Abstract
All homeotherms utilize thermogenesis to maintain core body temperature, ensuring that cellular functions and physiologic processes can ensue in cold environments1-3. In the prevailing model, when the hypothalamus senses cold temperatures, it triggers sympathetic discharge, resulting in the release of noradrenaline in brown adipose tissue (BAT) and white adipose tissue (WAT)4,5. Acting via the β3-adrenergic receptors, noradrenaline induces lipolysis in white adipocytes6, whereas it stimulates the expression of thermogenic genes, such as PPARγ coactivator 1a (Ppargc1a), uncoupling protein 1 (Ucp1), and acyl-CoA synthetase long-chain family member 1 (Acsl1), in brown adipocytes7-9. However, the precise nature of all the cell types involved in this efferent loop is not well established. Here we report an unexpected requirement for the interleukin 4 (IL4)-stimulated program of alternative macrophage activation in adaptive thermogenesis. Cold exposure rapidly promoted alternative activation of adipose tissue macrophages, which secrete catecholamines to induce thermogenic gene expression in BAT and lipolysis in WAT. Absence of alternatively activated macrophages impaired metabolic adaptations to cold, whereas administration of IL4 increased thermogenic gene expression, fatty acid mobilization, and energy expenditure, all in a macrophage-dependent manner. We have thus discovered a surprising role for alternatively activated macrophages in the orchestration of an important mammalian stress response, the response to cold.
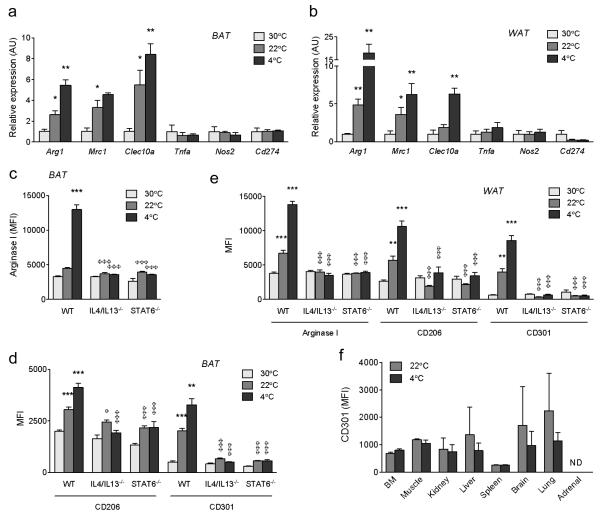
Mice housed at thermoneutral temperature of 30 °C do not require adaptive thermogenesis, whereas those housed in colder environments depend on BAT thermogenesis to maintain their body temperature10. Thus, in order to understand the relationship between temperature and macrophage activation, we profiled the status of BAT and WAT macrophages in mice chronically housed at 30 °C (thermoneutrality), 22 °C (normal housing temperature), or after an acute challenge to 4 °C. Gene expression profiling revealed a progressive increase in the expression of alternative activation mRNAs11,12, including Arg1, Mrc1, and Clec10a, in BAT and WAT of mice exposed to colder temperatures (Fig. 1a, b). In contrast, expression of classical activation markers was unchanged by cold exposure (Fig. 1a, b). This correlation between alternative macrophage activation and exposure to colder environments was further verified using flow cytometry. In wild type mice, exposure to progressively lower temperatures increased expression of CD206 (Mrc1), CD301 (Clec10a) and arginase 1 (Arg1) proteins in BAT and WAT macrophages (Fig. 1c-e; Supplementary Fig. 1a-f). Importantly, disruption of IL4/IL13 signaling, as in IL4/IL13−/− and STAT6−/− mice13, completely abrogated the cold-induced increase in alternative activation of BAT and WAT macrophages, as assessed by expression of CD206, CD301, and Arg1 (Fig. 1c-e; Supplementary Fig. 1a-f). This was a specific defect in cold-induced alternative activation because loss of IL4/IL13 signaling did not introduce a classical activation bias in BAT and WAT macrophages (Supplementary Fig. 2a-d). Lastly, acute exposure of mice to 4 °C failed to induce alternative macrophage activation in other tissues, including skeletal muscle and liver (Fig. 1f), suggesting that BAT and WAT alternative activation is an adaptive response for acclimation to cold.
