph shows that seven of 10 melanoma cell lines are at least slightly more sensitive than the average. These data confirm in a larger and completely independent cell panel the previous finding that a majority of human melanoma cell lines show a higher-than-average sensitivity to KP46 in vitro [24].
Fig. 1.
Cytotoxicity of KP46 [tris(8-quinolinolato)gallium(III)] in 10 melanoma cell lines (black bars) and 15 other human tumor cell lines (hatched bars). The 50% inhibitory concentration (IC50) mean graph representing the deviations from the mean log IC50. Bars oriented to the left indicate a higher sensitivity, bars oriented to the right a lower sensitivity than the average. NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer.
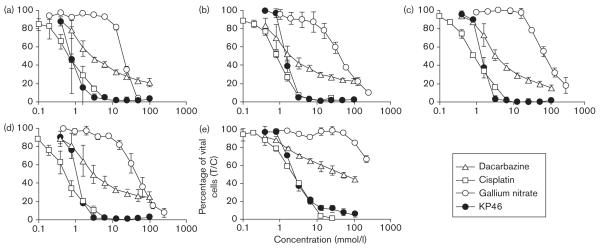
A comparison with gallium nitrate was drawn in five other human melanoma cell lines. The IC50 values of KP46 are in a similar range as those mentioned above (0.85–2.5 μmol/l), whereas those of gallium nitrate range between 20 and 72 μmol/l, with the exception of SK-MEL-28 cells in which the IC50 was not reached with a maximum concentration of 200 μmol/l. Thus, KP46 shows a 23-fold to greater than 83-fold higher potency than gallium nitrate, based on IC50 values. The high intrinsic gallium nitrate resistance of the cell line SK-MEL-28 does not affect sensitivity to KP46 in equal measure. Moreover, KP46 shows a higher cytotoxic potency and steeper concentration–effect curves than dacarbazine in these five cell lines, whereas the cytotoxicity of cisplatin is more comparable with that of KP46 (Fig. 2).
Fig. 2.
Concentration–effect curves of KP46 [tris(8-quinolinolato)gallium(III)], gallium nitrate, dacarbazine and cisplatin (MTT assay, 96 h exposure) in five human melanoma cell lines: 518A2 (a), 607B (b), A375 (c), MEL-JUSO (d), and SK-MEL-28 (e).
A comparison of 1 h and continuous exposure in 96-h cultures of the cell lines 518A2 and SK-MEL-28 revealed a clear time dependence of cytotoxicity in the case of KP46, which is, however, somewhat less pronounced than for both dacarbazine and cisplatin (Table 1). Thus, KP46 is even more cytotoxic than cisplatin in short-term exposure experiments. All three compounds require more than 1 h contact with cells to exert their full effect, indicating at the same time that the species (original compound and biotransformation products) present in the medium after 1 h contribute considerably to total cytotoxic activity. These findings prompted us to investigate this compound in primary cultures of freshly explanted melanoma samples obtained from human patients.
Table 1. Cytotoxicity of KP46, dacarbazine, and cisplatin in 96-h cultures of two human melanoma cell lines after exposure for 1 h or continuous exposure in the MTT assay.
| IC50 (μmol/l) |
||||
|---|---|---|---|---|
| Compound | 518A2 (1 h) |
518A2 (96 h) |
SK-MEL-28 (1 h) |
SK-MEL-28 (96 h) |
| KP46 | 9.4 ± 0.6 | 0.85 ± 0.20 | 15 ±2 | 2.5± 0.2 |
| Dacarbazine | 121 ± 18 | 2.5± 0.7 | > 400 | 50± 23 |
| Cisplatin | 16 ± 4 | 0.79 ± 0.36 | 56 ±24 | 2.2± 0.1 |
IC50, 50% inhibitory concentration; KP46, tris(8-quinolinolato)gallium(III); MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Growth-inhibitory effects in melanoma cells from primary explants
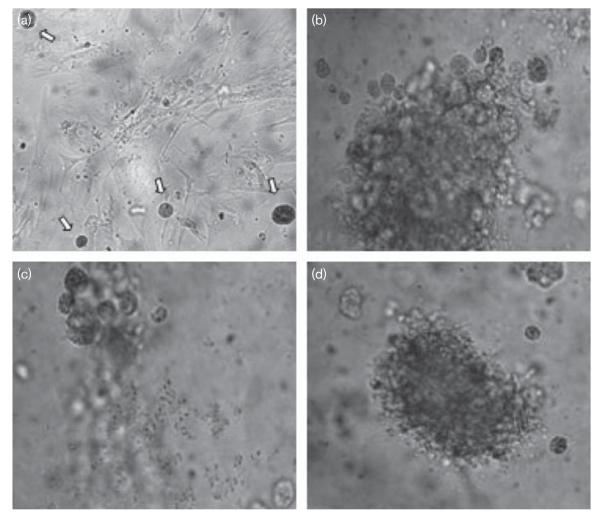
Colony growth of treated and untreated melanoma cells from primary explants was examined microscopically. Exemplary colonies are displayed in Fig. 3. Colony growth was effectively inhibited in a concentration-dependent manner by clinically relevant concentrations of KP46.
