Abstract
We conducted extensive surveillance for West Nile virus infection in equines and chickens in Guadeloupe in 2003–2004. We showed a high seroprevalence in equines in 2003 related to biome, followed by a major decrease in virus circulation in 2004. No human or equine cases were reported during the study.
Keywords: West Nile Virus, public health, flavivirus, equine, avian, guadeloupe, ELISA, epidemiology, caribbean
The recent introduction of West Nile virus (WNV, family Flaviviridae, genus Flavivirus) into the Caribbean region is a major public health concern, particularly because transmission of this virus probably occurs year-round in the neotropics. Since 2002, WNV activity has been detected in Guadeloupe (1), Mexico (2,3), the Dominican Republic (4), and Jamaica (5). The objectives of this study were to determine the spatial distribution of WNV in Guadeloupe, establish a surveillance system in humans and animals to detect clinical cases, and increase our understanding of WNV epidemiology in the neotropics.
The Study
The investigation was conducted in the Guadeloupe archipelago, which includes Guadeloupe (the main island), Marie Galante, Saint Martin, and Saint Barthelemy. Surveys of domestic birds (chickens) were performed in July 2003 and 2004 on 25 to 27 farms selected to cover the whole island; each farm contained 15–20,000 chickens from 1 month to 2 years of age. Exhaustive surveys were also conducted on equines in July 2003 and August 2004 (46 survey locations, 1–68 equines each, mean 10 equines).
Epitope-blocking enzyme-linked immunosorbent assays (ELISA) were performed by using the WNV-specific monoclonal antibody 3.1112G and flavivirus-specific monoclonal antibody 6B6C-1 (Chemicon, Temecula, CA, USA) as previously described (6,7). The ability of the test sera to block the binding of the monoclonal antibodies to WNV antigen was compared to the blocking ability of horse or chicken serum without antibody to WNV. An inhibition value >30% was considered to indicate the presence of viral antibodies. Plaque reduction neutralization tests (PRNTs) were performed as described previously (3) on serum samples that had antibodies to flaviviruses. PRNTs were performed with WNV and St. Louis encephalitis virus (SLEV, family Flaviviridae, genus Flavivirus). A serum sample was considered to have antibodies to WNV if it significantly inhibited the binding of monoclonal antibody 3.1112G by blocking enzyme-linked immunosorbent assay (ELISA) and had a 90% plaque reduction (PRNT90) titer to WNV that was at least 4-fold greater than the corresponding SLEV PRNT90 titer. A serum sample was considered to have antibodies to SLEV if it inhibited the binding of monoclonal antibody 6B6C-1 and had a PRNT90 titer to SLEV that was at least 4-fold greater than the corresponding WNV titer. A serum sample was considered to have antibodies to a flavivirus of undetermined origin if it contained epitope-blocking ELISA or neutralizing antibodies but did not meet the criteria for a WNV or SLEV infection.
Out of 487 equines (437 horses, 34 donkeys, 16 ponies) sampled in July 2003, 94 (19.3%) had antibodies to WNV, and 10 (2.1%) had antibodies to a flavivirus of undetermined origin (Table 1). In August 2004, of 431 equines (386 horses, 27 donkeys, 18 ponies), 70 (16.2%) had antibodies to WNV, and 11 (2.6%) had antibodies to a flavivirus of undetermined origin. WNV PRNT90 titers were between 1:20 and 1:1,280 (mean 1:260).
Table 1. West Nile virus (WNV) seroprevalence in equines and chickens in Guadeloupe, 2002–2004.
| Sample | Equines, n (%) |
Chickens, n (%) |
||||
|---|---|---|---|---|---|---|
| July 2002* | July 2003 | August 2004 | December 2002* | July 2003 | July 2004 | |
| WNV positive | 10 (2.8) | 94 (19.3) | 70 (16.2) | 11 (52.4) | 11 (1.7) | 5 (0.6) |
| Unknown flavivirus positive |
0 |
10 (2.1) |
11 (2.6) |
0 |
1 (0.2) |
2 (0.2) |
| Total tested | 360 | 487 | 431 | 21 | 656 | 801 |
*Data from the serosurveys conducted in 2002 have been presented elsewhere (1).
