Abstract
Background
Growing evidence suggests that motivation to engage in pain-coping strategies is a key predictor of how well a person adjusts to pain. According to the Motivational Model of Pain Self-Management, readiness to engage in pain self-management behaviors is influenced by beliefs about the importance of the behavior (importance) and the ability to carry out the behavior (self-efficacy).
Purpose
The purpose of this study was to test the Motivational Model of Pain Self-Management for exercise and task persistence pain-coping behaviors in a sample of 114 individuals with multiple sclerosis and chronic pain.
Methods
Measures included the Multidimensional Pain Readiness to Change Questionnaire-2 and measures of importance, self-efficacy, and coping behavior duration. Tests of mediation were conducted with two path analyses, one for each coping behavior.
Results
The effects of importance and self-efficacy beliefs on coping behaviors were mediated or partially mediated by readiness to engage in those behaviors.
Conclusions
These findings provide support for the Motivational Model of Pain Self-Management and have important implications for the development of treatments for chronic pain.
Keywords: Chronic pain, Multiple sclerosis, Exercise, Task persistence
Introduction
Multiple sclerosis is a progressive inflammatory demyelinating disease of uncertain etiology. As many as 350,000 individuals in the USA have been diagnosed with multiple sclerosis [1, 2]. The physical disability and fatigue that are frequent symptoms of multiple sclerosis have traditionally been the focus of clinical care. Chronic pain, however, is increasingly recognized as a common problem among individuals with multiple sclerosis, with the point prevalence of pain approaching 50% in some studies [3]. Moreover, for these individuals, chronic pain contributes to greater psychological distress [4, 5], health care utilization, physical disability, and reduced quality of life [3, 6] and is often inadequately managed [3, 7]. Although analgesic medications are the most commonly utilized pain management strategies, they do not typically provide complete or even necessarily adequate pain relief [5, 8, 9].
It is widely understood that how well individuals adapt to chronic pain largely depends on what they do to cope with or manage their symptoms. The broader pain literature indicates that long-term adaptation to chronic pain relies primarily on the individual’s ability to change behaviors and cognitions to mitigate their experience of pain and its effects on their lives [10–12]. For example, a willingness to engage in task persistence (i.e., a strategy in which one continues to work on a task despite pain) is a pain-coping response that has demonstrated consistent associations with greater positive adjustment in samples of individuals with chronic pain in general [13, 14] and in samples of individuals with multiple sclerosis in particular [15]. Exercise behavior is another key self-management strategy. Although most people diagnosed with multiple sclerosis do not participate in regular exercise [16, 17], for those with chronic pain and multiple sclerosis, exercise is a commonly endorsed pain management strategy [8] and is associated with lower pain, disability, fatigue, and depression and greater social support, self-efficacy, and quality of life [18–24]. However, as is true for almost any behavior, knowledge of which coping strategies are the most helpful in pain self-management is not sufficient to promote behavioral change. An individual must also have a degree of internal readiness to make behavioral changes and to maintain them. In fact, a number of studies have shown that a person’s readiness to adopt a given self-management strategy is a key predictor of completion of pain self-management programs and of positive response to program involvement [25–28]. Thus, a patient’s readiness to engage in self-management pain-coping strategies has important implications for treatment planning.
Jensen, Nielson, and Kerns [29] presented a model, the Motivational Model of Pain Self-Management, wherein patient motivation to engage in pain self-management behaviors was hypothesized to be an important predictor of adaptation to pain (see Fig. 1). Based on an expectancy value approach, readiness to change is influenced by (1) the patient’s perception of the importance of the coping behavior and (2) the patient’s perceived ability to adopt or use the coping response (i.e., self-efficacy). The primary outcomes of the model are engagement in adaptive and avoidance of maladaptive coping responses. These coping responses are then thought to contribute to patient function (or dysfunction). Recently, our research group found preliminary support for this motivational model in a sample of individuals with spinal cord injury (and spinal-cord-injury-related pain), where readiness to change exercise behavior mediated the association of both perceived importance of exercise and self-efficacy for exercise to reported exercise behavior [30].
Fig. 1.
The motivational model of pain self-management
The current study replicates and extends our previous research evaluating the Motivational Model of Pain Self-Management [31] in a sample of individuals with multiple sclerosis. Because the Motivational Model of Pain Self-Management makes predictions about specific pain-coping responses, in order to test the model, individual coping responses must be selected from among many different pain-coping responses. In the present study, we planned to study two coping responses in order to balance the need to limit the planned analyses (to control for alpha inflation associated with multiple experiment-wise tests) and the need for replicability (to allow for a test of the model for more than just one coping response). Based on the extant literature, we elected to study exercise and task persistence coping responses.
Methods
Procedures
A survey that included the study measures was mailed to a total of 381 individuals, identified through a combination of sources including study brochures and flyers, clinic referrals, and the mailing list of the University of Washington multiple sclerosis Research and Rehabilitation Training Center. Inclusion criteria included 18 years of age or older and diagnosis of multiple sclerosis by medical professional. Of the 381 surveys, 161 were returned, yielding a response rate of 42.3%. All participants signed an informed consent document approved by the University of Washington Institutional Review Board and were paid $25 for participation. Of the 161 individuals who returned the survey, 47 were either missing data on the variables of interest (n=5) or endorsed no pain in the past week (n=42) and were therefore excluded in subsequent analyses, yielding a final sample size of 114. Compared with those with complete data, the group of excluded cases was statistically comparable on demographic variables, age, education, employment, marital status, and level of mobility reported. The subgroup of excluded cases had a significantly higher proportion of males, 35% versus 17% in the group with complete data (χ2 (1, 161)=5.50, P=0.02), and significantly lower average pain (M(excluded)=0.50, SD=0.08, M(included)= 2.43, SD=0.22), t(161)=−16.31, P<0.01.