Fig. 3.
Single cells isolated from primary explanted melanoma and their colony growth after incubation for 3 weeks in the Human Tumor Cloning Assay using a double-layer soft-agar medium. (a) Untreated single cells (white arrows) after 5 days, accompanied by background growth of fibroblasts at an agar content of 0.25% in the upper layer; (b) colony formed by untreated cells after 3 weeks at an agar content of 0.3% in the upper layer, preventing fibroblast growth; (c) KP46 [tris(8-quinolinolato)gallium(III)]-treated cells (5 μmol/l) showing massive inhibition of colony formation; (d) Fotemustine-treated cells (5 μmol/l).
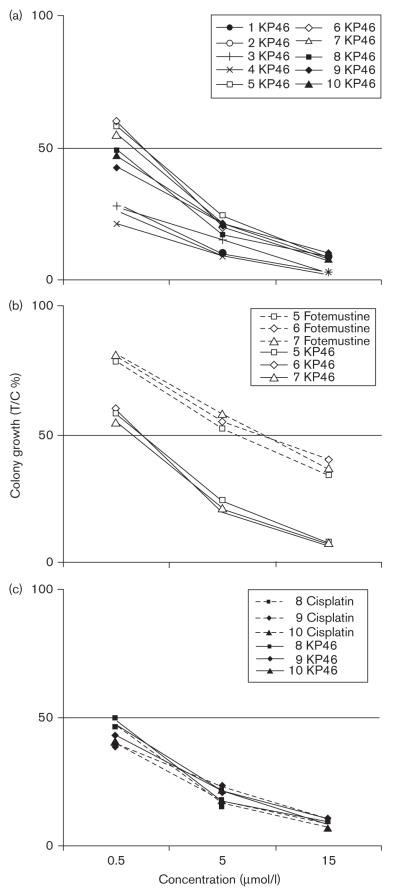
Inhibition by ≥ 50% was observed in 10 of 10 specimens at 15 μmol/l KP46, that is, the target steady-state plasma concentration that was postulated to be optimal based on pharmacological and toxicological considerations [25]; 10 of 10 specimens at 5 μmol/l KP46, approximating the highest steady-state gallium concentration in plasma of the patient having received the highest dose of KP46 (480 mg/m2/day) in the preceding phase I clinical study (Ulrich Jaehde, Institute of Pharmacy, University of Bonn, Germany, personal communication); and four of 10 specimens at 0.5 μmol/l KP46, corresponding to one-tenth of the latter concentration. Colony numbers were decreased to 2–10, 9–24, and 21–60% as compared with untreated control plates at these three concentrations, respectively (Fig. 4a). Direct comparison in three specimens showed that 5 μmol/l fotemustine, corresponding to the average peak plasma concentration in patients treated with the standard dose of 100 mg/m2 fotemustine [26], resulted in a 2.5-fold higher colony number than equimolar KP46 (Fig. 4b), whereas cisplatin had effects similar to KP46 in the same concentrations in three other specimens (Fig. 4c). Samples obtained from chemonaive patients (samples labeled with numbers 7, 8, and 9 in Fig. 3) did not show a higher sensitivity to KP46 than those from pretreated patients, suggesting that prior chemotherapy with dacarbazine or fotemustine is not disadvantageous in terms of sensitivity to KP46.
Fig. 4.