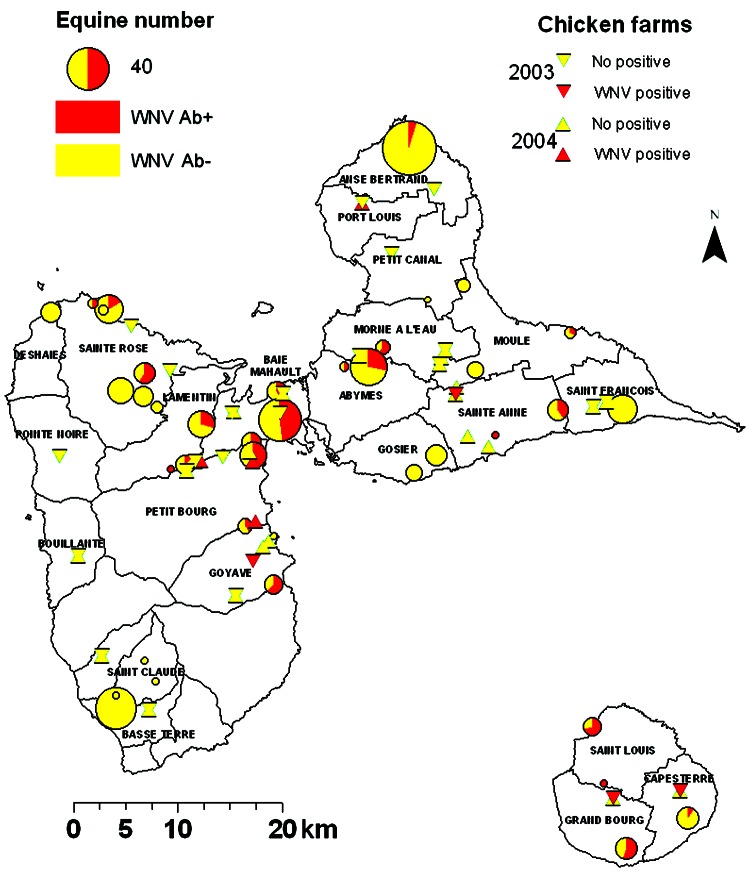
In 2003, WNV seroprevalence in the 46 equine centers was highly heterogeneous (0%–100%, chi-square test p<0.001, Figure 1). This heterogeneity was also found by county (0%–71%, p<0.001) and by island (p<0.001, Table 2).
Figure 1.
Results of West Nile virus (WNV) serosurveys in chickens and equines in Guadeloupe, 2003–2004. Equine centers are represented by circles, the sizes of which are proportional to the numbers of equines. The proportion of WNV-seropositive animals is represented in red. Chicken farms are represented by triangles (pointing down for 2003 survey, pointing up for 2004 survey). Red triangles denote farms where at least 1 seropositive chicken was identified. All other farms are denoted by yellow triangles. Ab, antibody.
Table 2. West Nile virus (WNV) seroprevalence in equines by island, July 2003.
| Island | WNV antibody positive, n (%) | No. tested |
|---|---|---|
| Guadeloupe main island | 80 (19.6) | 409 |
| Marie Galante | 13 (43.3) | 30 |
| Saint Martin | 1 (2.9) | 34 |
| Saint Barthelemy | 0 | 14 |
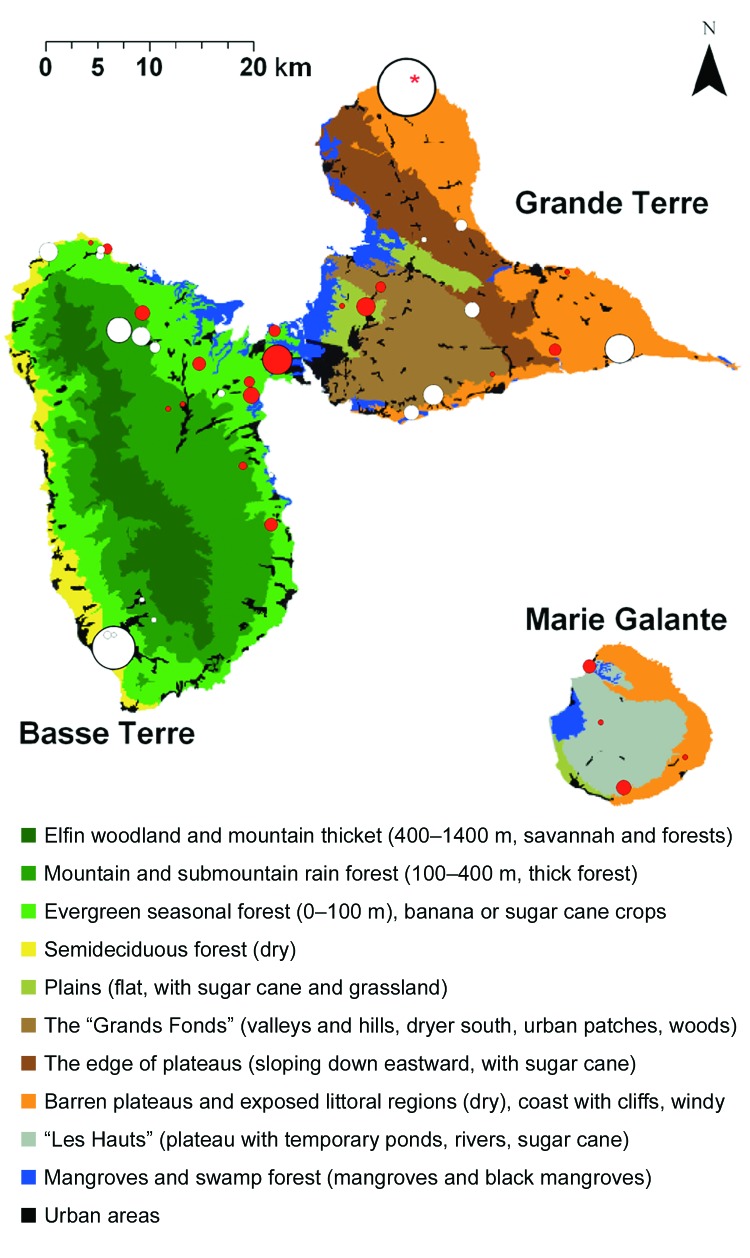
Figure 2 shows each equine center in relation to the ecologic area. Most WNV seropositive equines were located in evergreen forests characterized by a low altitude (≤100 m) and intensive farming of sugar cane in the vicinity of mangroves (Rhizophora mangle, Avicennia germinans, Laguncularia racemosa, Conocarpus erectus), back mangroves (marshy forests with Pterocarpus spp.), and swamps. Within Grande Terre, seropositive equines were mostly identified in the humid plains of Abymes or near small mangrove areas. In Marie Galante, nearly all seropositive equines were located near swamps, temporary ponds, or rivers. Conversely, no seropositive equines were found in the semideciduous forest (dry leeward coast of Basse Terre) and very few in the dry regions of Grande Terre.