Whereas in our initial study [30] we tested the mediation models for importance and self-efficacy with two separate series of linear regression models, in the present study, both predictors were tested in a single mediation model using path analysis. This procedure is preferable because it simultaneously estimates direct, indirect, and total effects within a single model. It is more accurate and informative from a theoretical perspective to simultaneously test all components of the proposed model rather than testing portions of the model separately.
Measures
Demographic Information
Information on participant gender, age, education level, employment status, marital status, and multiple sclerosis course/subtype was collected at the beginning of the questionnaire.
Disease-Related Information
Participants were asked to select the closest match to their multiple sclerosis course from a list of alternatives and to report their multiple sclerosis subtype (e.g., relapsing/ remitting, primary progressive, secondary progressive, etc.). Participant mobility was then assessed with the Gross Motor Function Classification System [32] on which each person classified their ability to “get around” on a 0 (I have no mobility limitations) to 5 (I have severely limited self-mobility even with the use of assistive technology (e.g., power mobility)) rating scale.
Pain Intensity
Participants were asked to indicate their average pain intensity during the past week and in the past 3 months on a 0 to 10 numerical rating scale, with zero indicating “no pain” and ten indicating “pain as bad as it could be.” The discriminant validity of this scale in measuring sensory rather than affective pain has recently been demonstrated in a sample of individuals with chronic musculoskeletal pain [33]. Pain was classified as mild (1–3), moderate (4–6), and severe (7–10) using a classification system validated across a broad range of pain conditions [34–37].
Pain Interference
Participants reported the number of days in the past week that “you have been kept from your usual activities because of pain.” There were also three pain interference items on which participants rated on an 11-point scale, 0 (“no interference”) to 10 (“unable to carry on any activities”), the extent to which, in the past week, their pain interfered with “daily activities,” “ability to take part in recreational, social, and family activities,” and “ability to work (including housework)” [38].
Readiness to Use Adaptive Coping and Avoid Use of Maladaptive Pain-Coping Responses
The revised version of the Multidimensional Pain Readiness to Change Questionnaire 2 [39], a 69-item measure that assesses willingness to use or not use nine pain-coping responses, including exercise (seven items) and task persistence (five items), was used in the present study. The Multidimensional Pain Readiness to Change Questionnaire 2 Exercise and Task Persistence scales have demonstrated adequate to excellent reliability across different samples of patients with chronic pain (overall alpha coefficients of 0.85 and 0.77, respectively) [36]. These scales have also demonstrated good concurrent validity, showing strong and significant associations with self-reported frequency of exercise and task persistence pain-coping behavior, respectively [40]. In the present sample, exercise (seven items) and task persistence (five items) subscales of the Multidimensional Pain Readiness to Change Questionnaire 2 also demonstrated adequate to excellent internal consistency (Cronbach’s α=0.86 and 0.73, respectively).
Perceived Importance
To measure a key component of the Motivational Model of Pain Self-Management, perceived importance related to self-management behaviors, six items were developed to assess perceived importance of exercise and task persistence, based on 0 (not at all important) to 10 (extremely important) numeric rating scales. For assessing perceived importance of exercise, the items were as follows: (1) “To what extent do you believe that regular exercise is important for managing your health and pain problem?”; (2) “To what extent have you experienced direct and immediate benefits of exercise (such as encouragement from someone important to you, or feeling better right after you exercise) in the past?”; (3) “To what extent do you currently receive encouragement or other benefits when you exercise?” Internal consistency for these three items was excellent (Cronbach’s α=0.80). Similarly, perceived importance of task persistence was assessed by using the average of three items: (1) “How important is it to you, in managing your health and pain problem, to keep going despite the pain?”; (2) “To what extent have you experienced direct and immediate benefits when you keep doing what you need to do despite pain in the past?”; (3) “To what extent do you currently receive encouragement or other benefits when you keep going despite pain?” Internal consistency for the task persistence scale was acceptable (Cronbach’s α=0.73).
Self-Efficacy
Self-efficacy for exercise and task persistence was assessed with one item each. For exercise, participants indicated “To what extent do you see yourself as having the resources (such as the time and energy) to exercise regularly if you choose to?” on a 0 (I am not capable of exercising regularly) to 10 (I am capable of exercising regularly) scale. For task persistence, participants indicated “To what extent do you see yourself as having the ability to keep going with what you need to do despite any pain you might feel?” [40] on a 0 (I am unable to keep going when I have pain) to 10 (I can always keep going despite pain) scale.
Self-Management Behaviors
Each self-management behavior was assessed with a single item that asked the number of months (0–12+) in a row that a patient had been “keeping going despite pain” (task persistence) or “exercising regularly” (exercise). Thus, this rating provides a continuous measure of the consistency of current exercise and task persistence behaviors, as opposed to overall amount that these coping responses are used in a given period of time. This methodology is consistent with the motivation model being tested, which predicts that current coping behavior will be associated with current self-efficacy and importance beliefs.