Inhibition of colony growth of primary explanted melanoma cells by KP46, cisplatin, and fotemustine, determined at various concentrations by the Human Tumor Cloning Assay in soft-agar medium. (a) Comparison of all ten KP46 [tris(8-quinolinolato)gallium(III)]-treated specimens; (b) comparison of KP46 and fotemustine, indicating thestronger cytostatic effect of the gallium complex; and (c) comparison of KP46 and cisplatin, indicating comparable activity. T/C, treated/control.
Cell cycle effects
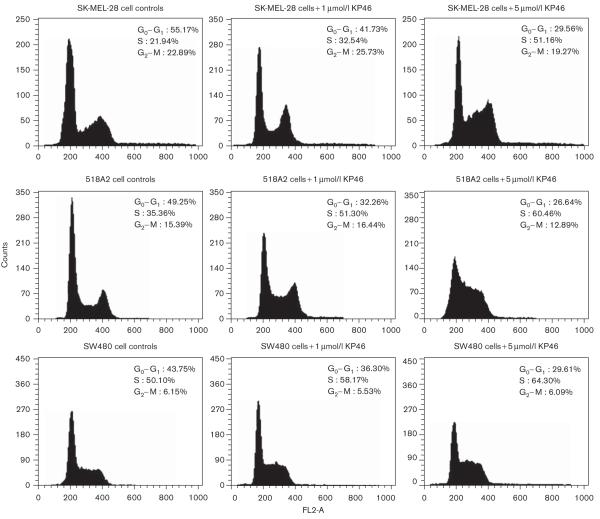
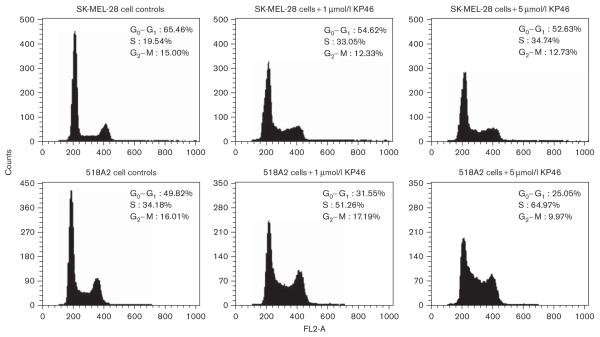
KP46 induces pronounced effects on the cell cycle distribution of both melanoma cell lines studied (518A2, SK-MEL-28). In the histograms depicted in Figs 5 and 6, an S-phase arrest is obvious after exposure for 24 and 48 h, respectively. The cell fraction in S phase increases in a concentration-dependent manner from 34–35% in untreated to 60–65% in KP46-treated (5 μmol/l) 518A2 cells, whereas the G0/G1 phase fraction decreases from 49–50% to 25–27%. In SK-MEL-28 cells, similar effects are discernible despite the somewhat lower KP46 sensitivity of these cells in the cytotoxicity assay, with the S-phase fraction increasing from 20–22 to 35–51% and the G0/G1 phase fraction decreasing from 55–65 to 30–35%. Concentration dependence is much more pronounced after 24 h than after 48 h exposure in the latter cell line. For comparison, the impact on the cell cycle was also studied in SW480 colon carcinoma cells, which show a relatively low sensitivity to KP46 similar to SK-MEL-28 cells in the MTT assay. KP46 induces an S-phase arrest in this cell line as well, but the effects are somewhat less pronounced, with an increase of the S-phase fraction from 50 to 64% and a concomitant decrease of the G0/G1 fraction from 44 to 30% after exposure for 24 h (Fig. 5).
Fig. 5.
Impact of KP46 [tris(8-quinolinolato)gallium(III)] (exposure time 24 h) on the cell cycle of the melanoma cell lines 518A2 and SK-MEL-28 and the colon carcinoma cell line SW480, as visualized by histograms obtained from fluorescence-assisted cell sorting upon propidium iodide staining of treated and control cells. Treatment with KP46 results in an increase of the cell fractions in the S phase and a concomitant decrease of cells in the G0/G1 phase. The two melanoma cell lines show a more massive S-phase arrest than the colon carcinoma cell line SW480.
Fig. 6.
Impact of KP46 [tris(8-quinolinolato)gallium(III)] (exposure time 48 h) on the cell cycle of the melanoma cell lines 518A and SK-MEL-28, measured by the same technique as in Fig. 5.
