Abstract
The complement system plays a major role in innate immune defenses against infectious agents, but exaggerated activation of complement can lead to severe tissue injury. Systemic (intravascular) activation of complement can, via C5a, lead to neutrophil (PMN) activation, sequestration and adhesion to the pulmonary capillary endothelium, resulting in damage and necrosis of vascular endothelial cells and acute lung injury (ALI). Intrapulmonary (intraalveolar) activation of complement can cause ALI that is complement and PMN-dependent, resulting in a cytokine/chemokine storm that leads to intense ALI. Surprisingly, C3−/− mice develop the full intensity of ALI in a C5a-dependent manner due to the action of thrombin that generates C5a directly from C5. There is conflicting evidence on the role of the second C5a receptor, C5L2 in development of ALI. There is accumulating evidence that C5a may suppress inflammatory responses or divert them from Th1 to Th2 responses, impacting the innate immune system. Finally, in experimental polymicrobial sepsis, there is evidence that many of the adverse outcomes can be linked to the roles of C5a and engagement of its two receptors, C5aR and C5L2. These observations underscore the diversity of effects of C5a in a variety of inflammatory settings.
Keywords: Complement, Anaphylatoxins, Immune complexes, Endotoxin, Sepsis, Septic shock
1 Acute Lung Vascular Injury Following Systemic Activation of Complement
Several years ago, before availability of C3−/− mice, purified naja naja cobra venom factor (CVF) was repetitively injected intraperitoneally over a period of 36 hr, resulting in nearly complete depletion of plasma C3 as measured quantitatively by immunochemical techniques. Such complement depletion resulted in greatly attenuated ischemia-reperfusion injury of hind limbs, or injury of kidney, heart, small bowel, to name a few examples (Seekamp et al. 1993; Seekamp and Ward 1993). Since later work indicated that in vivo neutralization of C5a had similar effects, it was assumed that C3 depletion prevented activation of C5, abolishing formation of C5a (reviewed, Collard et al. 1999; Hammerschmidt et al. 1980). This presumption was confirmed when it was shown that CVF isolated from naja haja cobra snakes (instead of CVF from naja naja cobra snakes) depleted C3 but did not activate C5 and did not cause acute lung vascular damage after vascular infusion (Till et al. 1987). In contrast, naja naja bolus CVF infusion (intravenous) caused rapid onset of extensive injury to the pulmonary vascular endothelium, leading to necrosis of endothelial cells and intraalveolar hemorrhage and flooding (Till et al. 1987). Such studies suggest that intravascular activation of complement can cause intense injury to the vascular endothelium, which is linked to PMN adherence to the endothelium associated with CD11b/CD18 activation on PMNs and rapid C5a-dependent expression of P-selectin on endothelial cell surfaces, the engagement of these adhesion molecules leading to intensification of microvascular injury due to close spatial proximity between PMNs and endothelial cells (Till et al. 1982).
In subsequent studies we demonstrated the mechanisms by which damage of endothelial cells in the presence of activated neutrophils (PMNs) occurs. Activated PMNs generate H2O2, which is freely permeable across the plasma membrane of endothelial cells. Production of H2O2 by activated PMNs is followed by O2• generation following conversion of xanthine dehydrogenase to xanthine oxidase in vascular endothelial cells, resulting in formation of O2•. O2 can react with Fe3+ from ferritin within endothelial cells, causing reduction to Fe2+ and release of Fe2+ into the cytosol of the endothelial cell. The interaction of Fe2+ with H2O2 within the endothelial cells results in formation of the highly-reactive and short-lived hydroxyl radical, HO• (Gannon et al. 1987; Varani et al. 1985). Prior depletion of iron within endothelial cells using the iron chelator, deferoxamine, or addition of allopurinol which blocks the enzymatic activity of xanthine oxidase, will both greatly attenuate the ability of activated PMNs to injure endothelial cells in vitro (reviewed, Till et al. 1991).
