Abstract
Background
Trichomoniasis vaginalis is now an important worldwide health problem. Metronidazole has so far been used in treatment, but the metronidazole-resistant strains and unpleasant adverse effects have been de-veloped. Myrrh is one of the oldest known medicinal plants used by the ancient Egyptians for medical purposes and for mummification. Commiphora molmol (Myrrh) proved safe for male reproductive organ which is the main habitat of T. vaginalis and this study aims to evaluate the efficacy of the herbal against T. vaginalis in females.
Methods
In the present study, 33 metronidazole-resistant T. vaginalis females were treated with a combined course of metronidazole and tinidazole. Those still resistant to the combined treatment were given C. molmol. Also, natural plant extract purified from pomegranate (Punica granatum, Roman) was in-vitro investigated for its efficacy against T. vaginalis on Diamond media.
Results
The anti-T. vaginalis activity of both P. granatum (in-vitro) and C. molmol (in-vivo) extracts gave promis-ing results.
Conclusion
The anti-T. vaginalis activity of P. granatum and C. molmol showed promising results indicating to sources of new anti-Ttrichomonas agents.
Keywords: Punica granatum, Commiphora molmol, Trichomonas vaginalis, Trichomoniasis, Treatment
Introduction
Although Trichomonas vaginalis was first described by Donne' in 1836, research on this organism did not begin until the 20th century. The research has been a progression of phases throughout the last 60 years and has gone from developing axenic culture and defining nutritional requirements to finding an effective treatment.[1] It was considered either a harmless vaginal colonizer or simply a minor nuisance.[2] Trichomoniasis was accounted to about half of all the curable sexually transmitted diseases worldwide.[3]The incidence of this sexually transmitted parasite has reached the epidemic levels in many countries.[4] Also, T. vaginalis survived in swimming pool, where human may acquire infection.[5]In USA, annual inci-dence of T. vaginalis reached 5 millions.[6] The general annual adult infection was 180-200 millions and being higher than that of gonorrhea, syphilis, and Chlamydia infections all together.[7] In many Arab countries, trichomoniasis was reported including Jordan,[8]Iraq,[9] Egypt,[10] Saudi Arabia,[11] Libya,[12] and Tunisia.[13] The wide diversion in subtypes of T. vaginalis isolates caused different clinical symptoms with diversity of innate immune responses.[14] The infection was always associated with other sexually-transmitted diseases (STDs) and a sensitive marker for high risk sexual behaviour.[15] T. vaginalis in males caused non-gonococcal urethritis,[16] but with serious complica-tions.[17] Also, T. vaginalis adherence was shown to mediate different gene expressions in human epithelial cells.[18] The premature rupture of membranes, lowbirth weight, preterm labor,[19] female infertility,[20] and postpartum infection, even in asymptomatic women were associated with trichomoniasis.[21] T. vaginalis is a factor in genesis and cause of cervical neoplasia,[22]
and progression of cervical carcinoma,[23] also phago-cytes sperm cells.[24] Unlike other STDs, T. vaginalis rate was more prevalent among women of all ages,[25] and half of them were asymptomatic[26] since trichomoniasis was a curable infection by a single dose metronidazole,[27] successful control of STDs was aided by sensitive, simple and rapid test(s). No doubt, treating patients lowered the overall disease prevalence and morbidity.[28] Metronidazole has so far been the most widely used drug for treating T. vaginalis,[29]but, metronidazole can lead to drug resistance and potential risks of mutagenesis and carcinogenicity.[30] In addition, its side effects such as headache, dry mouth, glossitis, and urticaria caused by lenity treatment or high doses have been described.[31] But, at least 5% of clinical trichomoniasis is caused by strains resistant to commonly used drugs.[32] Also, Hussien et al.[32] reported the presence of different strains of T. vaginalis. The lack of approved alternative therapies for T. vaginalis treatment means that higher and sometimes toxic doses of metronidazole were used.[33] Tinidazole (Fasigyn), a second-nitronidazole generation has shown to be an effective therapy in metronidazole-resistant T. vaginalis, with several advantages over metronidazole including greater in vitro potency against both sensitive and resistant strains of T. vaginalis, a more prolonged duration and improved patient tolerability.[34] Cross-resistance among mitronidazole doses occurred, and thus metronidazole resistant strain was treated with tinidazole but rapid development of tinidazole-resistant T. vaginalis due to the similarities of metabolic pathway of both.[4]
According to world health organization (WHO), more than 80% of the world's population relies on traditional medicine for their primary healthcare needs. Use of herbal medicines represents a long history of human interactions with the environment. Plants used for traditional medicine contain a wide range of substances that can be used to treat chronic as well as infectious diseases.35 The medical value of plants lies in some chemical substances that produce a definite physiological action on the human body. The most important of these bioactive compounds of plants are alkaloids, flavanoids, tannins, and phenolic compounds.36
Natural products are not only the basis for traditional or ethnic medicine, but also screening natural plant products provided highly successful new regimens for human welfare.37 Many new natural product groups have revealed anti-parasitic properties of surprising efficacy and selectivity.38 In the present study, Mirazid was given to metronidazole and tinidazole resistant T. vaginalis infected women. Also, the efficacy of Punica granatum extract against cultured T. vaginalis was evaluated.