Figure 2.
Ecologic map of Guadeloupe and West Nile virus (WNV)-positive equine centers. Basse Terre (southwest) is mainly mountainous (volcanic, highest point 1,467 m) and wet. Grande Terre (northeast) is flat (mainly <100 m) and dry. Marie Galante is flat (plateaus <200 m) but has more water than Grande Terre. The ecologic map was derived from "Carte écologique de la Guadeloupe," created by Alain Rousteau, University Antilles-Guyane. Equine centers with WNV-seropositive equines are represented by red circles (the size of each circle is proportional to the number of seropositive equines). Centers without WNV-seropositive equines are represented by white circles (the size of each circle is proportional to the number of equines tested). The red asterisk shows a site that contained 3 seropositive equines. All were race horses that travel frequently and thus may have been infected elsewhere.
Sixty-two equines that were WNV seronegative in December 2002 (1) were retested in July 2003, and all remained seronegative. Because of changes in the whole population (e.g., death, exportation), only 328 equines were tested in both July 2003 and August 2004. Of the 257 seronegative equines identified in 2003, 256 were seronegative in 2004, and 1 had been infected with an undetermined flavivirus. Thus, no WNV seroconversions were observed in equines between 2003 and 2004. Conversely, all the WNV-seropositive equines in 2003 were still positive in 2004.