Data Analysis
Prior to conducting meditational analyses, the distributions of the study variables were evaluated for normality. As in our previous examination of exercise in spinal cord injury patients [31], it appeared that exercise behavior in this sample also trended toward a bimodal distribution, with 22.3% of participants reporting 0 months of exercise and 43.85% reporting 12 or more consecutive months of exercise. However, for all variables, skew and kurtosis were below critical cutoff values [41], allowing us to proceed with the planned path analyses. Descriptive statistics were calculated, including means, standard deviations, and frequencies for demographic and clinical variables of interest and Pearson’s bivariate correlations of model variables (importance, self-efficacy, readiness, and self-management behaviors) with each other and with demographic and clinical (pain, pain interference) variables.
Mediational analyses were conducted with MPlus 5.21 software [42], using maximum likelihood estimation to simultaneously estimate model parameters and standard errors for a system of regression models. Two just-identified (saturated) path analyses were conducted, predicting exercise behavior and task persistence behavior. Each path analysis model was used to estimate the relation of the predictors (importance and self-efficacy) to behaviors (exercise or task persistence) directly and indirectly through the proposed mediator, readiness. The predictors, importance and self-efficacy, were specified as correlated in the model. We utilized the unique capacity of the MPlus program to calculate parameters, standard errors, confidence intervals for direct effects, total effect, and specific indirect paths with multiple mediational pathways in the model. Sobel’s z statistics [43] are reported for tests of indirect effects.
Results
Demographic and multiple-sclerosis-related descriptive information is presented in Table 1. Nearly one fifth (19%) of the participants endorsed chronic “severe” pain (i.e., ≥7/10) in the past week. Participants reported pain in an average of 5.66 (SD=3.34) body locations. These findings are generally consistent with previous data [4]. The most commonly reported pain sites were low back (51.2%), neck (46.3%), shoulder (40.5%), and hands (38.8%). In this sample, 22.3% of participants reported 0 months of regular exercise and 5.0% reported 0 months of using a task persistence approach. Mean durations for the group were 6.74 months (SD=5.21) for regular exercise behavior and 10.10 months (SD=3.76) for practicing task persistence.
Table 1.
Demographic and clinical data
| Variable | Mean | SD | Number | Percent |
|---|---|---|---|---|
| Age | 54.06 | 11.78 | 121 | |
| Average pain (last week) | 4.00 | 2.43 | 121 | |
| Mild (1–3) | 48.8 | |||
| Moderate (4–6) | 32.2 | |||
| Severe (7–10) | 19.0 | |||
| Average pain (last 3 months) | 4.11 | 2.63 | 121 | |
| Mild (1–3) | 45.5 | |||
| Moderate (4–6) | 33.8 | |||
| Severe (7–10) | 20.7 | |||
| Pain interference | ||||
| Days of interference | 1.4 | 2.2 | 114 | |
| Average interference | 3.0 | 2.7 | 114 | |
| Gender | ||||
| Male | 21 | 17.4 | ||
| Female | 100 | 82.6 | ||
| Marital status | ||||
| Married | 70 | 57.9 | ||
| Separated | 4 | 3.3 | ||
| Divorced | 24 | 19.8 | ||
| Living with partner | 5 | 4.1 | ||
| Never married | 9 | 7.4 | ||
| Widowed | 9 | 7.4 | ||
| Education | ||||
| <12th grade | 1 | 0.8 | ||
| High school or GED | 11 | 9.1 | ||
| Voc/tech school | 10 | 8.3 | ||
| Some college | 28 | 23.1 | ||
| College graduate | 47 | 38.8 | ||
| Graduate or Prof school | 24 | 19.8 | ||
| Employment status | ||||
| Full time | 21 | 18.4 | ||
| Part time | 10 | 8.8 | ||
| Retired | 33 | 28.9 | ||
| Unemployed | ||||
| Due to pain | 8 | 7.0 | ||
| Due to disability | 53 | 46.5 | ||
| MS course | ||||
| Relapsing–remitting | 61 | 50.4 | ||
| Secondary progressive | 30 | 24.8 | ||
| Primary progressive | 18 | 14.9 | ||
| Progressive-Relapsing | 12 | 9.9 | ||
Participants were able to select multiple responses for employment status, resulting in a column total that is greater than the sample size
Full bivariate correlation results can be found in Table 2. There were few notable correlations between demographic variables and model variables. Older age was related to greater self-efficacy for and duration of exercise behavior. Average pain intensity was not related to any study variable. Mobility, however, was significantly negatively correlated with self-efficacy for task persistence and for readiness for both exercise and task persistence behaviors. Due to the correlations of age and mobility with some of the predictors and the outcomes in each path analysis, age and mobility were included as covariates in the models. A number of significant correlations were found between measures of pain interference and model variables. Specifically, importance, self-efficacy, and readiness for task persistence were consistently related to less pain interference, across domains of pain interference. Task persistence behavior was related to fewer days of pain interference. Self-efficacy for exercise and exercise behavior were both correlated with less pain interference. Notably, all predictors in the models showed significant correlations of moderate magnitudes with the outcome coping behaviors.
Table 2.