Materials and Methods
The institutional review board of hospital approved this study. The study was registered at the Ministry of Scientific Research Academy of Scientific Research and Technology (292473).
We informed women to allow for an attrition rate (i.e. women who discontinue participation in the study entirely, including failure to complete all follow-up). Thus, [33] women were available to be studied.
The patients were recruited from hospitals of Cairo Curative Organization of Egypt. Potentially eligible patients were identified through hospitals registries for a 3-years retrospective inclusion, and through treating physicians, for the 2 years prospective inclusion (Patient who met the case definition of metronidazole resistant vaginal trichomoniasis were selected). A letter was sent for them while had been signed by their physician informing them of the study protocol and, if initially interested, asking them to complete a brief questionnaire to screen for trichomoniasis symptoms. Women who were assigned into control group were given the choice of undergoing the study program.
Metronidazole- resistant trichomoniasis was defined clinically as failure to respond to conventional therapy with oral metronidazole, 500 mg for 7 days (Total dose, 7 g). Patients in whom treatment failed and for whom re-infection from a sexual partner was a possibility were excluded from the study. "Failure to respond" was defined as persistence or recurrence (with 28 days) of symptoms and signs of vaginitis together with the following confirmatory laboratory feature of vaginal trichomoniasis: high vaginal pH, increased numbers of polymorphonclear leukocytes, and a visualization of motile trichomonads using microscopy. In vitro Trichomonas cultures of individual specimens were performed using Dimoned's medium Modified (Remel), and they were examined at 24 hr and 48 h for the presence of motile trichomonads.
Two vaginal swabs were obtained from the childbearing period trichomoniasis infected women by sterile vaginal swab. The first swab was obtained from the lateral wall of vagina and was used to make
a wet mount preparation on a glass slide with a drop of normal saline and looking for motile trichomonads.39 The second swab was obtained from the posterior fornix of the vagina and inoculated immediately after collection in Diamond media at 32oC and examined for motile trichomonades at 24, 48, and 96 hr of incubation.[40]The efficacy of metronidazole and tindazole was evaluated in 33 patients. Both drugs were given orally in a single dose of 2 g for a minimum of 3 days or extended as indicated. A combination of vaginal metronidazole and oral tinidazole was tried. The effectiveness of an oleo-resin extract derived from Myrrh, Commiphora molmol (Mirazid) was given to the metronidazole and tinidazole resistant females as two capsules (600 mg) for six to eight successive days on an empty stomach two hours before breakfast. All patients were seen immediately after treatment completion and again 4 to 6 week later. Patients were considered cured if all symptoms and signs of vaginal trichomoniasis resolved with therapy and the patients had negative results of microscopy at least 4 weeks after completion of therapy. In the majority of patients, a follow- up culture was performed. Patients were also instructed to notify investigators if symptoms returned after the final follow-up visit. Follow up was done both clinically and parasitologically by the examination of vaginal discharge as wet mount smear and culture on Diamond media.
Punica granatum fruits were selected from tree that was neither treated with any insecticide nor with plant fertilizer. The fruits were carefully washed with sterile distilled water. The peels of pomegranate fruits were manually removed, sun dried and powdered. Powder was extracted with a Soxhlet extractor using methanol for 24 hr.[41] Extract was filtered through a Whatman filter, No. 41 filter paper, for removal of peel particles (Solvent was removed under gentle pressure in rotary evaporator till dryness, and residue was stored at 4oC).[42] Extract was tested at different concentrations, diluted with sterile normal saline against cultured T. vaginalis. Control culture lacked extract. All culture-media were incubated at 37oC and examined for living T. vaginalis (Figure 1 and Figure 2). The results are expressed as means and values were evaluated by the Chi Square test and p<0.5 was considered significant.
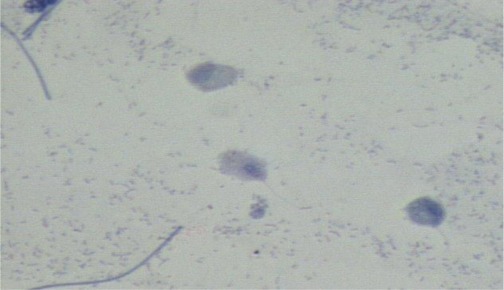
Figure 1.
Normal T. vaginalis stained by ZN
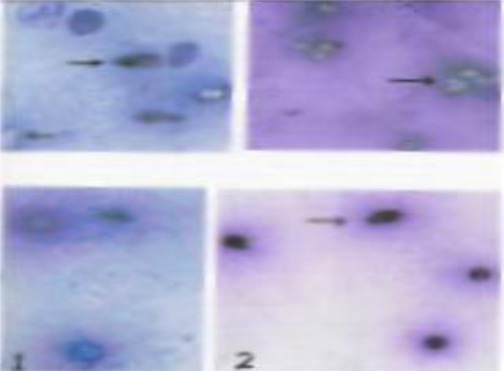
Figure 2.