Figure 1. Exposure to cold environment induces alternative activation of adipose tissue macrophages.
a, b, Real time-PCR analysis of markers of alternative and classical activation in BAT (a) and WAT (b) of wild type (WT) mice chronically housed at 30 °C, 22 °C, or acutely subjected to a 4 °C from 22 °C (n=4 per temperature). Expression of all genes is normalized to their relative expression at 30 °C in WT mice. c-e, Expression of alternative activation markers Arg-1, CD206, and CD301 was monitored by flow cytometry in BAT (c, d) and WAT (e) macrophages of wild type (WT), IL4/IL13−/−, and STAT6−/− mice housed at 30 °C, 22 °C, and 4 °C (n=4-5 per genotype and temperature). f, Alternative activation of tissue macrophages was monitored at 22 °C and 4 °C by quantifying expression of CD301. BM (bone marrow). *P < 0.05, **P < 0.01, ***P < 0.001 comparison between WT at 30 °C and 22 °C, or between 22 °C and 4 °C. φP < 0.05, φφφP < 0.001 comparison between WT and various knockout mice at the same temperature.
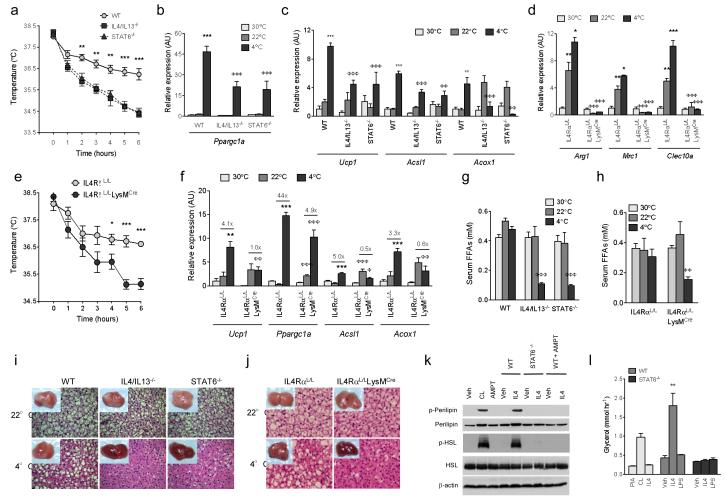
To investigate the importance of alternative macrophage activation in cold-induced thermogenesis, we challenged mice lacking alternatively activated macrophages to cold temperatures. Unlike wild type mice, IL4/IL13−/− and STAT6−/− mice dropped their core body temperature when exposed to temperatures of 4 °C (Fig. 2a). In wild type mice, to counteract the change in environmental temperature, thermogenic genes (Ppargc1□ and Ucp1) and the β-oxidation genes (Acox1 and Acsl1) were induced in BAT. This induction of thermogenic genes was blunted in BAT of IL4/IL13−/− and STAT6−/− mice (Fig. 2b, c). To determine whether the observed defects in cold-induced thermogenesis were a direct consequence of the loss of alternatively activated macrophages, we disrupted IL4/IL13 signalling in myeloid cells by breeding conditional IL4RαL/L with LysMCre mice14. BAT macrophages in IL4RαL/LLysMCre mice displayed impairment in alternative activation at 22 °C and 4 °C (Fig. 2d), which was sufficient to render mutant mice susceptible to cold-induced hypothermia (Fig. 2e). IL4RαL/LLysMCre mice also exhibited defects in expression of cold-inducible thermogenic genes, including Ucp1, Acox1, Acsl1, and Ppargc1a (Fig. 2f). Comparable results were obtained in a second model when macrophages were pharmacologically depleted in BAT using clodronate-containing liposomes (Supplementary Fig. 3a-e), which selectively deplete tissue macrophages and circulating monocytes but not neutrophils (Supplementary Fig. 4a, b). Moreover, expression of skeletal muscle mitochondrial genes implicated in thermogenesis was unaltered (Supplementary Fig. 5a), suggesting a primary defect in non-shivering thermogenesis. Serum triglyceride levels and expression of lipogenic genes in liver were similarly unchanged across the genotypes and temperatures (Supplementary Table 1 and Supplementary Fig. 5b). Lastly, defects in cold-induced thermogenesis were also observed in STAT6−/− mice on the C57BL/6J background (Supplementary Fig. 5c, d).
Figure 2. Cold-induced metabolic adaptations require alternatively activated macrophages.
