Abstract
Recent advances in biologically based ecosystem models of the coupled terrestrial, hydrological, carbon, and nutrient cycles have provided new perspectives on the terrestrial biosphere’s behavior globally, over a range of time scales. We used the terrestrial ecosystem model Century to examine relationships between carbon, nitrogen, and water dynamics. The model, run to a quasi-steady-state, shows strong correlations between carbon, water, and nitrogen fluxes that lead to equilibration of water/energy and nitrogen limitation of net primary productivity. This occurs because as the water flux increases, the potentials for carbon uptake (photosynthesis), and inputs and losses of nitrogen, all increase. As the flux of carbon increases, the amount of nitrogen that can be captured into organic matter and then recycled also increases. Because most plant-available nitrogen is derived from internal recycling, this latter process is critical to sustaining high productivity in environments where water and energy are plentiful. At steady-state, water/energy and nitrogen limitation “equilibrate,” but because the water, carbon, and nitrogen cycles have different response times, inclusion of nitrogen cycling into ecosystem models adds behavior at longer time scales than in purely biophysical models. The tight correlations among nitrogen fluxes with evapotranspiration implies that either climate change or changes to nitrogen inputs (from fertilization or air pollution) will have large and long-lived effects on both productivity and nitrogen losses through hydrological and trace gas pathways. Comprehensive analyses of the role of ecosystems in the carbon cycle must consider mechanisms that arise from the interaction of the hydrological, carbon, and nutrient cycles in ecosystems.
Keywords: climate, ecosystems, global change, nitrogen use efficiency, resource use efficiency
Global models of the terrestrial carbon cycle used in geochemical and assessment studies have generally lacked any serious representation of ecological processes or feedbacks and have been extrapolated into the future using a simple parameterization of the relationship between atmospheric CO2 and ecosystem carbon storage (1, 2). Biosphere models used to calculate surface water and energy exchanges in climate models commonly employ sophisticated representations of photosynthesis and respiration, but omit biogeochemical processes associated with the formation and turnover of organic matter (3, 4). Recently, process-based models for terrestrial biogeochemistry have been developed, based on theory linking climate, soil properties, and species- or growth form-specific traits to biogeochemical responses of plants and microorganisms. These models simulate the uptake and release of carbon in response to light, water, temperature, and nutrients (5–10). The roles of climate and nutrient limitations inherent in modern ecology (discussed in refs. 11 and 12) are important because the sensitivity of ecosystem models to climate change and increasing CO2 is strongly modulated by nutrients (13, 14). Response of modeled carbon storage to increasing CO2 and temperature is modified by increasing nutrient limitation (14, 15).
Large-scale patterns in terrestrial primary productivity, soil carbon, and soil metabolism can often be explained from simple equations using climate parameters (precipitation, actual evapotranspiration, solar radiation) (16–22). However, nutrients often limit terrestrial primary productivity in the sense that added nutrients lead to additional plant growth and carbon storage (15, 23). Current process-level models couple biophysical and biogeochemical limits to ecosystem processes explicitly (14, 24, 25). Recent work suggests that, in fact, biophysical and biogeochemical (nutrient) limitations to productivity and carbon storage may come into equilibrium with each other as ecosystems develop over time (24, 25). In this paper, we present a model-based analysis of the processes whereby water/energy and biogeochemical controls over ecosystem productivity and carbon storage converge, as a theoretical underpinning for the eventual quantitative analysis of terrestrial biogeochemical response to global change.
Model and Methods
In this study, we used the Century terrestrial ecosystem model, developed by Parton et al. (26) over the past decade. In the past few years the model has been extensively evaluated relative to observations along climate gradients (25, 27), at continental scales (25), globally (13, 28), and compared with remote sensing (12, 25). The model simulates the major pathways for water, carbon, and nitrogen exchange, including atmospheric and biological N inputs, and gaseous, combustion-related, and hydrological N losses (12, 13, 26, 28–31). Century explicitly partitions live biomass and organic matter (nonliving) into compartments defined by differing turnover times. For the live components, these correspond to leaves, fine roots, coarse roots, branches, and stems. For organic matter the model is based on isotopic and other evidence for multiple turnover times in detritus and soil organic matter (28, 32, 33). The model is integrated globally using gridded global climate, soils, and vegetation data sets with 0.5 degree resolution (24). Results (annual fluxes) shown in this paper are from a simulation of the Northern Hemisphere, using an updated version of Century (24).