ZN stained smear showing lethal effect of P. granatum on T. Vaginalis
Results
Review of medical records revealed 33 cases of met-ronidazole- resistant trichomoniasis seen during a 3 years period (2005-2008). The mean age of the female patient was 37.2 years (range= 25-58 years). Duration of vulvovaginal symptoms was from 4 months to 5 years. Seventy five percent of referred women had previously diagnosed resistant trichomoniasis, and 25% of the women were referred because of refractory vaginitis of unknown aetiology. Before referral, all patients had received and did not respond to multiple courses of oral metronidazole.
Fifty percent of patients (9/18) given oral tinidazole were cured and 73% of patients (11/15) given a combination of oral metronidazole and vaginal tinidazole were treated(Table 1) . Besides, This figure for patients who did not respond to combination of metronidazole and tinidazole oral or vaginal and were cured by Mirazid was 84.6% (11/13). In vitro susceptibility of isolates of T. vaginalis to P. granatum extract was determined. At pH=4.65, P. granatum showed a therapeutic effect against T. vaginalis, the organism was dead immediately in the
Table 1. Trichomoniasis cases treated by metronidazole and tinidazole.
| Drug used | Total | Cure | Not | %Cure |
| Tinidazole | 18 | 9 | 9 | 50 |
| Oral metronidazole + vaginal tinidazole | 15 | 11 | 4 | 73 |
| Total | 33 | 20 | 13 | 60.6 |
tube containing 50 mg and100 mg of the extract, and within 0.5 hour in the tube with 20 mg extract. At pH=6.00, however, P. granatum extract had no effect against the organism.
Discussion
There is a need for studies into other chemicals and/or medicinal plants or herbs for alternative regimens against T. vaginalis to be inexpensive, effective, safe to use and with short course of treatment. Globally, herbal remedies have been studied under rigorous controls and was technologically approved by authors in many countries.
Factors that predispose women to vulvovaginitis candidiasis and trichomoniasis are pregnancy, diabe-tes, HIV infection, higher dosage of oral antibiotics or corticosteroid use, immunosuppression, more patho-genic normal flora, and history of recurrent infection. Other factors that may increase the incidence include the use of perfumed feminine hygienic sprays, tropi-cal antimicrobial agents, and tight poorly ventilated clothing and under wear.[43]
Successful determination of biologically active compounds from plant material is largely dependant on the type of solvent used in the extraction proce-dure, properties of a good solvent in plant extraction that induces ease of evaporation at low heat, promo-tion of rapid physiologic absorption of the extract, a preservative action and inability to cause the extract to complex or dissociate.[44] The choice will also de-pend on targeted compounds. The most commonly used solvents for investigation of microbial activity in plants are methanol, ethanol, and water.[45] In this study methanol extract was used.
In the present study, P. granatum extract on T. vaginalis in Diamond media showed 100% efficacy in dilution up to 10%. On the other hand, extracts in the concentrations of 5%, 1% and 0.5% killed 40%, 25% and 10% of T. vaginalis respectively.
Metronidazole has a worldwide use within the last 2 years of its introduction, but the lack of surveillance data of vaginal trichomoniasis and clinical and mi-crobiological response to treatment, incidence of met-ronidazole resistance has spared. Lossick and Kent46 found that the high level resistance to metronidazole occurred in one out of 2000-3000 cases of vaginal trichomoniasis cases. Saurina et al.[47] studied the prevalence of in vitro metronidazole resistance among outpatients attended urban clinic, found that 3/118 (2.5%) of T. vaginalis isolates from 107 patients ex-hibited aerobic low level resistance. The development of drug resistance in human against commonly used treatments has necessitated a search for new anti-agent substances from other sources including plants.[48] Myrrh is an oleo-gum resin obtained from the stem of the herbal tree Commiphora molmol. It contains a resin (Myrrhin) which is a volatile oil (Myrrh), gum and a bitter principle.[49] Myrrh was used by Sumerians and Greeks to treat worms, stomach pain, flatulence particularly in children,[50] anti-inflammatory, anti-ulcer, anti-mutagenic, anti-cytotoxic, anti-carcinogenic,[51] and anti-diabetic prop-erties.[52] Also, C. myrrha and various other species of Commiphora are recognized to possess significant antiseptic, anesthetic, and anti-tumor properties.[53] Its safety and effectiveness was proved in the treatment of human schistosmoiasis,[54][55][56] fascioliasis of human,[57] and animals,[58] moniziasis,[59] strongyloidiasis,[60] heter-ophyiasis,[61] and both species of hymenolepiasis.[62] Also, myrrh has larvicidal action against larvae of both Culex pipiens and Aedes caspius,[54] molluscicidal action against Biomphalaria alexandrina, Bulinus truncatus and Lymnaea cailliaudi,[63] Bithynia connol-lyi, the snail vector of the trematod parasite Opisthor-chis sp.,[64] and Lymnaea natalensis.[65] C. molmol proved safe for male reproductive organs which is the main habitat of T. vaginalis.[66] Omar et al.[67] tested the safety of mirazid on adult male albino rats by assess-ment of serum levels of ALT, AST and bilirubin and histopathology of liver. They found a non-significant increase in these enzymes and bilirubin levels. Auffray[68] in France stated that essential oil of C. myrrha ha the best protection against squalene per oxidation, and that
sun care cosmetics should make use not only of free radical scavengers but also of singlet oxygen quenchers.