Effects on secondary structure of DNA
Alterations of DNA secondary structure can be easily detected by the changes in the electrophoretic mobility of a circular double-strand DNA plasmid (pTZ18u) that exists in a supercoiled and an open circular forms. The faster migration of the more compact supercoiled form in a neutral agarose gel is diminished by local untwisting of DNA upon interaction with intercalating or cross-linking agents. This is exemplified by the effect of 60 μmol/l cisplatin, which result in a marked retardation of the supercoiled plasmid already after incubation for 30 min. In addition, cisplatin causes a mobilization of the open circular form, which results in coalescence of the two forms of the plasmid after 30 min and 1 h incubation. This mobilization probably reflects bending of DNA as a result of bifunctional adducts [27]. In contrast, the mobility of neither the supercoiled nor the open circular form of the plasmid is altered by 60 μmol/l KP46 (Fig. 7), arguing against the formation of DNA cross-links. Similarly, no signs of strand breaks can be observed in the electropherograms. In conclusion, the lack of effects on DNA secondary structure argues against the induction of direct DNA damage by KP46.
Fig. 7.

Electropherograms of double strand DNA (plasmid pTZ18u) after incubation with 60 μmol/l cisplatin or KP46 [tris(8-quinolinolato)gallium(III)] for the indicated times as compared with untreated controls (c). In contrast to cisplatin, KP46 does not affect the mobility of the supercoiled (SC) form of the plasmid. C, control; OC, open circular.
Effects on cell morphology and induction of apoptosis
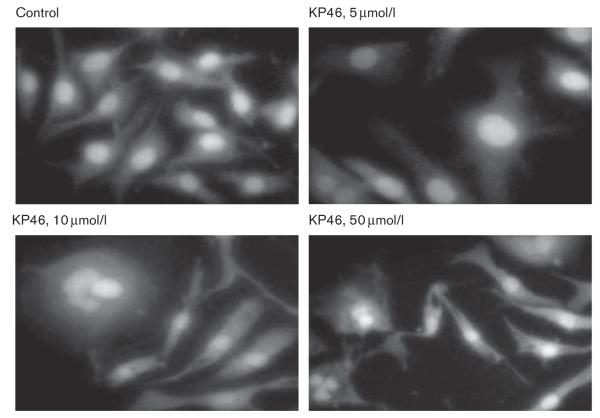
Induction of apoptosis by KP46 was visualized in SK-MEL-28 cells after exposure for 48 h by DAPI staining. Exposure to KP46 induced a frequent increase of the diameter of cells and nuclei, probably as a result of cell flattening (Fig. 8b). Apoptotic cells showed the typical morphology characterized by blebbing and fragmentation to clusters of smaller, sharply circumscribed chromatin-containing vesicles (Fig. 8c and d). Counting under the fluorescence microscope revealed a concentration-dependent increase of the percentage of apoptotic nuclei: 15.5% (5 μmol/l KP46), 27.5% (10 μmol/l KP46), and 43% (50 μmol/l KP46), whereas the number of apoptotic nuclei was negligible in untreated control cells.
Fig. 8.
Morphological features of apoptosis in SK-MEL-28 cells treated with KP46 [tris(8-quinolinolato)gallium(III)] (48 h), fixed in 4% paraformaldehyde and stained with DAPI (4′,6-diamidine-2′-phenylindole). Control cells show normal nuclear morphology, whereas an increasing percentage of KP46-treated cells show either bigger nuclei (5 μmol/l) or advanced nuclear segmentation (10 and 50 μmol/l).
Furthermore, apoptosis was verified by western blot analysis in KP46-treated 518A2 cells. The activation of caspase-9, the initiator caspase of the mitochondrial or intrinsic apoptotic pathway, and cleavage of PARP, the substrate of caspase-3, could be shown in 518A2 cells after treatment with KP46 (Fig. 9).
Fig. 9.
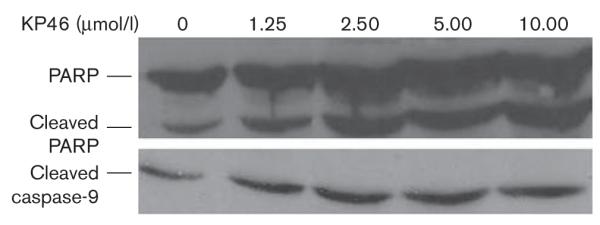
Western blot analysis of 518A2 cells treated with KP46 [tris(8-quinolinolato)gallium(III)] (0, 1.25, 2.5, 5, and 10 μmol/l) for 2 days as compared with untreated control, showing the activation of caspase-9 and cleavage of PARP [poly(ADP-ribose) polymerase] as indicators for apoptosis.