For this analysis the model was integrated using CO2 concentrations and nitrogen input rates deemed to be representative of the pre-industrial biosphere. For example, nitrogen inputs from precipitation were simulated to be 30–50% lower than current levels in moderately to severely polluted areas (34). We did this to simulate, for diagnostic purposes, a nearly steady-state biosphere. Much recent evidence suggests that the forcing due to increasing N deposition and frequency of ecosystem disturbance over the past 50–100 years may have resulted in non-steady-state N cycles in much of the world (34–36).
Equilibration of Nitrogen and Water Limitations
In ref. 24 we argued that spatial patterns of biophysical and nitrogen limitation are correlated because carbon and nitrogen fluxes are both strongly influenced by water and energy availability. This mechanism of equilibration is evident in Century because the model simulates the inputs and losses of N, rather than being calibrated to observed ecosystem N stocks (25). The equations in Century governing nitrogen fluxes include biophysical and soil biogeochemical processes. Atmospheric inputs of N are directly linked to precipitation (wet deposition). Biological nitrogen fixation is influenced by soil N and C availability and is assumed to be correlated with annual evapotranspiration (ET). The correlation is based on information indicating high rates of N fixation in humid tropical and temperate rain forests, and generally lower rates in mesic and arid systems, although the biogeography of nitrogen fixation is poorly known (23). It is noteworthy that, globally, patterns of N inputs through all processes are poorly known, and given their importance, require much more study (37). N inputs, as expected (summing biological and atmospheric processes) are strongly correlated with annual ET (Table 1).
Table 1.
Correlation structure emerging from key linkages between mechanisms shown in Fig. 1, as implemented in the simulation described in Model and Methods
| NMIN | NPP | NINPUT | NGAS | NO3 | DON | |
|---|---|---|---|---|---|---|
| NMIN | — | 0.90 | 0.54 | — | — | |
| ET | 0.67 | 0.71 | 0.96 | 0.71 | 0.33 | 0.00 |
| P-E | — | — | — | 0.74 | 0.05 | |
| NINPUT | — | — | 0.74 | — | — |
NMIN, nitrogen mineralization; NPP, net primary productivity; NINPUTs, nitrogen inputs; NGAS, trace gas losses of N; NO3, nitrate leaching; DON, organic nitrogen leaching; ET, evapotranspiration; P-E, precipitation minus ET. All correlations shown are significant at P < 0.05 (except for ET vs. DON).
Losses of nitrogen are controlled by soil moisture and water flux. Leaching losses of NO3 and dissolved organic N (DON) are directly controlled by the product of water flux and NO3/DON concentrations (28). Losses of N trace gases are linked to the rate of mineralization of NH4 and NO3 from organic matter, a rate that increases as temperature and soil moisture increase (28, 38). The proportional as well as absolute losses of gaseous N from inorganic N also increase with increasing soil moisture (30). Century simulates several pathways of N trace gas losses: the summed losses of N2, N2O, and NO from soil nitrification and denitrification are likewise highly correlated with ET (Table 1). This arises because of the strong first-order kinetic regulation of trace gas emissions with respect to soil inorganic N turnover.