The Pomegranate fruit has been used for centuries in ancient cultures for its medicinal purposes. It is widely consumed fresh and in beverage forms as juice and wine.[69] Properties attributed to its high con-tent of polyphenols, including ellagic acid in its free and bound forms, and other flavouroids.[70] In the last two decades, many authors dealt with P. granatum (Pomegranate) as a medicinal plant.[71] It is a shrub or small tree which several parts have been used by old Indian physicians. Nowadays, parts of pomegranate are used as an astringent, anti-microbial hemostatic, anti-diabetes, anti-helminthes,[72] anti-prostate cancer,[73] improved anti-oxidant function in elderly subjects,[74] anti-fungal peptide,[75] and anti-Candida,[76] mouth-anti-T. Gingivalis,[77] as heart-healthy juice,[78] and preven-tion of the cardiovascular diseases.[79] Dried per carp was decocted with other herbs and used to treat colic, dysentery, leucorrhoea,[80] and as larvicide against my-iasis producing larvae of Lucilia sericata.[81] Also, the rind of fruit and flower, combined with aromatics, such as cloves, cinnamon, coriander, pepper etc as bowel astringent in the diarrhea.[82] It was used exter-nally in treatment of the vaginal discharge, mouth sores, and throat infections.[83] Methanol extracts of P. granatum fruit exhibited a higher degree of antimi-crobial activity.[84] The fruit was successfully used to treat dysentery, diarrhea and gastralgia.[85] Voraavu Thikunchai et al.[86] reported that P. granatum contains 25% tannins which made it an effective astringent. In old medicine, the pomegranate as a pharmacy unto itself was used as an anti-parasitic agent, a blood ton-ic and to heal apathies and ulcers.[87]
This study shows statistically significant effects on resistant T. vaginalis strains. These proposed benefits, however, are in assays that are as yet invalidated, and further research is needed to prove the validity of these tests. In conclusion, the results in the present study support the two safe plant extracts (Commiphora molmol and Punica granatum) proved to be valuable agents in treating of T. vaginalis infection, and will form the basis for further investigation in the potential discovery of new natural bioactive compounds.
Acknowledgments
We thank the Gynaecology Clinic staffs and all the participants who shared their time for working on this study.
Footnotes
Conflict of interest:: None declared.
References
- 1.Poch F, Levin D, Levin S, Dan M. Modified thioglycolate medium: a simple and reliable means for detec-tion of Trichomonas vaginalis. J Clin Microbiol. 1996;34:2630–1. doi: 10.1128/jcm.34.10.2630-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira-Neves A, Benchimol M. Trichomonas vaginalis: in vitro sur-vival in swimming pool water sam-ples. Exp Parasitol 2008. 118:438–41. doi: 10.1016/j.exppara.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Hook EW 3rd. Trichomonas vaginalis--no longer a minor STD. Sex Transm Dis 1999. 26:388–9. doi: 10.1097/00007435-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lewis DA, Habgood L, White R, Barker KF, Murphy SM. Managing vaginal trichomoniasis resistant to high-dose metronidazole therapy. Int J STD AIDS . 1997;8:780–4. doi: 10.1258/0956462971919110. [DOI] [PubMed] [Google Scholar]
- 5.Pereira-Neves A, Benchimol M. Trichomonas vaginalis: In vitro sur-vival in swimming pool water sam-ples. Exp Parasitol 2008. 118:438–41. doi: 10.1016/j.exppara.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: Incidence and prevalence estimates. Perspect Sex Reprod Health 2004. 36:6–10. doi: 10.1363/3600604. [DOI] [PubMed] [Google Scholar]
- 7.Schwebke JR, Burgess D. richomoniasis. Clin Microbiol Rev 2004. 17:794–803. doi: 10.1128/CMR.17.4.794-803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morsy TA, El Dasouki ST. A study on vaginal trichomoniasis in Am-man, Jordan. J Egypt Soc Parasitol 1979. 8:279–282. [Google Scholar]
- 9.Mahdi NK, Gany ZH, Sharief M. Risk factors for vaginal trichomoniasis among women in Basra, Iraq. East Mediterr Health J. 2001;7:918–24. [PubMed] [Google Scholar]