Discussion
In this study, we showed that the tumor-inhibiting compound KP46 has strong antiproliferative activity in human melanoma cells. Compared with cell lines derived from various other solid tumors, the majority of melanoma cell lines displayed higher-than-average sensitivity to KP46. The cytotoxic potency of this compound is one to two orders of magnitude higher than that of gallium nitrate, similar to previous findings in nonmelanoma cell lines [12,13,27]. Considering the pharmacokinetic data provided by the prior phase I study, the sensitivity of melanoma cells was corroborated by the HTCA using freshly explanted samples of metastases obtained from surgically treated patients. In these experiments, KP46 effectively inhibited colony growth with a higher potency than the alkylating agent fotemustine and a similar potency as cisplatin. Absolute cytotoxic potency should not be overemphasized, however, without considering the importance of pharma-cokinetics. For instance, some features of dacarbazine (short half-life in vivo, hepatic activation) differ substantially from KP46 and make comparative predictions for the in-vivo situation very difficult. Still, the reported results show that KP46 is active in a reasonable range of concentrations, as will be specified below.
Although not adopted for routine chemosensitivity testing of individual patients because of feasibility reasons, the HTCA has been attested a high predictive power in a meta-analysis comprising a large number of cases (true sensitive: 69%; true resistant: 91%) [28]. Malignant melanoma, especially melanotic metastases, poses comparatively little problems in terms of processing to single-cell suspensions, growth rate, and distinction from nonmalignant cells and was among the first malignancies studied in this test system [29]. Obviously, the HTCA is a valuable research tool for preassessing the potential of investigational drugs in certain tumor entities, preferentially as a prephase II in-vitro screen, provided that the clinically achievable plasma concentration of the drug can at least be estimated. Conventionally, one-tenth of the clinical peak plasma concentration is being taken as either the single decisive or the lowest concentration for assessing sensitivity [30], which might, however, be too strict a criterion if a drug gradually accumulates in the tumor from a continuous steady-state plasma level.
In the case of KP46, which is given daily through the oral route, pharmacokinetic data from the phase I study suggest a steady-state gallium concentration in plasma of approximately 300 μg/l (4.3 μmol/l) toward the end of the 14-day administration period at the highest dose applied (480 mg/m2/day) (Ulrich Jaehde, Institute of Pharmacy, University of Bonn, Germany, personal communication). One-tenth of this gallium level is close to the lowest concentration chosen for the HTCA (0.5 μmol/l), which proved to be effective in four of 10 melanoma specimens. As dose escalation in the clinical trial had not been continued because of feasibility reasons and no unequivocal maximum tolerable dose had been reached [31], an increase of the clinical dose and prolongation of the period of administration is conceivable, which would result in an enhanced drug exposure in patients.
Given the poor phase II data with gallium nitrate in melanoma patients, the strong potency of KP46 in melanoma cells from both chemonaive as well as patients previously treated with several lines of chemotherapy is promising. These findings lend further support to the hypothesis that KP46 does not simply behave like a prodrug in relation to conventional gallium salts, but as an agent with its own mechanistic properties and a distinct activity profile. The reasons for the sensitivity of most melanoma cells remain elusive so far, but ongoing investigations on the anti-tumor mechanism of action and key targets of KP46 are expected to generate testable hypotheses.