Bivariate correlations of model variables with demographic and clinical variables
| Variable | Importance
|
Self-efficacy
|
Readiness
|
Self-management behavior
|
||||
|---|---|---|---|---|---|---|---|---|
| Ex | Task | Ex | Task | Ex | Task | Ex | Task | |
| Age | 0.06 | 0.09 | 0.25** | −0.01 | −0.08 | 0.03 | 0.22* | 0.09 |
| Gender | 0.20* | 0.08 | 0.07 | −0.04 | 0.13 | 0.17 | −0.01 | −0.09 |
| Education | 0.02 | 0.05 | 0.18* | −0.03 | 0.17 | −0.02 | 0.10 | −0.05 |
| Employment | 0.02 | −0.10 | 0.00 | −0.22* | −0.03 | −0.19* | 0.10 | −0.10 |
| Pain intensity | −0.11 | −0.12 | −0.15 | −0.07 | −0.01 | −0.10 | −0.07 | −0.04 |
| Mobility | −0.09 | −0.08 | −0.17 | −0.26** | −0.25** | −0.23* | 0.02 | −0.11 |
| Pain interference—extent to which pain interferes with activities or ability to participate | ||||||||
| Days in past week | −0.17 | −0.25** | −0.30** | −0.33** | −0.03 | −0.30** | −0.16 | −0.18* |
| Daily activities | −0.14 | −0.27** | −0.30** | −0.29** | −0.06 | −0.29** | −0.15 | −0.09 |
| Recreational, social, family | −0.19* | −0.25** | −0.35** | −0.18 | −0.06 | −0.21* | −0.21* | −0.03 |
| Ability to work | −0.19* | −0.28** | −0.37** | −0.28** | −0.06 | −0.34** | −0.21* | −0.08 |
Mobility was measured with the Gross Motor Function Classification System: Ex = Exercise; Task = Task Persistence; Employment = full or part time; yes = (1); no = (0)
P<0.05;
P<0.01
Mediational Analyses Predicting Exercise Behavior
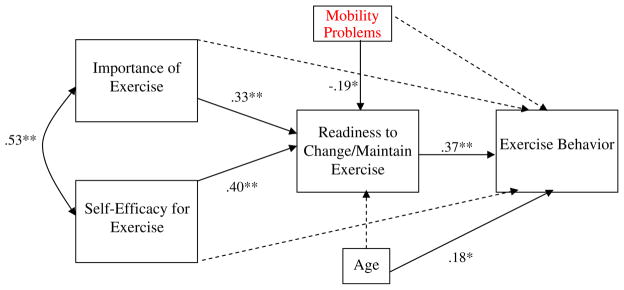
As can be seen in Fig. 2, the direct paths from importance and self-efficacy to exercise behavior were not significant (β=0.13, ns and β=0.14, ns, respectively). Consistent with expectations, the effects of importance and of self-efficacy on readiness to change/maintain exercise were significant (β=0.33, P<0.01 and β=0.40, P<0.01, respectively), and readiness, in turn, had a significant effect on exercise behavior (β=0.37, P<0.01). The specific indirect effects (i.e., mediated effects) of importance and self-efficacy on exercise by way of readiness were both significant (Sobel’s z=2.70, P<0.01, Sobel’s z=2.85, P<0.01, respectively). The model accounted for 49% of variance in readiness and 33% of variance in exercise behavior.
Fig. 2.
Path analysis model demonstrating the associations between importance of exercise, self-efficacy for exercise, readiness to change/maintain exercise, and exercise behavior, with covariates age and mobility problems. Coefficients are standardized path coefficients. *P<0.05, **P<0.01. Non-significant paths are depicted as dashed lines
Mediational Analyses Predicting Task Persistence
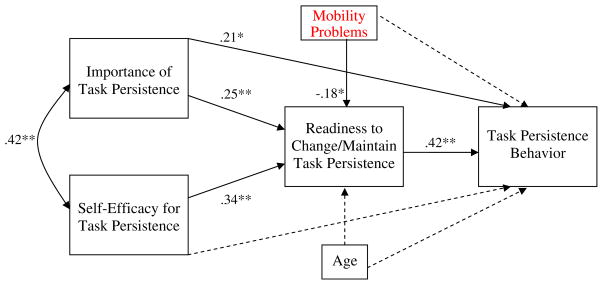
As shown in Fig. 3, the direct path from self-efficacy to task persistence behavior was not significant (β=0.01, ns), but the path from importance to task persistence was significant (β=.21, P=0.02). Consistent with expectations, the effects of importance and of self-efficacy on readiness to change/maintain task persistence were significant (β= 0.25, P<0.01 and β=0.34, P<0.01 respectively), and readiness, in turn, had a significant effect on task persistence behavior (β=0.42, P<0.01). The specific indirect effects (i.e., mediated effects) of importance and self-efficacy on task persistence through readiness were both significant (Sobel’s z=2.57, P=0.01, Sobel’s z=3.03, P<0.01,respectively). The model accounted for 32% of variance in readiness and 31% in task persistence behavior.
Fig. 3.
Path analysis model demonstrating the associations between importance of task persistence, self-efficacy for task persistence, readiness to change/ maintain task persistence, and task persistence behavior, with covariates age and mobility problems. Coefficients are standardized path coefficients. *P< 0.05, **P<0.01. Non-significant paths are depicted as dashed lines
For clarity in interpretation, zero-order correlation coefficients among key study variables are presented in Table 3.
Table 3.