a, Core body temperature of WT, IL4/IL13−/−, and STAT6−/− mice during a cold challenge at 4°C (n=8 per genotype and temperature). b, c, Real-time PCR analysis of thermogenic genes in BAT of WT, IL4/IL13−/−, and STAT6−/− mice housed at 30 °C, 22 °C or subjected to 4 °C cold challenge (n=4-5 per genotype and temperature). Expression of all genes is normalized to their relative expression at 30 °C in WT mice. d, Expression of alternatively activated mRNAs in BAT of IL4RαL/L and IL4RαL/LLysMCre mice housed at various temperatures (n=5 per genotype and temperature). e, Core body temperature of IL4RαL/L and IL4RαL/LLysMCre mice during exposure to 4 °C (n=5-6 per genotype and temperature). f, BAT of IL4RαL/L and IL4RαL/LLysMCre mice was analyzed by real-time PCR for expression of thermogenic and β-oxidation genes (n=5 per genotype and temperature). Expression of all genes is normalized to their relative expression at 30 °C in IL4RαL/L mice. g, Serum free fatty acid (FFA) levels in WT, IL4/IL13−/−, and STAT6−/− mice housed at 30 °C, 22 °C, and 4 °C (n=5-8 per genotype). h, Serum FFAs in IL4RαL/L and IL4RαL/LLysMCre mice housed at the three temperatures (n=5-11 per genotype). i, Representative gross and microscopic (haematoxylin and eosin staining) histology and of BAT from WT, IL4/IL13−/−, and STAT6−/− mice at 22 °C and after exposure to 4°C for 6 hours. j, Representative gross and microscopic (haematoxylin and eosin staining) histology of BAT from IL4RαL/L and IL4RαL/LLysMCre mice at 22 °C and after 6 hour exposure to 4°C. k, Immunoblot analysis for serine phosphorylated perilipin, total perilipin, serine phosphorylated-HSL and total HSL in 3T3-L1 adipocytes treated with PIA, CL-316243, IL4 or macrophage conditioned medium (± IL4 and AMPT) for 15 min. PIA (N6-phenylisopropyl adenosine), AMPT (α-methyl-p-tyrosine). l, Glycerol release by 3T3-L1 adipocytes after 6h treatment with PIA, CL-316243 (CL), IL4 or macrophage conditioned medium (n=5-7). *P < 0.05, **P < 0.01, ***P < 0.001 compared to comparison between WT or IL4RαL/L at 30 °C and those at 22 °C, or at 22 °C and 4 °C. φP < 0.05, φφP < 0.01, φφφP < 0.001 comparison between knockouts and WT or IL4RαL/L at the same temperature.
During cold exposure, β-adrenergic signalling in white adipocytes stimulates the release of free fatty acids to fuel uncoupled respiration in BAT1,6. Since WAT macrophages also undergo alternative activation upon cold challenge (Fig. 1b, e), we examined whether a defect in alternative macrophage activation was associated with impaired release of free fatty acids. Indeed, compared to wild type mice, circulating levels of free fatty acids were reduced by ~75% in IL4/IL13−/− and STAT6−/− mice (Fig. 2g). Serum free fatty acid levels were similarly reduced by ~65% in IL4RαL/LLysMCre mice at 4 °C (Fig. 2h). Consistent with reduced release of fatty acids, gross and microscopic histology revealed that all mutant mice impaired in alternative macrophage activation had exhausted their lipid stores in BAT (Fig. 2i, j). Correspondingly, mice deficient in IL4/IL13 signaling or macrophages lost less weight during the cold challenge (Supplementary Table 1).
To explore whether factors released by alternatively activated macrophages work in trans to stimulate lipolysis of stored triglycerides, we utilized differentiated 3T3-L1 cells to study triglyceride lipolysis in vitro. Treatment of adipocytes with conditioned-medium from alternatively activated macrophages induced phosphorylation of perilipin and hormone sensitive lipase (HSL), lipases that are phosphorylated by protein kinase A in response to adrenergic signalling (Fig. 2k)15. The phosphorylation of perilipin A releases CGI-58, allowing it to interact with Pnpla2 to enhance lipolysis of stored triglycerides16,17. Indeed, paralleling the increase in perilipin phosphorylation, triglyceride lipolysis, as quantified by glycerol release, was increased by ~4.5-fold in adipocytes treated with conditioned medium from alternatively activated macrophages (Fig. 2l). No significant increase in phosphorylation of perilipin, HSL, or triglyceride lipolysis was observed when adipocytes were exposed to conditioned medium from STAT6−/− macrophages (Fig. 2k, l). Together, these data suggest alternatively activated macrophages coordinate the thermogenic response during cold exposure by increasing BAT’s thermogenic capacity and mobilizing fatty acids to fuel uncoupled respiration.