- 10.Negm AY, el-Haleem DA. Detection of trichomoniasis in vaginal specimens by both conventional and modern molecular tools. J Egypt Soc Parasitol 2004. 34:589–600. [PubMed] [Google Scholar]
- 11.Alzanbagi NA, Salem HS, Al Braiken F. Trichomoniasis among women with vaginal discharge in Jeddah city, Saudi Arabia. J Egypt Soc Parasitol 2005. 35:1071–80. [PubMed] [Google Scholar]
- 12.Kassem HH, Majoud OA. Trichomoniasis among women with vaginal discharge in Benghazi city, Libya. J Egypt Soc Parasitol 2006. 36:1007–16. [PubMed] [Google Scholar]
- 13.Zribi M, Mansour KB, Abid F, Masmoudi A, Fendri C. Syndromic approach in Tunisian women: Bacteriological validation. Int J STD AIDS 2008. 19:112–4. doi: 10.1258/ijsa.2007.007140. [DOI] [PubMed] [Google Scholar]
- 14.Hussien EM, El-Sayed HZ, El-Moamly AA, Helmy MM, Shaban MM. Molecular characterization of Egyptian Trichomonas vaginalis clinical isolates by HSP70 restriction fragment length polymorphism. J Egypt Soc Parasitol 2005. 35:699–710. [PubMed] [Google Scholar]
- 15.James JA, Thomason JL, Gelbart SM, Osypowski P, Kaiser P, Hanson L. Is trichomoniasis often associated with bacterial vaginosis in pregnant adolescents? Am J Obstet Gynecol . 1992;166:859–63. doi: 10.1016/0002-9378(92)91350-j. [DOI] [PubMed] [Google Scholar]
- 16.Wilson A, Ackers JP. Urine culture for the detection of Trichomonas vaginalis in men. Br J Vener Dis 1980. 56:46–8. doi: 10.1136/sti.56.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benchimol M, de Andrade Rosa I, da Silva Fontes R, Burla Dias AJ. Trichomonas vaginalis adhere and phogocytose sperm cells: Adhesion seems to be a prominent stage during interaction. Parasitol Res 2008. 102:597–604. doi: 10.1007/s00436-007-0793-3. [DOI] [PubMed] [Google Scholar]
- 18.Kucknoor A, Mundodi V, Alderete JF. Trichomonas vaginalis adherence mediates differential gene expression in human epithelial cells. Cell Microbiol 2005. 7:887–97. doi: 10.1111/j.1462-5822.2005.00522.x]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotch MF, Pastorek JG 2nd, Nugent RP, Hillier SL, Gibbs RS, Martin DH, Eschenbach DA, Edelman R, Carey JC, Regan JA, Krohn MA, Klebanoff MA, Rao AV, Rhoads GG. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis 1997. 24:353–60. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 20.el-Shazly AM, Morsy TA, Dawoud HA. Human monisziasis expansa: The first Egyptian parasitic zoonosis. J Egypt Soc Parasitol 2004. 34:515–8. [PubMed] [Google Scholar]
- 21.Schwebke JR. Cost effective screening for trichomoniasis. Emerg Infect Dis. 2002;8:749;author reply 749–50. doi: 10.3201/eid0807.010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang ZF, Begg CB. Is Trichomonas vaginalis a cause of cervical neoplasia? Results from, a com-bined analysis of 24 studies. Int J Epidemiol. 23:682–90. doi: 10.1093/ije/23.4.682. [DOI] [PubMed] [Google Scholar]
- 23.Sayed el-Ahl SA, el-Wakil HS, Kamel NM, Mahmoud MS. A preliminary study on the relationship between Trichomonas vaginalis and cervical cancer in Egyptian women. J Egypt Soc Parasitol 2002. 32:167–78. [PubMed] [Google Scholar]
- 24.Benchimol M, de Andrade Rosa I, da Silva Fontes R, Burla Dias AJ. Trichomonas vaginalis adhere and phogocytose sperm cells: Adhesion seems to be a prominent stage during interac-tion. Parasitol Res 2008. 102:597–604. doi: 10.1007/s00436-007-0793-3. [DOI] [PubMed] [Google Scholar]
- 25.Bowden FJ, Garnett GP. Why is Trichmonas vaginalis ignored? Sex Transm Infect 1999. 75:372–4. [PubMed] [Google Scholar]
- 26.Krieger J, Alderete M, Tricho-monas vaginalis. , tricho-monaiasis. In: K.Holmes, F Sparling, S Lemon, et al. (eds), Sexually Transmitted Diseases, 3rd ed. Mc Graw-Hill, New York; 1999 [Google Scholar]
- 27.Okun N, Gronau KA, Hannah ME. Antibiotics for bacterial vaginosis or Trichomonas vaginalis in pregnancy: A systematic review. Obstet Gynecol 2005. 105:857–68. doi: 10.1097/01.AOG.0000157108.32059.8f. [DOI] [PubMed] [Google Scholar]