Bivariate correlations among model variables
| Variable | Importance | Self-efficacy | Readiness | Self-management behavior |
|---|---|---|---|---|
| Exercise | ||||
| Importance | 1.0 | 0.53* | 0.55* | 0.41* |
| Self-efficacy | 0.53* | 1.0 | 0.57* | 0.46* |
| Readiness | 0.55* | 0.57* | 1.0 | 0.50* |
| Self-management behavior | 0.41* | 0.46* | 0.50* | 1.0 |
| Task persistence | ||||
| Importance | 1.0 | 0.41* | 0.41* | 0.40* |
| Self-efficacy | 0.41* | 1.0 | 0.46* | 0.31* |
| Readiness | 0.41* | 0.47* | 1.0 | 0.51* |
| Self-management behavior | 0.40* | 0.31* | 0.51* | 1.0 |
p<0.01
Discussion
Questions about what motivates human behavior have been central to the study of psychology since its inception. Within the subfield of health psychology, a great deal of attention has focused on developing and testing models of health behaviors in particular. A prominent group of health behavior models based on expectancy value theory, Social Cognitive Theory [44], the Health Belief Model [45], and the Protection Motivation Theory [46, 47], highlights the role of cognitions in influencing behavior. The Motivational Model of Pain Self-Management, like the above-mentioned models of health behavior, emphasizes the importance of an individual’s beliefs and values in understanding health behaviors. What is unique about this model is that it borrows from the clinically based Motivational Interviewing [48] approach to understanding behavior and presents readiness, or the motivation for a given behavior in the context of competing motivations, as a key mediator between expectations and values and behavioral outcomes. This study provides support for the overall model, which is not surprising, given the robustness of expectancy value theories in explaining health behaviors. Notably, however, in addition to providing support for the expectancy value part of the model, the findings also supported the importance of readiness as a mediator between beliefs and behaviors.
Adding to our previous work applying the Motivational Model of Pain Self-Management for exercise behaviors in persons with spinal cord injury [31], results from this study provide further support for the model in terms of both exercise and task persistence behaviors in persons with multiple sclerosis and pain. The majority of the study participants reported that they believed that exercise and task persistence behaviors were important and that they were confident in their ability to maintain or change these behaviors. For both self-management outcomes, perceived importance of and self-efficacy for each behavior were found to be positively associated with self-reports of the duration of engaging in exercise and task persistence behaviors. As predicted, we found that the associations of self-efficacy and perceived importance to exercise and task persistence were mediated by readiness to change the behavior. In the case of the importance of task persistence, the mediated effect through readiness was significant even as the direct path from importance to task persistence behavior was also significant, indicating partial mediation. These data support the Motivational Model of Pain Self-Management and suggest that beliefs regarding the perceived importance and self-efficacy for a particular pain management behavior (e.g., exercise) are related to changes in one’s readiness to engage in these behaviors. In turn, this readiness predicts actual self-reported behavior.
These data also indicate that two subscales taken from the Multidimensional Pain Readiness to Change Questionnaire 2 [39], a measure of the readiness to begin using pain-coping strategies, can be effectively used to test the Motivational Model of Pain Self-Management. In addition to exercise and task persistence (tested in this study), the Multidimensional Pain Readiness to Change Questionnaire 2 measures readiness to adopt an additional seven pain self-management strategies—relaxation, cognitive control, avoiding asking for assistance, pacing, avoiding rest, assertiveness, and body mechanics. To more fully understand the proposed motivational model, especially for outcomes that are cognitive or social in nature, the Multidimensional Pain Readiness to Change Questionnaire 2 could be used in future studies to test the Motivational Model for Pain Self-Management for these other pain self-management outcomes.
Although results of this study contribute to a growing literature highlighting the importance of motivational factors in pain management, several limitations are worth noting. Most notably, the cross-sectional design of this study and retrospective recall of self-management behaviors severely limit our ability to draw causal conclusions from our findings and therefore to determine that self-efficacy and importance cause readiness, which then causes behavior. It is possible, for example, that those who reported greater exercise and task persistence endorsed higher ratings of importance, self-efficacy, and readiness because they were already engaging in those behaviors. Our understanding of the causal chain of events that lead from cognitions about value and expectancy to pain self-management behaviors would benefit from future studies that utilize longitudinal and/or interventional study design.
Additional design limitations include the fact that we relied on a self-report survey to measure self-management behaviors that were rated retrospectively. To improve measurement reliability and validity, future studies should utilize direct observation of behavioral outcomes and/or real-time measurements of behaviors. Similarly, our use of single-item measures of self-efficacy and exercise and task persistence behaviors increases the risk of type II errors (not identifying an association as significant when in fact it exists in the population), due to limitations in their reliability. However, despite the potential for not finding significant relationships due to this limitation, significant associations were found, and the study hypotheses were supported. The items that measured duration of task persistence and exercise were additionally limited by the fact that these behaviors were measured in consecutive months. This means that someone who had exercised 11 of the past 12 months, but not in the past month would be rated as having fewer months of consecutive exercise than someone who just started and maintained an exercise program in the past month. Though it is likely that current self-management behaviors are highly related to current ratings of importance of and readiness and self-efficacy for those behaviors, it is also possible that previous levels of these behaviors are highly related to current beliefs and attitudes. It is recommended that in the future, researchers use multiple-item scales that measure more than just the consecutive duration of the behaviors (e.g., duration, frequency, and intensity of the behaviors over a given time period) when possible. The response rate of 42.3% in this study, although consistent with other cross-sectional survey studies in multiple sclerosis samples, may have introduced sampling bias. Individuals who were highly motivated or active at the time of recruitment may have been more likely to participate. We have no information on those who did not respond to the invitation to participate and, therefore, are unable to examine differences between responders and non-responders.
Our sample was also rather homogenous, consisting mainly of Caucasian women who reported relatively high levels of exercise and task persistence behaviors. This may limit the generalizability of our data to other populations with pain secondary to disability, including individuals with multiple sclerosis who are male, physically inactive/low functioning, and/or part of a minority group. Future studies should include more diverse samples made up of individuals, with a range of demographic and clinical characteristics. Also, we did not include a screen for cognitive function, and it is possible that cognitive impairment associated with multiple sclerosis lesions may have affected the ability of participants to comprehend and adequately respond to study questionnaires.