In July 2003, of 656 chickens, 11 (1.7%) had antibodies to WNV, and 1 had antibodies to an undetermined flavivirus. Three (0.5%) WNV-seropositive chickens were found on the main island of Guadeloupe and 8 (14.8%) on Marie Galante (Figure 1). In July 2004, of 801 chickens, 5 (0.6%) had antibodies to WNV, and 2 had antibodies to an undetermined flavivirus. All seropositive chickens were on the main island of Guadeloupe (Figure 1). WNV PRNT90 titers were between 1:20 and 1:1,280 (mean 1:519).
A surveillance system was established in equines, domestic and wild birds, and humans. Equine surveillance was performed by veterinarians. Awareness of WNV was increased in the public, hunters, and horse and poultry owners with educational leaflets and with the help of veterinarians and natural reserve wardens. In 2003 and 2004, veterinarians reported 4 horses that exhibited signs of WNV-like illness (ataxia, weakness of limbs); the horses were tested at the onset of clinical signs and 15 days later. All were negative for WNV by epitope-blocking ELISA and PRNT, and other causes were eventually determined. Clinical signs were not observed in any equines with antibodies to WNV. Abnormal death and paralysis were reported in domestic chickens in 5 farms. In these farms, serum specimens from 23 chickens were analyzed for evidence of WNV infection, and 1 chicken was seropositive.
Human surveillance was performed by the public hospitals of Guadeloupe. In humans, suspected cases were defined as neurologic symptoms of encephalitis or meningoencephalitis or acute neurologic symptoms with fever. In 2003, the hospital reported 9 suspected cases of clinical WNV infection in Guadeloupe (Cecile Herrmann-Storck, pers. comm.). Antibodies to WNV were not detected in the sera of any of these patients, and another cause was determined.
Conclusions
A high seroprevalence (19.3%) for WNV was detected in equines in 2003. Seropositive equines and chickens were commonly found near mangroves, which contain many species of wild birds and mosquitoes. Culex nigripalpus and Ochlerotatus taeniorhynchus are common in Guadeloupe and are particularly abundant in mangrove areas. Culex species are the major amplification vectors of WNV in the United States (8,9) and may also be vectors of WNV in Guadeloupe.
Results of the equine and avian serosurveys suggest that transmission of WNV decreased dramatically in 2003 and 2004 in comparison to 2002. No equine seroconversion occurred between January 2003 and August 2004, and seroprevalence in chickens was low in 2003 (1.7%) and 2004 (0.6%). In comparison, 10 of 21 chickens in Goyave were seropositive for WNV in 2002, although the sample size was small (1). In the tropics, where temperature is favorable year-round, changes in rainfall can substantially affect the size of vector populations (10). Cx. nigripalpus and Oc. taeniorhynchus need heavy rains or changes in water level to develop (11). Therefore, the 7-month drought (half of the usual rainfall) observed between November 2002 and May 2003 was probably responsible for a major decrease in the mosquito population. If Cx. nigripalpus is involved in WNV transmission in Guadeloupe, a decrease in its population could explain reduced virus circulation. Alternatively, the number of nonimmune resident birds may have decreased.
No dead wild bird was reported to the veterinary services in Guadeloupe in 2003 or 2004, although dead bird carcasses are presumably difficult to find in areas with dense vegetation. No abnormal death rate was noted in anthropophilic birds either.
Although an efficient passive surveillance system was implemented in humans and equines, no clinical cases of WNV infection were observed. This situation is considerably different from that observed in the United States but mimics the situation in Mexico, where only a few human and equine cases were observed, despite a seroprevalence of 29% in equines in 2004 (12). Cross-protection conferred by other flaviviruses could explain the difference with the North American situation. In fact, we found some equines that were considered to have been infected with an unknown flavivirus. Alternatively, virus mutations could explain a change in virulence and the absence of clinical cases. Indeed, recent studies identified attenuated WNV in Texas (13) and southern Mexico (14). Isolation and characterization of WNV in Guadeloupe would help clarify these issues.
Acknowledgments
We thank Rosalie Aprelon, Carène Pagesy, and Valérie Pinarello for technical assistance; the veterinarians of Guadeloupe for equine serum sampling; the Antilles-Guyane Cellule Inter-Regionale d'Epidémiologie and Direction de la Santé et du Développement Social for their active collaboration on WNV surveillance; and Barry Beaty for helpful advice.