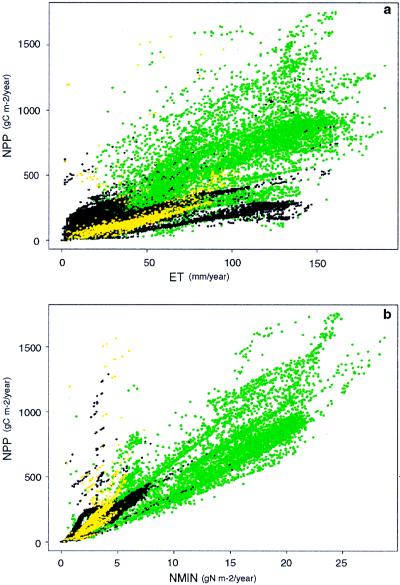
A key index of soil inorganic N turnover, N mineralization, is likewise strongly correlated with ET (Table 1). As noted in ref. 24, the correlation of N mineralization and ET, though strong, varies among ecosystem types, as is evident for other processes (see Fig. 2). Trace gas losses show similar patterns (data not shown), indicating ecosystem type-specific relationships between biophysical controls and N trace gas emissions, a factor not widely recognized (24). Spatial patterns of nitrate N leaching (data not shown) show strong dependence on ecosystem type, with many systems showing no or low losses; here we computed correlations for systems with non-zero leaching losses. Leaching losses are less directly related to ET, perhaps because ET is a poor predictor of available water below the rooting zone. Nitrate leaching is, however, strongly correlated with precipitation minus ET (P-E), which is related to the amount of water available for movement below the rooting zone (Table 1). Organic N leaching only occurs in a small fraction of grid cells (≈10%) and generally at low rates. It is poorly correlated with either ET or P-E (Table 1). The low leaching losses of N from many of the world’s ecosystems in this simulation of a preindustrial biosphere are consistent with Hedin et al. (35), who suggested that undisturbed ecosystems may have very low losses compared with the bulk of extant ecosystems. The results indicate significant correlation between key fluxes in the nitrogen budget and biophysical controls, although ecosystem-specific processes such as organic N leaching add some variability to patterns of equilibration.
Figure 2.
Results from an integration of Century for the Northern Hemisphere. Simulations used standard global climate, soils, and vegetation type distribution data sets and were carried out globally on an 0.5 × 0.5 degree grid. Points indicated in green are for forest ecosystems, yellow indicates grasslands, and black indicates “mixed” ecosystems that include both grasses and trees or shrubs (such as savannas). (a) The relationship between ET and NPP (r2 = 0.71). (b) The relationship between nitrogen mineralization and NPP (r2 = 0.90).
In Century the potential for carbon fixation increases as evapotranspiration increases via an equation that constrains primary production based on moisture available for transpiration (28). This equation integrates precipitation, energy, and soil hydrological constraints over the water flux in evapotranspiration. ET is linked to both precipitation, soil properties and radiation, as radiant energy is the driving force for ET. Thus, ET, which together with soil hydrological properties, controls the partitioning of soil moisture into runoff and fluxes back to the atmosphere or to depths below the rooting zone. Primary production also requires nitrogen to form organic matter meeting critical C/N ratios for wood, foliage, and roots. On an annual time scale most plant-available N is derived from nitrogen mineralization, which arises from organic matter turnover (decomposition); rates of N mineralization range from 0.2 to 30 g⋅m2⋅yr−1, greatly exceeding inputs in most cases. N inputs range from 0.5 to 1.5 g⋅m2⋅yr−1. Whereas N availability can vary substantially from year to year, the natural nitrogen budget changes on centennial time scales, as inputs and losses are small fractions of soil N stocks, which typically exceed 500 g⋅m2.
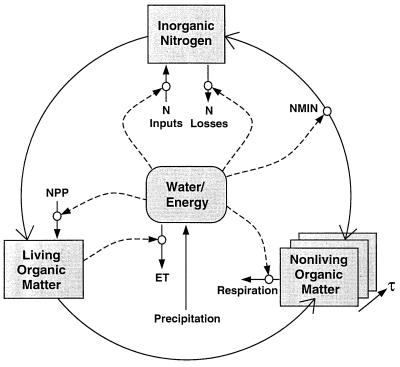
As a consequence of the tight coupling of the water/energy fluxes and nitrogen budget in Century, strong correlations between ET, nitrogen availability, and net primary productivity appear in global Century simulations (see Fig. 2). The correlations arise because water and energy fluxes controls both carbon and nitrogen fluxes (Fig. 1). These fluxes of carbon and nitrogen are mutually interdependent through the dual requirements of nitrogen in the formation of organic matter and of the role of organic matter decomposition in nitrogen mineralization. As water flux increases, N flux increases (inputs and losses), and likewise, the potential for carbon fixation increases. As carbon fixation increases, the amount of the N flux that can be captured in organic matter increases. As more nitrogen is captured in organic matter, its subsequent turnover also contributes to plant available N, allowing more plant productivity. Thus, water/energy and nutrient limitation of plant primary productivity and ecosystem carbon storage tend to “equilibrate” in near-steady-state ecosystems, as illustrated by the spatial patterns of correlation in Fig. 2.