- 28.Chuachoowong R, Shaffer N, Siriwasin W, Chaisilwattana P, Young NL, Mock PA, Chearskul S, Waranawat N, Chaowanachan T, Karon J, Simonds RJ, Mastro TD. Short-course antenatal zidovudine reduces both cervicovaginal human im-munodeficiency virus type 1 RNA levels and risk of perinatal transmission. Bangkok Collaborative Perinatal HIV Transmission Study Group. J Infect Dis 2000. 181:99–106. doi: 10.1086/315179. [DOI] [PubMed] [Google Scholar]
- 29.Houang ET, Ahmet Z, Lawrence AG. Successful treatment of four patients with recalcitrant vaginal trichomoniasis with a combination of zinc sulfate douche and metronidazole therapy. Sex Transm Dis 1997. 24:116–9. doi: 10.1097/00007435-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Diseases: Over view and Estimates. Geneva. 1999 [Google Scholar]
- 31.Klebanoff MA, Carey JC, Hauth JC, Hillier SL, Nugent RP, Thom EA, Ernest JM, Heine RP, Wapner RJ, Trout W, Moawad A, Leveno KJ, Miodovnik M, Sibai BM, Van Dorsten JP, Dombrowski MP, O'Sullivan MJ, Varner M, Langer O, McNellis D, Roberts JM, National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med 2001. 345:487–93. doi: 10.1056/NEJMoa003329. [DOI] [PubMed] [Google Scholar]
- 32.El-Moamly AM, Rashad SM.M. Trichomonas vaginalis antigens in vaginal and urine specimens by immunochromatography, compared to culture and microscopy. J Egypt Soc Parasitol . 2008;38:573–84. [PubMed] [Google Scholar]
- 33.Hussien EM, El-Sayed HZ, El-Moamly AA, Helmy MM, Shaban MM. Molecular characterization of Egyptian Trichomonas vaginalis clinical isolates by HSP70 restriction fragment length polymorphism. J Egypt Soc Parasitol 2005. 35:699–710. [PubMed] [Google Scholar]
- 34.Wendel KA, Workowski KA. Trichomoniasis challenges to appropriate management. Clin Infect Dis. 2007;44:S123–9. doi: 10.1086/511425. [DOI] [PubMed] [Google Scholar]
- 35.Diallo D, Hveem B, Mohamoud MA, Betge G, Paulsen BS, Maiga A. An ethanobotanical survey of herbal drugs of Gourma district, Mali. Pharmaceutical Biology 1999. 37:80–91. doi: 10.1076/phbi.37.1.80.6313. [DOI] [Google Scholar]
- 36.Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 2005. 4:685–8. [Google Scholar]
- 37.Hussain A, Virmami OP, Popli SP. 1992. Dictionary of Indian Medicinal Plants. Lucknow, India, CIMAP. 2008:384. [Google Scholar]
- 38.Kayser O, Kiderlen AF, Croft SL. Natural products as antiparasitic drugs. Parasitol Res . 2003;90:S55–62. doi: 10.1007/s00436-002-0768-3. [DOI] [PubMed] [Google Scholar]
- 39.Garcia LS. Diagnostic Medical Parasitology. 4th edition ASM Press, Washington DC, 2001 [Google Scholar]
- 40.Diamond LS. The establishment of various trichomonads of animals and men in axenic culture. J Parasitol 1957. 43:488–90. doi: 10.2307/3274682. [DOI] [PubMed] [Google Scholar]
- 41.Negi PS, Jayaprakasha GK, Jena BS. Antioxidant and anti mutagenic activities of pomegranate peel extracts. Food Chem 2003. 80:393–7. doi: 10.1016/S0308-8146(02)00279-0. [DOI] [Google Scholar]
- 42.Li YC, Guo J, Yang J, Wei JX, Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem 2005. :[In Press]. [Google Scholar]
- 43.Steben M. Sexually transmitted diseases. The American college of Obstetricians, and Gynecologist. Washington. 1996 [Google Scholar]
- 44.Hughesi. Science in Africa Magazine. Issue 56, 2002 [Google Scholar]
- 45.Rojas JJ, Ochoa VJ, Ocampo SA, Muñoz JF. Screening for antimicrobial activity of ten medicinal plants used in Colombian Folkovic medicine: a possible alternative in treatment of non nosocomial infection. BMC Complement Altern Med 2006. 6:2. doi: 10.1186/1472-6882-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lossick JG, Kent HL. Trichomoniasis: trends in diagnosis and management. Am J Obstet Gynecol 1991. 165:1217–22. doi: 10.1016/s0002-9378(12)90730-9. [DOI] [PubMed] [Google Scholar]
- 47.Saurina G, McCormack WM, Landman D. A study of the prevalence of resistant trichomoniasis and response to treatment in Brooklyn, NY (abstract 088). In: 13th Meeting of Inter Soc Trans Dis Res (Denver) . 1999:11–14. [Google Scholar]