The data presented here indicate that KP46 induces a cell cycle arrest in the S phase in melanoma cells, consistent with the assumed inhibition of ribonucleotide reductase [13]. However, unequivocal evidence for the involvement of this enzyme is still lacking in the case of this compound. Furthermore, KP46 induces a cell morphology suggesting a classic apoptotic process. Induction of apoptosis was also corroborated on the molecular level by western blots showing cleavage of the caspase substrate PARP. More specifically, the involvement of the mitochondrial apoptotic pathway could be substantiated by the detection of caspase-9 activation. These findings are consistent with those reported for gallium nitrate in lymphoma cells, which include activation of the proapoptotic protein Bax and release of cytochrome c from mitochondria [32]. The upstream events triggering the mitochondrial pathway remain to be identified, but direct DNA damage is unlikely to be involved, as no effects on electrophoretic mobility of a supercoiled DNA plasmid could be observed in cell-free experiments, virtually excluding strand breaks and cross-links. In parallel with the higher cytotoxic potency, both induction of cell cycle arrest and apoptosis require much lower concentrations in the case of KP46 than those reported for gallium nitrate which are mostly in the 10−4 mol/l range in cell lines originating from hematological malignancies [6,32]. More detailed studies on the molecular basis of the antiproliferative activity of KP46 in cancer cells as well as its dissimilarity from and advantages over gallium salts are ongoing and will be the subject of a separate publication.
With respect to a possible clinical evaluation, applicability in combination with established drugs is an important issue. The synergistic effects of the KP46/cisplatin combination as observed in vitro [33] might be relevant for the melanoma setting, as cisplatin is part of the chemotherapeutic armamentarium for the treatment of metastatic melanoma. As the toxicity profiles of combined drugs should be nonoverlapping, additional nephrotoxicity must be avoided when combining a drug with cisplatin. Fortunately, KP46 is largely devoid of the nephrotoxicity of gallium nitrate; as the preclinical toxicological studies in mice and rats showed mild nephrotoxicity only at high doses of KP46 [31].
In conclusion, the strong antiproliferative effects of the gallium complex KP46 in melanoma cells, in particular in the HTCA in primary melanoma explants, warrant further studies of this orally bioavailable compound in this malignancy. It is desirable to study the compound in comparison with and combination with established agents in an appropriate in-vivo model with predictive power for clinical therapy to preassess in various settings its potential of complementing the as yet small spectrum of antimelanoma drugs.
Acknowledgements
The authors are indebted to Dr Nikolaus Lilgenau (Department of Dermatology, Rudolfstiftung Hospital, Academic Teaching Hospital, Medical University of Vienna) for providing the samples of surgical specimens of melanoma metastases. They are also grateful to Dr Stefanie Zorbas-Seifried and Professor Dr Haralabos Zorbas (formerly Max Planck Institute of Biochemistry, Martinsried, Germany) for generous delivery of knowledge and materials for the plasmid interaction studies and to Dr Peter Unfried, Dr Susanna Slaby, and Maria Grazia Ferri-Mendoza (Institute of Inorganic Chemistry, University of Vienna, Austria) for the synthesis of KP46, cisplatin and dacarbazine, respectively. This study was supported by the Austrian Research Promotion Agency (FFG; project no. 811591), the Austrian Science Fund (FWF; project no. L212-B11), and the Austrian Council for Research and Technology Development.
Footnotes