Figure 1.
Schematic illustration of the coupling of water, nitrogen cycling, and carbon in ecosystems. Principle features of these coupled controls are that (i) water controls the inputs and outputs of nitrogen, (ii) increasing net primary productivity (NPP) allows more of the N flux through the system to be captured into organic matter, (iii) increasing organic N stocks allow for more N mineralization, supporting more NPP, and (iv) increasing precipitation both allows more NPP and more N cycling, thus water and nutrient limitation of NPP tend to become correlated.
The relationships of NPP and N availability with ET are modulated by other factors that influence turnover times. The relationships between NPP, ET, and N are modified by ecosystem type-specific factors that control resource use efficiencies. Effectively these are the carbon-to-nutrient stoichiometry of plants and microorganisms, and water use efficiency (or organic matter produced per unit water transpired) (Fig. 2). Ecosystems with wider C/N ratios in plant tissue have higher NPP per unit N mineralization (higher nitrogen use efficiencies). Systems with lower C/N ratios in leaf and root tissues have higher rates of N cycling per unit ET. C/N ratios reflect both plasticity in foliar and root composition, and more significantly, changes in allocation between high and low-N tissues (wood vs. leaves or roots). Although large-scale patterns arise from system-level interactions of the biogeochemical and hydrological cycles, substantial variation is induced by species- and growth form-specific traits related to allocation patterns and C/N ratios. The correlation of variables in Fig. 2 indicates the extent to which NPP and nitrogen cycling are controlled by system-level dynamics: the vertical scatter indicates roughly the extent to which system-specific ecological traits influence NPP and nitrogen cycling.
The model results are consistent with observations of large-scale correlations of NPP with direct or derived climate variables, but also with experimental evidence of nutrient limitation. The modulation of the water-carbon-nitrogen system by species- and/or growth form-specific traits implies that large-scale dynamics are influenced by population dynamics on time scales longer than the life spans of individual plants (years-centuries). The relationship of biogeochemistry to population dynamics is outside the scope of this paper, but see refs. 39 and 40.
Conclusions
We hypothesized that water and nitrogen limitations of NPP are correlated at steady-state because of the control of carbon and nitrogen fluxes by the water budget. We further hypothesized that these correlations arise because of the system-level structure of interactions among the water, carbon, and nitrogen cycles. In model simulations, the correlations between biophysical and nutrient limitations to NPP and carbon storage arise because both carbon and nitrogen fluxes (ecosystem inputs and outputs) are influenced by water and energy availability. This model-based analysis is consistent with the widespread reports of strong correlations of climate with ecosystem processes, suggesting dominant climate controls, and strong experimental evidence that nutrient additions can increase productivity and carbon storage. The tight correlations among N budgetary fluxes (Table 1) suggest that atmospheric trace gas composition may be affected by changes to either climate or N inputs via air pollution or fertilization.
Predicted ecosystem behavior becomes more complex when biophysical and nutrient constraints are considered together as compared with purely biophysical formulations (24, 41, 42). The biophysical effects of temperature, moisture, and radiation on photosynthesis, plant respiration, and evapotranspiration can be simulated with relatively few ecosystem-specific controls (4). Nutrient cycling is additionally coupled to spatial patterns of N inputs (34–36) and to patterns of plant allocation of carbon and nitrogen among roots, wood, and leaves. These allocation patterns, in turn, influence the distribution among long and short-lived compartments of living (e.g., wood vs. leaves) and detrital organic matter. As more of a system’s organic matter becomes tied up in long-lived compartments, fewer nutrients are available for rapid recycling, and nutrient limitation becomes tied to processes with longer time scales, such as soil carbon turnover or tree mortality and wood decomposition.