- 48.Erdogrul OT. Antibacterial activities of some plants extracts used in folk medicine. Pharmaceutical Biology 2002. 40:269–73. doi: 10.1076/phbi.40.4.269.8474. [DOI] [Google Scholar]
- 49.al-Awadi F, Fatania H, Shamte U. The effect of a plants mixture extract on liver gluconeogenesis in streptozotocin induced diabetic rats. Diabetes Res 1991. 18:163–8. [PubMed] [Google Scholar]
- 50.Michie CA, Cooper E. Frankincense and myrrh as remedies in children. J R Soc Med. 1991;84:602–5. doi: 10.1177/014107689108401011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.al-Harbi MM, Qureshi S, Raza M, Ahmed MM, Afzal M, Shah AH. Gastric antiulcer and cytoprotective effect of Commiphora molmol in rats. J Ethnopharmacol 1997. 55:141–50. doi: 10.1016/S0378-8741(96)01488-2. [DOI] [PubMed] [Google Scholar]
- 52.Al-Awadi FM, Gumaa KA. Studies on the activity of individual plants of an antidiabetic plant mixture. Acta Diabetol Lat 1987. 24:37–41. doi: 10.1007/BF02732051. [DOI] [PubMed] [Google Scholar]
- 53.Nomicos EY. Myrrh: medical marvel or myth of the Magi? Holist Nurs Pract. 2007;21:308–23. doi: 10.1097/01.HNP.0000298616.32846.34. [DOI] [PubMed] [Google Scholar]
- 54.Massoud AM, Labib IM. Larvicidal activity of Commiphora molmol against Culex pipiens and Aedes caspius larvae. J Egypt Soc Parasitol 2000. 30:101–15. [PubMed] [Google Scholar]
- 55.El Baz MA, Morsy TA, El Bandary MM, Motawea SM. Clinical and parasitological studies on the efficacy of Mirazid in treatment of schistosomiasis haematobium in Tatoon, Etsa Center, El Fayoum Governorate. J Egypt Soc Parasitol 2003. 33:761–76. [PubMed] [Google Scholar]
- 56.Soliman OE, El-Arman M, Abdul-Samie ER, El-Nemr HI, Massoud A. Evaluation of myrrh (Mirazid) therapy in fascioliasis and intestinal schistosomiasis in children: immunological and parasitological study. J Egypt Soc Parasitol. 2004;34:941–66. [PubMed] [Google Scholar]
- 57.Abo-Madyan AA, Morsy TA, Motawea SM, Morsy AT. Clinical trial of Mirazid in treatment of human fascioliasis, Ezbet El-Bakly (Tamyia Center) Al-Fayoum Governorate. J Egypt Soc Parasitol 2004. 34:807–18. [PubMed] [Google Scholar]
- 58.Haridy FM, El-Sherbiny GT, Morsy TA. Some parasitic flukes infecting farm animals in Al-Santa Center, Gharbia Governorate, Egypt. J Egypt Soc Parasitol 2006. 36:259–64. [PubMed] [Google Scholar]
- 59.el-Shazly AM, Morsy TA, Dawoud HA. Human monisziasis expansa: the first Egyptian parasitic zoonosis. J Egypt Soc Parasitol . 2004;34:515–8. [PubMed] [Google Scholar]
- 60.Massoud AM, El-Shazly AM, Awad SE, Morsy AT, Sadek GS, Morsy TA. New trends in diagnosis and treatment of chronic intestinal strongyloidiasis stercoralis in Egyptian patients. J Egypt Soc Parasitol 2006. 36:827–44. [PubMed] [Google Scholar]
- 61.Massoud AM, El-Shazly AM, Morsy TA. Mirazid (Commiphora molmol) in treatment of human heterophyiasis. J Egypt Soc Parasitol. 2007;37:395–410. [PubMed] [Google Scholar]
- 62.Massoud AM, Shazly AM, Shahat SA, Morsy TA. Mirazid in treatment of human hymenolepiasis. J Egypt Soc Parasitol 2007. 37:863–76. [PubMed] [Google Scholar]
- 63.Allam AF, el-Sayad MH, Khalil SS. Laboratory assessment of the molluscicidal activity of Commiphora molmol (Myrrh) on Biomphalaria alexandrina, Bulinus truncatus and Lymnaea cailliaudi. J Egypt Soc Parasitol. 2001;31:683–90. [PubMed] [Google Scholar]
- 64.Shoukry NM. Effect of Commiphora molmol on Bithynia connollyi with special reference to their morphology and medical importance. J Egypt Soc Parasitol 2006. 36:701–12. [PubMed] [Google Scholar]
- 65.Massoud AM, El-Shazly AM, Na-gaty IM, Morsy TA. Commiphora molmol extracts as plant molluscicide against Lymnaea natalensis. J Egypt Soc Parasitol 2007. 37:437–48. [PubMed] [Google Scholar]
- 66.Massoud AM, El-Ashmawy IM, Naser MA, Salama OM. Mirazid: a new schistosomicidal agent derived from Myrrh: Studies on its influence on male reproductive organs and bile flow. Alex J Pharmacol Sci 2002. 16:82–7. [Google Scholar]