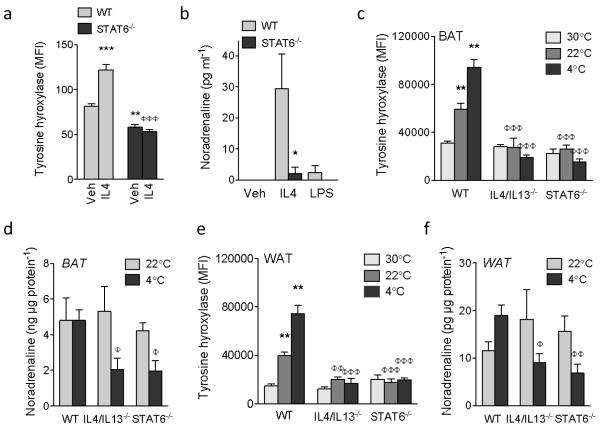
The requirement for alternatively activated macrophages in fatty acid mobilization and thermogenic gene induction prompted us to investigate whether WAT and BAT macrophages might be an important source of catecholamines. In this regard, catecholamine production by classically activated macrophages has previously been shown to promote inflammation-induced injury18,19. Intracellular staining for Th (tyrosine hydroxylase), Ddc (dopa decarboxylase), and Dbh (dopamine β-hydroxylase) revealed that all three catecholamine-synthesizing enzymes were induced in macrophages upon stimulation with IL4 (Supplementary Fig. 6a-f). This induction was a bona fide part of alternative activation because IL4 failed to induce Th, the rate-limiting step in the synthesis of catecholamines20, in macrophages lacking STAT6 (Fig. 3a; Supplementary Fig. 6g). Congruent with this, stimulation of macrophages with IL4, but not lipopolysaccharide (LPS), increased secretion of noradrenaline and adrenaline into the culture medium in a STAT6-dependent manner (Fig. 3b; Supplementary Fig. 7a). Furthermore, treatment of wild type macrophages with α-methyltyrosine, a specific inhibitor of tyrosine hydroxylase18, inhibited secretion of noradrenaline into the culture medium, and abrogated its lipolytic activity on cultured adipocytes (Fig. 2k; Supplementary Fig. 7b, c).
Figure 3. Alternatively activated macrophages produce catecholamines.
a, Expression of tyrosine hydroxylase in WT and STAT6−/− peritoneal macrophages treated with vehicle (veh) or IL4, n=5 per genotype and condition. b, Noradrenaline secretion by WT and STAT6−/− bone-marrow-derived macrophages stimulated with IL4 or LPS (n=5 per genotype and condition). c, e Tyrosine hydroxylase expression in BAT (c) and WAT (e) macrophages of WT and STAT6−/− mice at 30 °C, 22 °C and 4 °C (n=5 per genotype and temperature). d, f, Noradrenaline content of BAT (d) and WAT (f) at 22 °C and 4 °C of WT and STAT6−/− mice (n=4-5 per genotype and temperature). *P < 0.05, **P < 0.01, ***P < 0.001 compared to WT. φP < 0.05, φφP < 0.01, φφφP < 0.001 compared to WT with IL4 at 4 °C samples.
Next, we examined catecholamine synthesis by adipose tissue macrophages. At thermoneutrality (30 °C), expression of Th in BAT and WAT macrophages was the lowest (Fig. 3c, e). Th expression progressively increased as mice were exposed to colder temperatures (Fig. 3c, e), and was restricted to Ly6Clo-midCD301+ alternatively activated BAT and WAT macrophages (Supplementary Fig. 8a, b). Consistent with this, loss of IL4/IL13 signaling abrogated cold-induced expression of Th in BAT and WAT macrophages (Fig. 3c, e) and reduced noradrenaline content of these adipose tissues by ~50-60% in IL4/IL13−/− and STAT6−/− mice (Fig. 3d, f and Supplementary Table 2). This decrease in BAT and WAT catecholamine content was a direct consequence of loss of alternative activation because similar changes were observed in IL4RαL/LLysMCre mice. Specifically, cold exposure failed to induce Th in BAT and WAT macrophages (Supplementary Fig. 9a, c), resulting in reduction of noradrenaline content of BAT and WAT by 70-80% in IL4RαL/LLysMCre mice (Supplementary Fig. 9b, d). In contrast, catecholamine content of serum and other tissues was unaffected (Supplementary Table 3). Finally, IL4 stimulation of primary human monocytes or U937 cells enhanced their alternative activation, catecholamine production, and lipolytic activity on adipocytes, suggesting conservation of these pathways across species (Supplementary Fig. 10a-h).
These data prompted us to investigate whether β3-adrenergic agonist CL-316243 can rescue the thermogenic defect in IL4/IL13−/− mice21. Indeed, a single injection of CL-316243 increased core body temperature and thermogenic gene expression in IL4/IL13−/− mice (Supplementary Fig. 11a, b). The restoration of core body temperature by CL-316243 also normalized weight loss and BAT histology in IL4/IL13−/− mice housed at 4 °C (Supplementary Fig. 11c-e), including the reappearance of lipid droplets in brown adipocytes. The increased accumulation of lipid droplets likely resulted from enhanced mobilization of free fatty acids and induction of lipogenic genes, such as Lpl, Hmgcs1 and Dgat1, in BAT of IL4/IL13−/− mice administered CL-316243 (Supplementary Fig. 11 f, g; Supplementary Fig. 12 a, b). Hence, alternatively activated macrophages are an unexpected source of noradrenaline that sustains the metabolic adaptations to cold.