Nutrient-mediated processes assume increasing importance as ecosystem behavior is considered on interannual and longer time scales because they can cause lagged responses to climate change and variability (24). Models such as those discussed by Sellers et al. (3) describe the behavior of the “fast” carbon-water-energy system (43); biogeochemical models add the consequences of slower processes such as soil carbon and biomass accumulation, and allocation patterns between leaves, roots, and wood. Whereas even biophysical models may have “memory” over one to two years through soil moisture storage, biogeochemical models can simulate lagged effects over decades through the decomposition of wood or soil organic matter. Here we identify “equilibration” of the nitrogen budget with water/energy and carbon fluxes as an additional process causing decadal and longer time scale behavior.
Just as perturbations to the climate system can cause the nitrogen and carbon systems to respond (4, 24), perturbations of the N inputs from air pollution or fertilization will also cause long-lived ecosystem changes (34, 36, 44). Analyses of the past interannual variability of the carbon cycle, and of its potential future behavior, must consider mechanisms that act through the coupled water/energy, carbon, and nitrogen cycles.
Acknowledgments
We acknowledge the assistance of Rebecca McKeown, Melannie Hartmann, and Hank Fisher with conducting and analyzing global Century runs, with special thanks to Becky for her exceptional effort in completing the global calculations for this paper. Dennis Ojima, Beth Holland, Alan Townsend, and Jason Neff all helped conceive of or design the model experiments. This research was supported by the National Aeronautics and Space Administration Earth Observing System Interdisciplinary Science Program, and by the National Center for Atmospheric Research. The National Center for Atmospheric Research is sponsored by the National Science Foundation.
ABBREVIATIONS
- ET
evapotranspiration
- NPP
net primary productivity
References
- 1.Enting I G, Wigley T M L, Heimann M. Future Emissions and Concentrations of Carbon Dioxide: Key Ocean/Atmosphere/Land Analyses. Commonwealth Scientific and Industrial Research Organization, Australia: Division of Atmospheric Research; 1994. , Tech. Paper No. 31. [Google Scholar]
- 2.Siegenthaler U, Joos F. Tellus B. 1992;44:186–207. [Google Scholar]
- 3.Sellers P J, Bounoua L, Collatz G J, Randall D A, Dazlich D A, Los S O, Berry J A, Fung I, Tucker C J, Field C B, Jensen T G. Science. 1996;271:1402–1406. [Google Scholar]
- 4.Sellers P J, Dickinson R E, Randall D A, Betts A K, Hall F G, Berry J A, Collatz G J, Denning A S, Mooney H A, Nobre C A, Sato N, Field C B, Henderson-Sellers A. Science. 1997;275:502–509. doi: 10.1126/science.275.5299.502. [DOI] [PubMed] [Google Scholar]
- 5.Farquhar G D, Von Caemmerer S, Berry J A. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- 6.Melillo J M, Naiman R J, Aber J D, Linkins A E. Bull Mar Sci. 1984;35:341–356. [Google Scholar]
- 7.Bloom A J, Chapin F S, III, Mooney H A. Annu Rev Ecol Syst. 1985;16:363–393. [Google Scholar]
- 8.Chapin F S, III, Bloom A J, Field C B, Waring R H. BioScience. 1987;37:49–57. [Google Scholar]
- 9.Nobel P S. Physicochemical and Environmental Plant Physiology. San Diego: Academic; 1991. [Google Scholar]
- 10.Running S W, Nemani R R. Clim Change. 1991;19:349–368. [Google Scholar]
- 11.Schulze E D, De Vries W, Hauhs M, Rosén K, Rasmussen L, Tann O-C, Nilsson J. Water Air Soil Pollut. 1989;48:451–456. [Google Scholar]