2 Complement in Experimental and Clinical Acute Lung Injury
In the literature dealing with acute lung injury (ALI) or acute respiratory distress syndrome (ARDS), C5a has been found in BAL fluids along with a substantial number of neutrophils, suggesting the possibility that C5a presence in lung may be related to the buildup of PMNs in the alveolar compartment and that products of PMNs may directly cause ALI, involving both the vascular and alveolar epithelial barriers (Hammerschmidt et al. 1980; Pittet et al. 1997; Solomkin et al. 1985). Endotoxemia in mice has been linked to the appearance of C5a in plasma, but, when LPS is given intratracheally, the result is ALI with alveolar hemorrhage and fibrin deposition together with abundant accumulation of PMNs, all of which have been shown to be associated with the requirements for migration inhibitory factor (MIF) and LTB4 receptors (Donnelly et al. 1997; Makita et al. 1998; Nishihira 2000; Rittirsch et al. 2008a). Surprisingly, in recent studies of LPS-induced ALI, no C5a could be detected in BAL fluids, although, when LPS was injected intraperitoneally, C5a appeared in the plasma (Rittirsch et al. 2008a). Furthermore, ALI after intratracheal administration of LPS was fully expressed in C5−/− mice, quantitatively the same as ALI developing in C5+/+ mice. Collectively, the data suggest that LPS-induced ALI is complement-independent but requires the participation of MIF and receptors (BLT1) for LTB4. In the setting of endotoxemia, C5a appears to be required for the acute febrile response (Barton and Warren 1993; Li et al. 2005). The reason for the independence of the requirement for C5a in LPS-induced ALI may be related to the high levels in lung of C1 esterase inhibitor and surfactant A, which sharply limit activation of complement in the lung (Watford et al. 2000, 2001), or the problem could be the lack of adequate amounts of complement proteins in the alveolar compartment to generate needed levels of C5a.
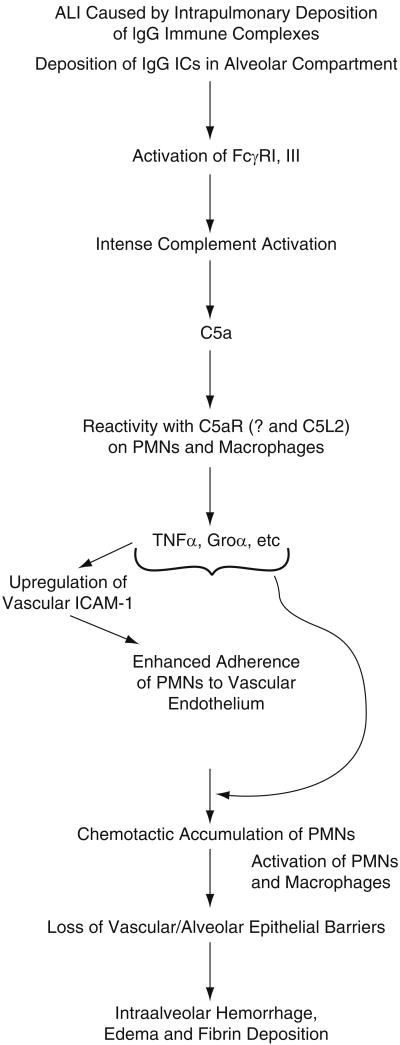
It should be noted that in the setting of ALI induced by intrapulmonary deposition of IgG immune complexes, there is robust engagement of Fc receptors (FcγRI/III) (Ravetch and Clynes 1998) as well as complement activation as measured by buildup of C5a in BAL fluids and suppression of IgG-induced ALI by the use of C5−/− mice (Larsen et al. 1981) or by use of neutralizing antibody to C5a (see Fig. 1, Huber-Lang et al. 2006; Mulligan et al. 1996; Ward 1996). Generation of C5a within lung sets the stage for the appearance of TNFα, suppression of which reduces PMN buildup in the lung and protects from ALI, perhaps due to the lack of upregulation of vascular ICAM-1, which is needed for full adhesive interactions between endothelial cells and PMNs etc. (Warren et al. 1989).
Fig. 1.
Pathophysiology describing development of acute lung injury induced introdent lung following intrapulmonary deposition of IgG immune complexes.