Finally, it is important to note that pain in multiple sclerosis likely comes from a number of sources, including those that are either directly related to multiple sclerosis or its treatment (e.g., multiple-sclerosis-associated neuropathic pain, pain secondary to muscle spasm), indirectly related (e.g., shoulder or low-back pain that develops as a result of long-term wheel chair use), or coincident (e.g., preexisting low-back pain). In this study, we simply asked about “current pain” and may have missed important information regarding the source and nature of the problem. Future work would benefit from a more nuanced approach that includes these distinctions.
Despite these limitations, however, this study adds to the literature that highlights the role of patient readiness to adopt coping behaviors. An individual’s value of and perceived ability to engage in adaptive pain-coping strategies predict his or her motivation to adopt or maintain these strategies; this motivation or readiness level, in turn, predicts actual self-management behavior. These findings also hold some positive news in that they suggest that many individuals with multiple sclerosis and chronic pain place high value on exercise and feel highly capable of engaging in an exercise program.
These findings also contain important clinical implications for the treatment of individuals with chronic pain. Average rates of exercise and task persistence were relatively high for the sample as a whole. However, the fact that nearly a quarter of the sample (22.3%) reported 0 months of exercise suggests that patient readiness to engage in self-management strategies seems a promising target for psychosocial interventions. Existing psychotherapeutic strategies can be used to target perceived importance of and self-efficacy for coping. For example, education-based treatment strategies can be used to inform individuals about the known potential for benefits of certain adaptive pain-coping approaches. In fact, a recent meta-analysis indicated that some education-based treatments to increase exercise were effective among chronically ill individuals [49]. Cognitive techniques can also be used to counteract pessimistic and self-defeating beliefs about the value of or ability to engage in a given pain-coping strategy. Various treatments delivered in group format offer the potential for improved self-efficacy by group members modeling positive change, providing social reinforcement for adaptive changes (i.e., operant conditioning) and teaching practical aspects of behavior change to each other.
One basic assumption of the model tested in this study, as with other models of pain self-management, is that engaging in behaviors that promote adaptive coping and refraining from other behaviors that thwart adaptive coping will ultimately result in optimal adaptation to living with chronic pain. Clinicians and patients with chronic pain alike may expect or hope that adaptive self-management behaviors will result in reduced pain intensity in addition to other benefits such as improved mental, social, and physical health. Because the main aim of this paper was to determine whether there was support for the Motivational Model of Pain Self-Management in individuals with multiple sclerosis for exercise and task persistence behaviors, markers of positive adaptation to chronic pain were not included in the model. However, correlational results do suggest that model variables are related to some markers of positive adjustment to chronic pain. Pain intensity was not significantly correlated with any model variables. But, model variables, including self-management behaviors, were related to less pain interference across multiple domains. This suggests that, for individuals with multiple sclerosis, factors related to self-management behaviors such as exercise and task persistence may play more of a role in reducing the impact of pain on a person’s ability to be an active participant in their life than on their perceived pain intensity per se. This hypothesis, however, needs to be further examined in future research.
Motivational interviewing strategies can also be used to help patients resolve ambivalence about changing behavior and to address perceived barriers to behavior change [39]. For example, one strategy used in motivational interviewing is to elicit and reinforce positive statements about one’s ability to engage in behavior change. These characteristics of motivational interviewing make it an especially promising strategy for improving readiness to engage in managing one’s own pain. A handful of clinical trials have examined the effects of motivational interviewing for medication adherence [50] and general healthy lifestyle changes [51] among individuals with multiple sclerosis. However, no study has examined the effects of motivational interviewing on pain self-management behaviors, and no treatment based on the Motivational Model of Pain Self-Management has been established. Findings from this study indicate that development and evaluation of treatments based on this theory-based model are warranted.
Acknowledgments
This research was supported by grant number P01 HD33988 from the National Institutes of Health, National Institute of Child Health and Human Development (National Center for Medical Rehabilitation Research). It was also supported in part by a grant from the National Multiple Sclerosis Society grant number MB 0008, awarded to Dawn Ehde, Ph.D.
The authors gratefully acknowledge the contributions of David Mackinnon, Ph.D., Arizona State University, who provided statistical guidance, and Kevin Gertz, Katherine Raichle, Travis Osborne, Amy Hoffman, Natalie Brown, Joseph Skala, Emily Phelps, Laura Nishimura, Lindsay Washington, Tyler Einheuser, Silvia Amtmann, and Eric Weitz, University of Washington Department of Rehabilitation Medicine, who assisted in data collection and database management. The primary findings of this paper were previously presented as a paper and poster at the 12th Annual Meeting of Rehabilitation Psychology, February 2010, Tucson, Arizona.
Footnotes
Conflict of Interest Statement The authors have no conflict of interest to disclose.
Contributor Information
Anna L. Kratz, Email: alkratz@uw.edu, Department of Rehabilitation Medicine, University of Washington, HMC BX359612, Seattle, WA 98104, USA.
Ivan R. Molton, Department of Rehabilitation Medicine, University of Washington, HMC BX359612, Seattle, WA 98104, USA.
Mark P. Jensen, Department of Rehabilitation Medicine, University of Washington, HMC BX359612, Seattle, WA 98104, USA.
Dawn M. Ehde, Department of Rehabilitation Medicine, University of Washington, HMC BX359612, Seattle, WA 98104, USA.