A hallmark of cold-induced thermogenesis is an increase in uncoupled respiration and energy expenditure by noradrenaline10. Since we observed that IL4 driven alternatively activated macrophages release noradrenaline in BAT and WAT in response to cold, we next examined metabolic effects of IL4 in wild type mice. Injection of IL4 induced alternative activation and Th expression in BAT and WAT macrophages (Supplementary Fig. 13 a, b). As expected, the strongest effects of IL4 were observed at thermoneutrality, when basal alternative activation and Th expression was the lowest. However, administration of IL4 was sufficient to augment alternative activation and Th expression in mice housed at 22 °C and 4 °C (Supplementary Fig. 13a, b). Concomitant with induction of alternative activation, noradreanline content and thermogenic gene expression in BAT, and fatty acid levels in serum increased after administration of IL4 (Supplementary Fig. 13 c, d, e). Lastly, albeit to a much lower degree, administration of IL4 enhanced expression of Th in alternatively activated macrophages taking residence in other tissues, including liver, spleen, lung, and bone marrow (Supplementary Fig. 14a-f; Supplementary Fig. 15a, b).
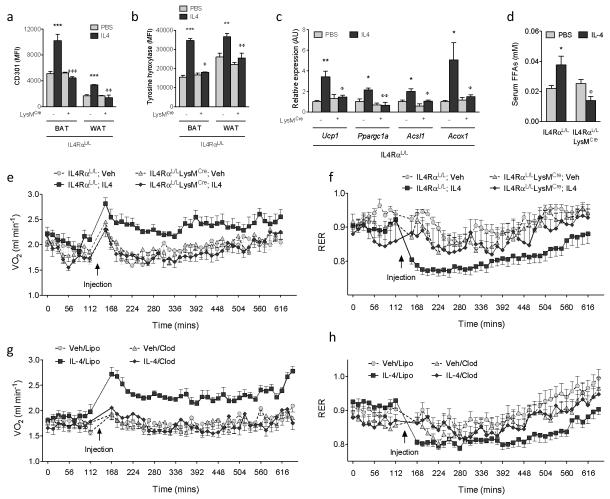
We next investigated whether acute administration of IL4 to adapted animals could enhance oxygen consumption in a macrophage-dependent manner. As shown in Figure 4a, administration of IL4 promoted alternative activation of BAT and WAT macrophages in IL4RαL/L but not IL4RαL/LLysMCre mice. This was accompanied by an increase in expression of Th in BAT and WAT macrophages, resulting in induction of thermogenic genes and release of free fatty acids (Fig. 4b-d). Furthermore, quantification of energy expenditure revealed that injection of IL4 rapidly increased oxygen consumption in IL4RαL/L but not IL4RαL/LLysMCre mice (Fig. 4e). Importantly, consistent with a shift from carbohydrate to fatty acid metabolism, administration of IL4 decreased the respiratory exchange ratio (RER) in IL4RαL/L mice (Fig. 4f). These changes in energy expenditure were independent of alterations in locomotor activity (Supplementary Fig. 15c). Furthermore, in wild type mice, the stimulatory effect of IL4 on energy expenditure showed a marked dependence on macrophages, as IL4 failed to raise oxygen consumption or decrease RER in mice treated with clodronate-containing liposomes (Fig. 4g, h; Supplementary Fig. 15d). These findings thus provide direct evidence that actions of alternatively activated macrophages in BAT and WAT orchestrate the metabolic programs that constitute adaptive thermogenesis.
Figure 4. Alternative activation of macrophages increases energy expenditure.
a,b, Expression of alternative activation marker CD301 (a) and TH (b) in adipose tissue macrophages from IL4RαL/L and IL4RαL/LLysMCre mice treated with vehicle (Veh) or IL4 for 6 hours at 22 °C (n=4-5 per genotype and condition). c, Real-time PCR for thermogenic genes in BAT of IL4RαL/L and IL4RαL/LLysMCre mice treated with Veh or IL4 for 6 hours at 22 °C (n=4-5 per genotype and condition). d, Serum free fatty acid (FFA) levels in IL4RαL/L and IL4RαL/LLysMCre mice treated with Veh or IL4 for 6 hours at 22 °C (n=4-5 per genotype and condition). e, f, Quantification of energy expenditure in IL4RαL/L and IL4RαL/LLysMCre mice treated with vehicle (Veh) or IL4 (n=7-9 per genotype and condition). (e) Oxygen consumption (VO2) and (f) respiratory exchange ratio (RER). g, h, Quantification of energy expenditure in WT mice after macrophage depletion (n=8 per condition). Mice were injected with empty liposomes (Lipo) or clodronate-containing liposomes (Clod) 24 hours prior to energy expenditure studies. All data were collected during the light cycle. *P < 0.05, **P < 0.01, ***P < 0.001 compared to IL4RαL/L with Veh. φP < 0.05, φφP < 0.01, φφφP < 0.001 compared to IL4RαL/L with IL4.