- 12.Schimel D S, Kittel T G F, Parton W J. Tellus AB. 1991;43:188–203. [Google Scholar]
- 13.Schimel D S, Braswell Jr B H, Holland E A, McKeown R, Ojima D S, Painter T H, Parton W J, Townsend A R. Global Biogeochem Cycles. 1994;8:279–293. [Google Scholar]
- 14.VEMAP Participants. Global Biogeochem Cycles. 1995;9:407–438. [Google Scholar]
- 15.Schimel D S. Global Change Biol. 1995;1:77–91. [Google Scholar]
- 16.Leith H. In: Primary Productivity of the Biosphere. Leith H, Whittaker R B, editors. New York: Springer; 1975. pp. 237–263. [Google Scholar]
- 17.Uchijima Z, Seino H. J Agric Meteorol. 1985;40:43–352. [Google Scholar]
- 18.Sala O E, Parton W J, Joyce L A, Lauenroth W K. Ecology. 1988;69:40–45. [Google Scholar]
- 19.Potter C S, Randerson J T, Field C B, Matson P A, Vitousek P M, Mooney H A, Klooster S A. Global Biogeochem Cycles. 1993;7:811–841. [Google Scholar]
- 20.Gifford R M. Aust J Plant Physiol. 1994;21:1–15. [Google Scholar]
- 21.Zak D R, Tilman D, Parmenter R R, Rice C W, Fisher F M, Vose J, Milchunas D, Martin C W. Ecology. 1994;75:2333–2347. [Google Scholar]
- 22.Post W M, Pastor J, Zinke P J, Stangenberger A G. Nature (London) 1985;317:613–616. [Google Scholar]
- 23.Vitousek P M, Howarth R W. Biogeochemistry. 1991;13:87–115. [Google Scholar]
- 24.Schimel D S, Braswell B H, McKeown R, Ojima D S, Parton W J, Pulliam W. Global Biogeochem Cycles. 1996;10:677–692. [Google Scholar]
- 25.Schimel, D. S., VEMAP Participants & Braswell, B. H. (1997) Ecol. Monogr., in press.
- 26.Parton W J, Schimel D S, Cole C V, Ojima D S. Soil Sci Soc Am J. 1987;51:1173–1179. [Google Scholar]
- 27.Townsend A R, Vitousek P M, Trumbore S E. Ecology. 1995;76:721–733. [Google Scholar]
- 28.Parton W J, Ojima D S, Cole C V, Schimel D S. Quantitative Modeling of Soil Forming Processes. Madison, WI: Soil Science Society of America; 1994. pp. 147–167. [Google Scholar]
- 29.Schimel D S, Parton W J, Kittel T G F, Ojima D S, Cole C V. Clim Change. 1990;17:13–25. [Google Scholar]
- 30.Parton W J, Stewart J W B, Cole C V. Biogeochemistry. 1988;5:109–131. [Google Scholar]
- 31.Ojima D S, Schimel D S, Parton W J, Owensby C E. Biogeochemistry. 1994;24:67–84. [Google Scholar]
- 32.Trumbore S E. Global Biogeochem Cycles. 1993;7:275–290. doi: 10.1029/2018GB005950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parton W J, Scurlock J M O, Ojima D S, Schimel D S, Hall D O. Global Change Biol. 1995;1:13–22. [Google Scholar]
- 34.Townsend A R, Braswell B H, Holland E A, Penner J E. Ecol Appl. 1996;6:806–814. [Google Scholar]
- 35.Hedin L O, Armesto J J, Johnson A H. Ecology. 1995;76:493–509. [Google Scholar]
- 36.Holland, E. A., Braswell, B. H., Lamarque, J.-F., Townsend, A., Sulzman, J. M., Müller, J.-F., Dentener, F., Brasseur, G., Levy, H., II, Penner, J. E. & Roelofs, G. (1997) J. Geophys. Res., in press.
- 37.Galloway J N, Levy H, II, Kasibhatla P S. Ambio. 1994;23:120–123. [Google Scholar]
- 38.Holland E A, Townsend A R, Vitousek P M. Global Change Biol. 1995;1:115–123. [Google Scholar]
- 39.Schimel D S. In: Biotic Interactions and Global Change. Kareiva P M, Kingsolver J G, Huey R B, editors. Boston: Sinauer; 1993. pp. 45–54. [Google Scholar]
- 40.Pastor J, Post W M. Biogeochemistry. 1986;2:3–27. [Google Scholar]
- 41.Pastor J, Post W M. Clim Change. 1993;23:111–119. [Google Scholar]
- 42.Bolker B M, Pacala S W, Bazzaz F A, Canham C D, Levin S A. Global Change Biol. 1995;1:373–381. [Google Scholar]
- 43.Sellers P, Schimel D. Global Planet Change. 1993;7:279–297. [Google Scholar]
- 44.Aber J D, Nadlehoffer J K, Steudler P A, Melillo J M. BioScience. 1989;39:378–386. [Google Scholar]