Warren R. Nielson, Department of Medicine, University of Western Ontario, London, ON, Canada, Department of Psychology, University of Western Ontario, London, ON, Canada, Beryl and Richard Ivey Rheumatology Day Programs, St. Joseph’s Health Care, London, ON, Canada.
References
- 1.Anderson DW, Ellenberg JH, Leventhal CM, et al. Revised estimate of the prevalence of multiple sclerosis in the United States. Ann Neurol. 1992;31:333–336. doi: 10.1002/ana.410310317. [DOI] [PubMed] [Google Scholar]
- 2.Reingold SC. Prevalence estimates for MS in the United States and evidence of an increasing trend for women. Neurology. 2002;59:294. doi: 10.1212/wnl.59.2.294. author reply 294–295. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: Systematic review and proposed classification. Pain. 2008;137:96–111. doi: 10.1016/j.pain.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12:629–638. doi: 10.1177/1352458506071346. [DOI] [PubMed] [Google Scholar]
- 5.Hadjimichael O, Kerns RD, Rizzo MA, Cutter G, Vollmer T. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain. 2007;127:35–41. doi: 10.1016/j.pain.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Khan F, Pallant J. Chronic pain in multiple sclerosis: Prevalence, characteristics, and impact on quality of life in an Australian community cohort. J Pain. 2007;8:614–623. doi: 10.1016/j.jpain.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Thompson AJ. Symptomatic treatment in multiple sclerosis. Curr Opin Neurol. 1998;11:305–309. doi: 10.1097/00019052-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Heckman-Stone C, Stone C. Pain management techniques used by patients with multiple sclerosis. J Pain. 2001;2:205–208. doi: 10.1054/jpai.2001.23133. [DOI] [PubMed] [Google Scholar]
- 9.Archibald CJ, McGrath PJ, Ritvo PG, et al. Pain prevalence, severity and impact in a clinic sample of multiple sclerosis patients. Pain. 1994;58:89–93. doi: 10.1016/0304-3959(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 10.Bradley L. Cognitive-behavioral therapy for chronic pain. In: Gatchel R, Turk DC, editors. Psychological Approaches to Pain Management: A Practitioner’s Handbook. New York: Guilford; 1996. [Google Scholar]
- 11.Loeser J, Turk DC. Multidisciplinary pain management. In: Loeser J, Batler S, Chapman C, Turk DC, editors. Bonica’s Management of Pain. Philadelphia: Lippincott Williams and Wilkins; 2003. [Google Scholar]
- 12.Novy DM, Nelson DV, Francis DJ, Turk DC. Perspectives of chronic pain: An evaluative comparison of restrictive and comprehensive models. Psychol Bull. 1995;118:238–247. doi: 10.1037/0033-2909.118.2.238. [DOI] [PubMed] [Google Scholar]
- 13.Hirsh AT, Kupper AE, Carter GT, Jensen MP. Psychosocial factors and adjustment to pain in individuals with postpolio syndrome. Am J Phys Med Rehabil. 2010;89:213–224. doi: 10.1097/PHM.0b013e3181c9f9a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raichle KA, Hanley M, Jensen MP, Cardenas DD. Cognitions, coping, and social environment predict adjustment to pain in spinal cord injury. J Pain. 2007;8:718–729. doi: 10.1016/j.jpain.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osborne TL, Jensen MP, Ehde DM, Hanley MA, Kraft G. Psychosocial factors associated with pain intensity, pain-related interference, and psychological functioning in persons with multiple sclerosis and pain. Pain. 2007;127:52–62. doi: 10.1016/j.pain.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: A meta-analysis. Mult Scler. 2005;11:459–463. doi: 10.1191/1352458505ms1188oa. [DOI] [PubMed] [Google Scholar]
- 17.Motl RW, Snook EM, McAuley E, Scott JA, Douglass ML. Correlates of physical activity among individuals with multiple sclerosis. Ann Behav Med. 2006;32:154–161. doi: 10.1207/s15324796abm3202_13. [DOI] [PubMed] [Google Scholar]
- 18.Barrett CL, Mann GE, Taylor PN, Strike P. A randomized trial to investigate the effects of functional electrical stimulation and therapeutic exercise on walking performance for people with multiple sclerosis. Mult Scler. 2009;15:493–504. doi: 10.1177/1352458508101320. [DOI] [PubMed] [Google Scholar]
- 19.Motl RW, Gosney JL. Effect of exercise training on quality of life in multiple sclerosis: A meta-analysis. Mult Scler. 2008;14:129–135. doi: 10.1177/1352458507080464. [DOI] [PubMed] [Google Scholar]
- 20.Snook EM, Motl RW. Effect of exercise training on walking mobility in multiple sclerosis: A meta-analysis. Neurorehabil Neural Repair. 2009;23:108–116. doi: 10.1177/1545968308320641. [DOI] [PubMed] [Google Scholar]
- 21.Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: A longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87:935–943. doi: 10.1016/j.apmr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: Looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90:420–428. doi: 10.1016/j.apmr.2008.09.558. [DOI] [PubMed] [Google Scholar]
- 23.Motl RW, McAuley E. Pathways between physical activity and quality of life in adults with multiple sclerosis. Health Psychol. 2009;28:682–689. doi: 10.1037/a0015985. [DOI] [PubMed] [Google Scholar]
- 24.Motl RW, McAuley E. Longitudinal analysis of physical activity and symptoms as predictors of change in functional limitations and disability in multiple sclerosis. Rehabil Psychol. 2009;54:204–210. doi: 10.1037/a0015770. [DOI] [PubMed] [Google Scholar]