Part of this study was presented at the AACR-NCI-EORTC International Conference ‘Molecular Targets and Cancer Therapeutics’, 22-26 October 2007, San Francisco, California, USA: Valiahdi SM, Jakupec MA, Marculescu R, Berger W, Rappersberger K, Marculescu R and Keppler BK. A new gallium complex exerts strong activity against primary explanted melanoma cells and cell cycle perturbation in metastatic melanoma cell lines. Proceedings of the AACR-NCI-EORTC International Conference ‘Molecular Targets and Cancer Therapeutics’ 2007. p. 155 (abstract no. A278)
References
- 1.Van der Wall H, McLaughlin AF, Southee AE. Gallium scintigraphy in tumor diagnosis and management. In: Murray IPC, Ell PJ, editors. Nuclear medicine in clinical diagnosis and treatment. 2nd ed Vol. 2. Churchill Livingston; Edinburgh: 1998. pp. 813–829. [Google Scholar]
- 2.Kirkwood JM, Myers JE, Vlock DR, Neumann R, Ariyan S, Gottschalk A, et al. Tomographic gallium-67 citrate scanning: useful new surveillance for metastatic melanoma. Ann Intern Med. 1982;97:694–699. doi: 10.7326/0003-4819-97-5-694. [DOI] [PubMed] [Google Scholar]
- 3.Kagan R, Witt T, Bines S, Mesleh G, Economou S. Gallium-67 scanning for malignant melanoma. Cancer. 1988;61:272–274. doi: 10.1002/1097-0142(19880115)61:2<272::aid-cncr2820610213>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Straus DJ. Gallium nitrate in the treatment of lymphoma. Semin Oncol. 2003;30(Suppl 5):25–33. doi: 10.1016/s0093-7754(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 5.Einhorn L. Galium nitrate in the treatment of bladder cancer. Semin Oncol. 2003;30(Suppl 5):34–41. doi: 10.1016/s0093-7754(03)00174-x. [DOI] [PubMed] [Google Scholar]
- 6.Hedley DW, Tripp EH, Slowiaczek P, Mann GJ. Effect of gallium on DNA synthesis by human T-cell lymphoblasts. Cancer Res. 1988;48:3014–3018. [PubMed] [Google Scholar]
- 7.Chitambar CR, Matthaeus WG, Antholine WE, Graff K, O’Brien WJ. Inhibition of leukemic HL60 cell growth by transferrin-gallium: effects on ribonucleotide reductase and demonstration of drug synergy with hydroxyurea. Blood. 1988;72:1930–1936. [PubMed] [Google Scholar]
- 8.Bernstein LR. Mechanisms of therapeutic activity for gallium. Pharmacol Rev. 1998;50:665–682. [PubMed] [Google Scholar]
- 9.Chitambar CR. Apoptotic mechanisms of gallium nitrate: basic and clinical investigations. Oncology. 2004;18(Suppl 10):39–44. [PubMed] [Google Scholar]
- 10.Casper ES, Stanton GF, Sordillo PP, Parente R, Michaelson RA, Vinceguerra V. Phase II trial of gallium nitrate in patients with advanced malignant melanoma. Cancer Treat Rep. 1985;69:1019–1020. [PubMed] [Google Scholar]
- 11.Collery P, Keppler B, Madoulet C, Desoize B. Gallium in cancer treatment. Crit Rev Oncol Hematol. 2002;42:283–296. doi: 10.1016/s1040-8428(01)00225-6. [DOI] [PubMed] [Google Scholar]
- 12.Thiel M, Schilling T, Gey DC, Ziegler R, Collery P, Keppler BK. Tris(8-quinolinolato)gallium(III), a novel orally applied antitumor gallium compound. Contrib Oncol. 1999;54:439–443. [Google Scholar]
- 13.Jakupec MA, Heffeter P, Pongratz M, Fremuth M, Horvath Z, Unfried P, et al. Depletion of cellular dNTP pools, cell cycle arrest and apoptosis induced by the oral gallium complex KP46 (FFC11) Clin Cancer Res. 2005;11(24 Suppl):9159s. [Google Scholar]
- 14.Hofheinz RD, Dittrich C, Jakupec MA, Drescher A, Jaehde U, Gneist M, et al. Early results from a phase I study on orally administered tris(8-quinolinolato)gallium(III) (FFC11, KP46) in patients with solid tumors - a CESAR study (Central European Society for Anticancer Drug Research - EWIV) Int J Clin Pharmacol Ther. 2005;43:590–591. doi: 10.5414/cpp43590. [DOI] [PubMed] [Google Scholar]
- 15.Collery P, Domingo JL, Keppler BK. Preclinical toxicity and tissue gallium distribution of a novel antitumour gallium compound: tris(8-quinolinolato)gallium(III) Anticancer Res. 1996;16:687–692. [PubMed] [Google Scholar]
- 16.Dhara SC. A rapid method for the synthesis of cis-[Pt(NH3)2Cl2] Indian J Chem. 1970;8:192–193. [Google Scholar]
- 17.Shealy YF, Krauth CA, Montgomery JA. Imidazoles. I. Coupling Reactions of 5-Diazoimidazole-4-carboxamide. J Org Chem. 1962;27:2150–2154. [Google Scholar]