- 67.Omar A, Elmesallamy Gel-S, Eassa S. Comparative study of the hepatotoxic, genotoxic and carcinogenic effects of praziquantel distocide and the natural myrrh extract Mirazid on adult male albino rats. J Egypt Soc Parasitol 2005. 35:313–29. [PubMed] [Google Scholar]
- 68.Auffray B. Protection against singlet oxygen, the main actor of sebum squalene peroxidation during sun exposure, using Commiphora myrrha essential oil. Int J Cosmet Sci 2007. 29:23–9. doi: 10.1111/j.1467-2494.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- 69.Longtin R. The pomegranate: nature's power fruit? J Natl Cancer Inst 2003. 95:346–8. doi: 10.1093/jnci/95.5.346. [DOI] [PubMed] [Google Scholar]
- 70.Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem 2000. 48:4581–9. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 71.De M, Krishna De A, Banerjee AB. Antimicrobial screening of some Indian species. Phytother Res 1999. 13:616–8. doi: 10.1002/(SICI)1099-1573(199911)13:7<616::AID-PTR475>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 72.Machado TB, Leal CR, Amerl ACF, Santos KRN, Silva MG, Kuster RM. Anti-microbial ellagitannin of Punica granatum fruits. J Brazil Chem Soc 2002. 13:606–10. doi: 10.1590/S0103-50532002000500010. [DOI] [Google Scholar]
- 73.Rettig MB, Heber D, An J, Seeram NP, Rao JY, Liu H, Klatte T, Belldegrun A, Moro A, Henning SM, Mo D, Aronson WJ, Pantuck A. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-kappaB-dependent mechanism. Mol Cancer Ther 2008. 7:2662–71. doi: 10.1158/1535-7163.MCT-08-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo C, Wei J, Yang J, Xu J, Pang W, Jiang Y. Pomegranate juice is potentially better than apple juice in improving anti-oxidant function in elderly subjects. Nutr Res 2008. 28:72–7. doi: 10.1016/j.nutres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Guo G, Wang HX, Ng TB. Pome-granin, an anti-fungal peptide from pomegranate peels. Protein Pept Lett 2009. 16:82–5. doi: 10.2174/092986609787049330. [DOI] [PubMed] [Google Scholar]
- 76.Tayel AA, El-Tras WF. Anticandidal activity pomegranate peels extract aerosol as an applicable sanitizing method. Mycoses 2010. 53:117–22. doi: 10.1111/j.1439-0507.2008.01681.x. [DOI] [PubMed] [Google Scholar]
- 77.DiSilvestro RA, DiSilvestro DJ, DiSilvestro DJ. Pomegranate extract mouth rinsing effects on saliva measures relevant to gingivalis risk. Phytother Res 2009. 23:1123–7. doi: 10.1002/ptr.2759. [DOI] [PubMed] [Google Scholar]
- 78.Basu A, Penugonda K. Pomegranate juice: A heart healthy fruit juice. Nutr Rev 2009. 67:49–56. doi: 10.1111/j.1753-4887.2008.00133.x. [DOI] [PubMed] [Google Scholar]
- 79.Rosenblat M, Aviram M. Paraoxonases role in the preven-tion of cardiovascular diseases. Bio factors 2009. 35:98–104. doi: 10.1002/biof.16. [DOI] [PubMed] [Google Scholar]
- 80.Duke JA, Ayensu ES. Medicinal Plants of China Reference Publication, Inc. ISBNO-917256. 1985. pp. 2–4. [Google Scholar]
- 81.Mazyad SA, El-Serougi AO, Morsy TA. The efficacy of the volatile oils of three plants for controlling Lucilia sericata. J Egypt Soc Parasitol 1999. 29:91–100. [PubMed] [Google Scholar]
- 82.Blatter E, Cains JF, Mhaskar KS. Indian Medicinal Plants. 2nd ed. Uttranched, India :Oriental Enterprises. 2001:1506–1551. [Google Scholar]
- 83.Brown D. Encyclopedia of Herbs and their Uses. Dorling Kindersley London., ISBNO 7513-020-31. 1995 [Google Scholar]
- 84.Prashanth D, Asha MK, Amit A. Antibacterial activity of P. granatum. Fitoterapia 2001. 72:171–3. doi: 10.1016/S0367-326X(00)00270-7. [DOI] [PubMed] [Google Scholar]
- 85.Warrier PK, Nambiar VPK, Ra-mankutty C. Indian medicinal plants a compendium of 500 species. Hyderabad, India: Orient Longman Private Ltd. Hyderabad. 2002. pp. 396–402. [Google Scholar]
- 86.Voravuthikunchai SP, Sririrak T, Limsuwan S, Supawita T, Lida T, Honda T. Inhibitory effect of active compounds from Punica granatum per carp on verocytotoxicn production by Entero hemorrhagic E. coli.O157: H7. J Hlth Sci 2005. 51:590–6. [Google Scholar]
- 87.Jurenka JS. Therapeutic applications of pomegranate (Punica granatum L. A review. Altern Med Rev. 2008;13:128–44. [PubMed] [Google Scholar]