The data presented here show that alternatively activated macrophages participate in vivo in the regulation of adaptive and facultative aspects of non-shivering thermogenesis. In a macrophage-dependent manner, the administration of IL4 raises energy expenditure in a facultative manner, whereas adaptation to lower temperatures is associated with polarization of BAT and WAT macrophages to the alternative state. Moreover, the secretion of noradrenaline by alternatively activated macrophages allows these cells to coordinate the thermogenic response in animals experiencing cold stress. Thus, we propose that, in addition to the sympathetic nerves, cells of the hematopoietic system, such as alternatively activated macrophages, constitute a second, parallel circuit for controlling non-shivering thermogenesis.
METHODS SUMMARY
Male mice, 8-12 weeks old, were used in all experiments. Breeding pairs of wild type and STAT6−/− mice on BALB/cJ background were purchased from the Jackson Laboratory, and IL4/IL13−/−, IL4RαL/L, and LysMCre mice on the BALB/cJ background were obtained from the Locksley or Brombacher laboratories. For cold challenge experiments, mice were fed ad lib and individually housed in cages that had been pre-chilled at 4 °C8. Core body temperature was monitored hourly by a rectal temperature probe (Physitemp). For the thermoneutrality experiments, mice were adapted to 30 °C in laboratory incubator (Darwin Chambers) for 2-4 weeks prior to experimentation. For rescue experiments, the β3-adrenergic agonist CL-316243 (Sigma) was injected intraperitoneally at 0.1mgkg−1 30 min before the cold challenge. Tissues were harvested at the end of 6 h cold challenge, and processed for RNA and protein analyses. To deplete macrophages, mice were injected intraperitoneally with two doses of clodronate-containing or empty liposomes (400 μl and 100 μl at 24 h and 30 min, respectively, prior to initiation of experiment)22. Depletion was confirmed by flow cytometric analysis of monocytes and macrophages in blood, adipose tissues, and spleen. Cohorts of ≥ 4 mice per genotype or treatment were assembled for all in vivo studies, which were repeated 2-3 independent times. All data are presented as mean ± s.e.m.
METHODS
Flow cytometry and immunoblot analysis
Adipose tissues were minced and digested with collagenase I (3 mgml−1, Worthington) for 45 min at 37 °C in a shaker (400 rpm). The digested cell suspension was centrifuged at 1,600 rpm for 5 min to separate stromal-vascular fraction from adipocytes. Pelleted cells were resuspended in FACS buffer (PBS containing 5% FBS and 1% L-glutamine) and passed through a 40 μm strainer (BD Biosciences) to remove large cellular debris. Antibodies directed against mouse CD3, B220, Ly6G, CD45, CD49b, CD11c, and F4/80 (Biolegend); Siglec F (BD Biosciences); FcεR1 (eBioscience); tyrosine hydroxylase (Origene); Ly6C, dopamine β-hydroxylase, and dopa decarboxylase (Abcam); arginase-1 (Santa Cruz Biotech); and anti-rabbit and anti-mouse IgG (Invitrogen) were used for flow cytometric analysis. Samples were stored in FACS buffer with 1% paraformaldehyde at 4 °C prior to analysis. Data was acquired on LSRII (BD Biosciences) and data analysis was performed using FlowJo (Treestar). For analysis of mitochondrial proteins in muscle, soleus muscle from mice housed at 22 C and 4 C was lysed using TissueLyser II (Qiagen), and antibodies directed against cox-1 (Invitrogen) or Cpt1b (Alpha Diagnostic International) were used to detect mitochondrial proteins.