- 25.Biller N, Arnstein P, Caudill MA, Federman CW, Guberman C. Predicting completion of a cognitive-behavioral pain management program by initial measures of a chronic pain patient’ s readiness for change. Clin J Pain. 2000;16:352–359. doi: 10.1097/00002508-200012000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Heapy A, Otis J, Marcus KS, et al. Intersession coping skill practice mediates the relationship between readiness for self-management treatment and goal accomplishment. Pain. 2005;118:360–368. doi: 10.1016/j.pain.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Kerns RD, Rosenberg R. Predicting responses to self-management treatments for chronic pain: application of the pain stages of change model. Pain. 2000;84:49–55. doi: 10.1016/S0304-3959(99)00184-0. [DOI] [PubMed] [Google Scholar]
- 28.Williams DA. Psychological and behavioural therapies in fibro-myalgia and related syndromes. Best Pract Res Clin Rheumatol. 2003;17:649–665. doi: 10.1016/s1521-6942(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 29.Jensen MP, Nielson WR, Kerns RD. Toward the development of a motivational model of pain self-management. J Pain. 2003;4:477–492. doi: 10.1016/s1526-5900(03)00779-x. [DOI] [PubMed] [Google Scholar]
- 30.Molton IR, Jensen MP, Nielson W, Cardenas D, Ehde DM. A preliminary evaluation of the motivational model of pain self-management in persons with spinal cord injury-related pain. J Pain. 2008;9:606–612. doi: 10.1016/j.jpain.2008.01.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molton IR, Jensen MP, Nielson W, Cardenas D, Ehde DM. A preliminary evaluation of the motivational model of pain self-management in persons with spinal cord injury related pain. Journal of Pain. 2010;9:606–612. doi: 10.1016/j.jpain.2008.01.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 33.Huber A, Suman AL, Rendo CA, et al. Dimensions of “unidimensional” ratings of pain and emotions in patients with chronic musculoskeletal pain. Pain. 2007;130:216–224. doi: 10.1016/j.pain.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman DL, Sadosky A, Dukes EM, Alvir J. How do changes in pain severity levels correspond to changes in health status and function in patients with painful diabetic peripheral neuropathy? Pain. 2010;149:194–201. doi: 10.1016/j.pain.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Jensen MP, Smith DG, Ehde DM, Robinsin LR. Pain site and the effects of amputation pain: Further clarification of the meaning of mild, moderate, and severe pain. Pain. 2001;91:317–322. doi: 10.1016/S0304-3959(00)00459-0. [DOI] [PubMed] [Google Scholar]
- 36.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 37.Zelman DC, Hoffman DL, Seifeldin R, Dukes EM. Development of a metric for a day of manageable pain control: Derivation of pain severity cut-points for low back pain and osteoarthritis. Pain. 2003;106:35–42. doi: 10.1016/s0304-3959(03)00274-4. [DOI] [PubMed] [Google Scholar]
- 38.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 39.Nielson WR, Jensen MP, Ehde DM, Kerns RD, Molton IR. Further development of the multidimensional pain readiness to change questionnaire: The MPRCQ2. J Pain. 2008;9:552–565. doi: 10.1016/j.jpain.2008.01.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen MP, Turner JA, Romano JM, Nielson WR. Chronic Pain Coping Inventory: Professional Manual. Lutz: Psychological Assessment Resources; 2008. [Google Scholar]
- 41.Curran PJ, West SG, Finch JF. The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychol Meth. 1996;1:16–29. [Google Scholar]
- 42.Muthen LK, Muthen BO. MPlus User’s Guide. 5. Los Angeles: Muthen & Muthen; 1998–2007. [Google Scholar]
- 43.Sobel M. Asymptotic confidence intervals for indirect effects in structural equation models. Socio Meth. 1982;13:290–312. [Google Scholar]
- 44.Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs: Prentice Hall; 1986. [Google Scholar]
- 45.Janz NK, Becker MH. The Health Belief Model: A decade later. Health Educ Q. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 46.Rogers R. A protection motivation theory of fear appraisals and attitude change. J Psychol. 1975;91:93–114. doi: 10.1080/00223980.1975.9915803. [DOI] [PubMed] [Google Scholar]
- 47.Rogers R. Cognitive and physiological processes in fear-based attitude change: A revised theory of protection motivation. In: Cacioppo J, Petty R, editors. Social Psychophysiology: A Sourcebook. New York: Guilford; 1983. pp. 153–176. [Google Scholar]
- 48.Miller W, Rollnick S. Motivational Interviewing: Preparing People to Change. 2. New York: Guilford; 2002. [Google Scholar]
- 49.Conn VS, Hafdahl AR, Brown SA, Brown LM. Meta-analysis of patient education interventions to increase physical activity among chronically ill adults. Patient Educ Couns. 2008;70:157–172. doi: 10.1016/j.pec.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berger BA, Liang H, Hudmon KS. Evaluation of software-based telephone counseling to enhance medication persistency among patients with multiple sclerosis. J Am Pharm Assoc (2003) 2005;45:466–472. doi: 10.1331/1544345054475469. [DOI] [PubMed] [Google Scholar]
- 51.Bombardier CH, Cunniffe M, Wadhwani R, et al. The efficacy of telephone counseling for health promotion in people with multiple sclerosis: A randomized controlled trial. Arch Phys Med Rehabil. 2008;89:1849–1856. doi: 10.1016/j.apmr.2008.03.021. [DOI] [PubMed] [Google Scholar]