- 18.Berger W, Hauptmann E, Elbling L, Vetterlein M, Kokoschka EM, Micksche M. Possible role of the multidrug resistance-associated protein (MRP) in chemoresistance of human melanoma cells. Int J Cancer. 1997;71:108–115. doi: 10.1002/(sici)1097-0215(19970328)71:1<108::aid-ijc18>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Dornetshuber R, Heffeter P, Kamyar MR, Peterbauer T, Berger W, Lemmens-Gruber R. Enniatin exerts p53-dependent cytostatic and p53-independent cytotoxic activities against human cancer cells. Chem Res Toxicol. 2007;20:465–473. doi: 10.1021/tx600259t. [DOI] [PubMed] [Google Scholar]
- 20.Elbling L, Berger W, Weiss RM, Printz D, Fritsch G, Micksche M. A novel bioassay for P-glycoprotein functionality using cytochalasin D. Cytometry. 1998;31:187–198. doi: 10.1002/(sici)1097-0320(19980301)31:3<187::aid-cyto6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Dittrich C. Cloning of solid tumors. Springer; Wien: 1987. [Google Scholar]
- 22.Heffeter P, Jakupec MA, Körner W, Wild S, von Keyserlingk NG, Elbling L, et al. Anticancer activity of the lanthanum compound [tris(1,10-phenanthroline)lanthanum(III)] trithiocyanate (KP772; FFC24) Biochem Pharmacol. 2006;71:426–440. doi: 10.1016/j.bcp.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Zorbas-Seifried S, Jakupec MA, Kukushkin NV, Groessl M, Hartinger CG, Semenova O, et al. Reversion of structure-activity relationships of antitumor platinum complexes by acetoxime but not hydroxylamine ligands. Mol Pharmacol. 2007;71:357–365. doi: 10.1124/mol.106.030726. [DOI] [PubMed] [Google Scholar]
- 24.Valiahdi SM, Jakupec MA, Marculescu R, Keppler BK. Tris(8-quinolinolato)gallium(III) exerts strong antiproliferative effects in melanoma cells. Metal Ions Biolo Med. 2006;9:282–286. [Google Scholar]
- 25.Jakupec MA, Keppler BK. Gallium and other main group metal compounds as antitumor agents. In: Sigel A, Sigel H, editors. Metal ions in biological systems. Vol. 42. M. Dekker; New York: 2004. pp. 425–462. [PubMed] [Google Scholar]
- 26.Dumontet C, Jaubert J, Sebban C, Bouafia F, Ardiet C, Tranchand B, et al. Clinical and pharmacokinetic phase II study of fotemustine in refractory and relapsing multiple myeloma patients. Ann Oncol. 2003;14:615–622. doi: 10.1093/annonc/mdg158. [DOI] [PubMed] [Google Scholar]
- 27.Collery P, Lechenault F, Cazabat A, Juvin E, Khassanova L, Evangelou A, Keppler B. Inhibitory effts of gallium chloride and tris(8-quinolinolato)gallium III on A549 human malignant cell line. Anticancer Res. 2000;20:955–958. [PubMed] [Google Scholar]
- 28.Hanauske AR, Von Hoff DD. In vitro and in vivo predictive tests. In: Holland JF, Frei E, Bast RC, Kufe DW, Pollock RE, Weichselbaum RR, editors. Cancer medicine. B. C. Decker; Hamilton: 2000. pp. 585–588. [Google Scholar]
- 29.Meyskens FL, Loescher L, Moon TE, Takasugi B, Salmon SE. Relation of in vitro colony survival to clinical response in a prospective trial of single-agent chemotherapy for metastatic melanoma. J Clin Oncol. 1984;2:1223–1228. doi: 10.1200/JCO.1984.2.11.1223. [DOI] [PubMed] [Google Scholar]
- 30.Von Hoff DD, Clark GM, Stogdill BJ, Sarosdy MF, O’Brien MT, Casper JT, et al. Prospective clinical trial of a human tumor cloning system. Cancer Res. 1983;43:1926–1931. [PubMed] [Google Scholar]
- 31.Collery P, Jakupec MA, Kynast B, Keppler BK. Preclinical and early clinical development of the antitumor gallium complex KP46 (FFC11) Metal Ions Biolo Med. 2006;9:521–524. [Google Scholar]
- 32.Chitambar CR, Wereley JP, Matsuyama S. Gallium-induced cell death in lymphoma: role of transferrin receptor cycling, involvement of Bax and the mitochondria, and effects of proteasome inhibition. Mol Cancer Ther. 2006;5:2834–2843. doi: 10.1158/1535-7163.MCT-06-0285. [DOI] [PubMed] [Google Scholar]
- 33.Jakupec MA, Collery P, Keppler BK. Synergistic antiproliferative effects of tris(8-quinolinolato)gallium(III) (KP46) in combination with platinum drugs in ovarian and colon carcinoma cells. Metal Ions Biol Med. 2008;10:110–115. [Google Scholar]