Macrophage culture and stimulation
Bone marrow-derived macrophages (BMDMs) were cultured as previously described23. Classical or alternative activation was induced in BMDMs by stimulation with lipopolysaccharide (10 ngml−1) or IL4 (10 ngml−1) for 24 hours, respectively. Macrophages were elicited into the peritoneal cavity by injection of thioglycollate (3 ml, BD Biosciences). To promote alternative activation of elicited macrophages, mice were given a single injection of IL4 (2 μg) complexed to anti-IL4 antibody (10 μg) three days after injection of thioglycollate, and elicited macrophages were recovered 24 h later. After washing two times, elicited macrophages were cultured in low glucose (LG)-DMEM with 3% BSA and macrophage-conditioned medium was collected 24 h later. Human monocytes, which were isolated from whole blood by magnetic purification with CD14 microbeads (Miltenyi Biotech), and the human macrophage cell line U937 were cultured in RPMI with 5% FBS and 1 % L-glutamine and stimulated with lipopolysaccharide (10 ngml−1) or IL4 (10 ngml−1) for 24 hours to induce classical or alternative activation, respectively. Condition media were collected and supplemented with 3% BSA for lipolysis assays. In some experiments, α-methyl-p-tyrosine (AMPT, 2 mM, Sigma) was added to cultured macrophages to inhibit tyrosine hydroxylase.
Quantitative RT-PCR
Tissues were homogenized in Trizol (Invitrogen), total RNA was isolated using RNeasy kit (Qiagen), and used as template for cDNA synthesis (Origene). Quantitative PCR reactions were carried out in triplicate using the CFX384 real-time PCR detection system (Bio-Rad). Relative expression level of mRNAs was calculated by the comparative threshold cycle method using 36B4 as an internal control24. The following primers were used in these studies: Il1: (Forward-GAAGAAGAGCCCATCCTCTG, Reverse-TCATCTCGGAGCCTGTAGTG); Il6: (Forward-AGTCCGGAGAGGAGACTTCA, Reverse-TTGCCATTGCACAACTCTTT); Acsl1: (Forward-TGGGGTGGAAATCATCAGCC, Reverse-CACAGCATTACACACTGTACAACGG); Acox: (Forward-GGTGGACCTCTGTCTTGTTCA, Reverse-AAACCTTCAGGCCCAAGTGAG); Ucp1: (Forward-GTGAAGGTCAGAATGCAAGC, Reverse-AGGGCCCCCTTCATGAGGTC); Ppargc1a: (Forward-CAACATGCTCAAGCCAAACCAACA, Reverse-CGCTCAATAGTCTTGTTCTCAAATGGG); Tnf: (Forward-CCAAGGCGCCACATCTCCCT, Reverse-GCTTTCTGTGCTCATGGTGT); Nos2: (Forward-ACCTTGGTGAAGGGACTGAG, Reverse-TCCGTTCTCTTGCAGTTGAC); Arg1: (Forward-AGACCACAGTCTGGCAGTTG, Reverse-CCACCCAAATGACACATAGG); Mrc1: (Forward-TGATTACGAGCAGTGGAAGC, Reverse-GTTCACCGTAAGCCCAATTT); Clec10a: (Forward-CTCTGGAGAGCACAGTGGAG, Reverse-ACTTCCGAGCCGTTGTTCT); 36B4: (Forward-GAGACTGAGTACACCTTCCCAC, Reverse-ATGCAGATGGATCAGCCAGG).
Catecholamines and lipids
Catecholamines (Rocky Mountain Diagnotics), free fatty acids (Biovision), and glycerol (Abcam) were quantified in duplicate as per the manufacturers’ protocols. For catecholamine ELISAs, tissues were homogenized by sonification in homogenization buffer (1N HCl, 0.25M EDTA, 1M Na2S2O5), and cellular debris was pelleted by centrifugation at 13,000 rpm for 15 min at 4 °C. The cleared homogenates were collected and stored at −80 °C prior to quantification. All samples were normalized to total tissue protein concentration.
Adipocyte differentiation and lipolysis
The 3T3-L1 pre-adipocytes were grown in high glucose Dulbecco’s modified Eagle’s medium (HG-DMEM) supplemented with BCS (10%). Two days after confluence, differentiation was induced with insulin (10 μg/ml), dexamethasone (1 μM), and 3-isobutyl-1-methylxanthine (0.5 mM) in HG-DMEM containing FBS (10%). All subsequent media changes (every 2 days) were performed using HG-DMEM supplemented with FBS (10%) and insulin (10 μg/ml). For the lipolysis studies, differentiated adipocytes were cultured in low glucose (LG)-DMEM supplemented with BSA (3%) for 16 h prior to stimulation with vehicle, CL-316243 (1 μM), IL4 (10 ngml−1) or macrophage conditioned medium for 15 min or 6 h to quantify phospho-HSL, phospho-Perilipin or glycerol release, respectively. Basal lipolysis was quantified in presence of N6-phenylisopropyl adenosine (PIA, 1 μM). Glycerol release into the culture medium was quantified using Free Glycerol Assay Kit (Abcam). For immunoblot analysis, treated adipocytes were lysed in lysis buffer (20 mM Tris-HCl, pH 7.5, 100 mM KCl, 0.1% Nonidet P-40, 1 mM EDTA, and 10% glycerol containing 1 mM phenylmethylsulfonyl fluoride, 1% protease inhibitor cocktail, and 1% phosphatase inhibitor cocktails I/II for 30 min at 4 °C. Total cellular protein extracts were separated on SDS–polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane (Bio-Rad), and incubated with antibodies directed against HSL, serine 660 phosphorylated HSL (Cell Signaling), perilipin, or serine 552 phosphorylated perilipin (Vala Sciences). After incubation with appropriate secondary antibodies, proteins were detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific).