PRNT diagnostic testing was made possible by grant U50 CCU820510 from the Centers for Disease Control and Prevention.
Biography
Dr. Lefrançois is a veterinarian and member of the Control of Exotic and Emerging Animal Diseases research unit at the Centre de Coopération Internationale en Recherche Agronomique pour le Développement–Département Délevage et Médecine Vétérinaire research center in Guadeloupe. His research focuses on diagnosis and surveillance of several animal diseases in the Caribbean.
Footnotes
Suggested citation for this article: Lefrançois T, Blitvich BJ, Pradel J, Molia S, Vachiéry N, Pallavicini G, et al. West Nile virus surveillance, Guadeloupe, 2003–2004. Emerg Infect Dis [serial on the Internet]. 2005 Jul [date cited]. http://dx.doi.org/10.3201/eid1107.050105
References
- 1.Quirin R, Salas M, Zientara S, Zeller H, Labie J, Murri S, et al. West Nile virus, Guadeloupe. Emerg Infect Dis. 2004;10:706–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blitvich BJ, Fernandez-Salas I, Contreras-Cordero JF, Marlenee NL, Gonzalez-Rojas JI, Komar N, et al. Serologic evidence of West Nile virus infection in horses, Coahuila State, Mexico. Emerg Infect Dis. 2003;9:853–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Salas I, Contreras-Cordero JF, Blitvich BJ, Gonzalez-Rojas JI, Cavazos-Alvarez A, Marlenee NL, et al. Serologic evidence of West Nile virus infection in birds, Tamaulipas State, Mexico. Vector Borne Zoonotic Dis. 2003;3:209–13. 10.1089/153036603322662192 [DOI] [PubMed] [Google Scholar]
- 4.Komar O, Robbins MB, Klenk K, Blitvich BJ, Marlenee NL, Burkhalter KL, et al. West Nile virus transmission in resident birds, Dominican Republic. Emerg Infect Dis. 2003;9:1299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupuis AP II, Marra PP, Kramer LD. Serologic evidence of West Nile virus transmission, Jamaica, West Indies. Emerg Infect Dis. 2003;9:860–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blitvich BJ, Bowen RA, Marlenee NL, Hall RA, Bunning ML, Beaty BJ. Epitope-blocking enzyme-linked immunosorbent assays for detection of West Nile virus antibodies in domestic mammals. J Clin Microbiol. 2003;41:2676–9. 10.1128/JCM.41.6.2676-2679.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blitvich BJ, Marlenee NL, Hall RA, Calisher CH, Bowen RA, Roehrig JT, et al. Epitope-blocking enzyme-linked immunosorbent assays for the detection of serum antibodies to West Nile virus in multiple avian species. J Clin Microbiol. 2003;41:1041–7. 10.1128/JCM.41.3.1041-1047.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould LH, Fikrig E. West Nile virus: a growing concern? J Clin Invest. 2004;113:1102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis. 2002;8:1385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiter P. Weather, vector biology, and arboviral recrudescence. In: Monath TP. The arboviruses: epidemiology and ecology. Volume 1. Boca Raton (FL): CRC Press. 1988. p. 245–55. [Google Scholar]
- 11.Day JF, Curtis GA. When it rains, they soar—and that makes Culex nigripalpus a dangerous mosquito. American Entomologist. 1994;40:162–7. [Google Scholar]
- 12.Centro Nacional de Vigilancia Epidemiológica. Diagnóstico de laboratorio para VON hasta el 9 de diciembre de 2004. [database on the Internet]. [cited 2005 May 13]. Available from http://www.cenave.gob.mx/von/archivos/ResumenCASOSVON.xls
- 13.Davis CT, Beasley DWC, Guzman H, Siirin M, Parsons RE, Tesh RB, et al. Emergence of attenuated West Nile virus variants in Texas, 2003. Virology. 2004;330:342–50. 10.1016/j.virol.2004.09.016 [DOI] [PubMed] [Google Scholar]
- 14.Beasley DWC, Davis CT, Estrada-Franco J, Navarro-Lopez R, Campomanes-Cortes A, Tesh RB, et al. Genome sequence and attenuating mutations in West Nile virus isolate from Mexico. Emerg Infect Dis. 2004;10:2221–4. [DOI] [PMC free article] [PubMed] [Google Scholar]