Energy expenditure
Oxygen consumption, RER, and activity were quantified in 12-week-old male mice of various genotypes fed ad libitum using CLAMS (Columbus Instruments). Following acclimatization to CLAMS cages for 48 hours, mice were given an intraperitoneal injection of recombinant IL4 (45.5 μg/kg body weight) at 11 am. Consumption rates of O2 (VO2) and release of CO2 (VCO2) were monitored for ~8 hours every 14 minutes. Locomotor activity, the number of x-axis beam breaks, was monitored every minute. Data was collected during light cycle (9 am~7 pm).
Statistical analysis
All data are presented as mean ± s.e.m and analyzed using Prism (Graphpad). Statistical significance was determined using the Student’s t-test and two-way ANOVA. A p value of < 0.05 was considered to be statistically significant, and is presented as * (p < 0.05), ** (p < 0.01), or *** (p < 0.001).
Supplementary Material
Acknowledgments
We thank members of the Chawla laboratory, A. Loh, and C-H. Lee for valuable comments on the manuscript, and F. Kraemer for guidance on in vitro lipolysis assays. This work was supported by grants from: NIH (DK076760, HL076746, DK094641), Larry L. Hillblom Foundation Network Grant and an NIH Director’s Pioneer Award (DP1OD006415) to A.C. Support was provided by Stanford Graduate Fellowship (K.D.N) and A-STAR Fellowship (Y.P.G). All animal care was in accordance with Stanford University’s A-PLAC and UCSF’s IACUC guidelines.
Footnotes
Supplementary Information accompanies the paper on Nature’s website (http://www.nature.com).
Author Contributions K.D.N. and Y.Q. performed the experiments with assistance from X.C., J.M., T.D., Y.P.G., and L.M.; F.B., R.M.L, and A.C. were involved in project planning; K.D.N., Y.Q., and A.C. designed the experiments, analyzed the data, and wrote the manuscript.
Competing interests statement The authors declare that they have no competing financial interests.
References
- 1.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. doi:10.1152/physrev.00015.2003 84/1/277 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 3.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. doi:nrd3138 [pii] 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. doi:nn2027 [pii] 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. doi:expphysiol.2007.041848 [pii] 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nedergaard J, Bengtsson T, Cannon B. New powers of brown fat: fighting the metabolic syndrome. Cell Metab. 2011;13:238–240. doi: 10.1016/j.cmet.2011.02.009. doi:S1550-4131(11)00053-2 [pii] 10.1016/j.cmet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Ellis JM, et al. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab. 2010;12:53–64. doi: 10.1016/j.cmet.2010.05.012. doi:S1550-4131(10)00188-9 [pii] 10.1016/j.cmet.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enerback S, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. doi:10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 9.Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 10.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214:242–253. doi: 10.1242/jeb.050989. doi:214/2/242 [pii] 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 11.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 12.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. doi:10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 14.Herbert DR, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 15.Watt MJ, et al. Reduced plasma FFA availability increases net triacylglycerol degradation, but not GPAT or HSL activity, in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287:E120–127. doi: 10.1152/ajpendo.00542.2003. doi:10.1152/ajpendo.00542.2003 00542.2003 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Haemmerle G, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. doi:312/5774/734 [pii] 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 17.Lass A, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. doi:S1550-4131(06)00114-8 [pii] 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Flierl MA, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. doi:nature06185 [pii] 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- 19.Brown SW, et al. Catecholamines in a macrophage cell line. J Neuroimmunol. 2003;135:47–55. doi: 10.1016/s0165-5728(02)00435-6. doi:S0165572802004356 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Zhou QY, Quaife CJ, Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995;374:640–643. doi: 10.1038/374640a0. doi:10.1038/374640a0. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida T, Sakane N, Wakabayashi Y, Umekawa T, Kondo M. Anti-obesity and anti-diabetic effects of CL 316,243, a highly specific beta 3-adrenoceptor agonist, in yellow KK mice. Life Sci. 1994;54:491–498. doi: 10.1016/0024-3205(94)00408-0. [DOI] [PubMed] [Google Scholar]
- 22.Kosteli A, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. doi:42845 [pii] 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odegaard JI, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odegaard JI, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.