3 Lessons Learned in C3−/− Mice
The genetic-based knockout of FcγRI/III, as expected, substantially reduced the intensity of IgG immune complex-induced ALI in lungs of mice (Sylvestre et al. 1996). Earlier work indicated that IgGIC-induced ALI was attenuated in C5−/− mice (Larsen et al. 1981). In spite of intrapulmonary neutralization of C5a greatly attenuating ALI (Gao et al. 2006), the finding of fully expressed ALI after IgGIC deposition in C3−/− mice led to the conclusion that this form of ALI was independent of complement activation (Sylvestre et al. 1996). However, it was subsequently shown that C3−/− mice undergoing IgGIC deposition had C5a in their BAL fluids and that neutralization of C5a in these mice markedly attenuated ALI (Huber-Lang et al. 2006). The absence of C3 in these KO mice inferred that no complement activation pathway could be engaged under such conditions. On the basis of earlier work, we showed that neutral proteases in PMNs could directly generate C5a in the presence of C5 (Ward and Hill 1970). More recently, we demonstrated that lung macrophages, when activated, expressed a neutral protease that would cleave C5 to form C5a (Huber-Lang et al. 2006). In the case of C3−/− mice, the co-instillation into lung of recombinant hirudin (a potent thrombin inhibitor) together with the IgG antibody to bovine serum albumin greatly reduced generation of C5a. In addition, it was shown that thrombin could catalyze the cleavage of C5 and amino acid sequencing confirmed that the peptide formed by this interaction of C5 with thrombin was authentic C5a. In addition, we showed that C3−/− mice had supernormal levels of plasma thrombin (Huber-Lang et al. 2006). All in all, these studies suggested that, in the absence of C3, mice compensate by producing supernormal levels of thrombin in liver, allowing formation of C5a at various tissue/organ sites. Recent studies also suggest that several activated clotting factors (reviewed, Amara et al. 2010; Markiewski et al. 2007) also have the ability to generate C5a from C5, emphasizing interactions between the clotting and complement cascades (see below).
A very recent study has extended the spectrum of activated human clotting factors that can generate complement anaphylatoxins (C3a, C5a) in the presence of purified human C3 or C5 (Amara et al. 2010). As described above, a few years ago it was demonstrated that thrombin (Factor IIa) can react with C5 to generate C5a (Huber-Lang et al. 2006). It has recently been shown that the following activated clotting and fibrinolytic factors (F) can generate (in rank order) both C3a and C5a when exposed to human C3 or C5: FXa > plasmin > thrombin > FIXa > FXIa. In the case of FXa, selective inhibitors (fondaparinux, enoxaparin) suppressed generation of the anaphylatoxins. Such data reinforce the existence of crosstalk between the complement, clotting and fibrinolytic cascades. Obviously, in the setting of human plasma, the ability to rank order these various factors and relate them to anaphylatoxin generation is extremely difficult especially because of various endogenous inhibitors present in human plasma.
4 Role of C5aR and C5L2 in IgGIC ALI
As indicated above, it was shown many years ago that IgGIC-induced ALI was greatly reduced in intensity in C5−/− mice (Larsen et al. 1981). The two receptors for C5a have been characterized over the past decade (Cain and Monk 2002; Gerard and Gerard 1991; Gerard et al. 2005; Kalant et al. 2003, 2005; Lee et al. 2008; Okinaga et al. 2003). C5aR is a traditional G-protein coupled receptor that, when ligated to C5a, proceeds through the MAPK signaling pathways, resulting in phagocyte (PMNs, macrophages) responses such as rapid Ca2+ transients, chemotaxis, respiratory burst, oxidant production and secretion of granule enzymes. Such innate immune responses are designed to confront invading micro-organisms, resulting in their localization and destruction. The story with the other C5a receptor, C5L2 is much less well understood. C5L2 is reported to react with C5a, C5a des Arg, and C3a des Arg, the last being also referred to as “acylation-stimulating protein.” It is clear that ligation of C5L2 does not result in appearance of Ca2+ transients even though C5L2 has very high binding affinity to the complement-derived anaphylatoxins. The lack of Ca2+ signaling is due to an amino acid substitution in the DRY region of the third intracellular loop of C5L2, which prevents interactions of the receptor with G-proteins. The localization of receptor protein for C5aR is unequivocally on the outer cell membrane of phagocytes. After ligation of C5aR with C5a, the receptor is internalized, stripped of C5a, and some (< 50%) of the receptor is recycled to the cell membrane. The story of C5L2 is quite complicated. It appears that, in the non-activated PMN, most of the C5L2 receptor is present within cytosolic granules (Bamberg et al. 2010; Cain and Monk 2002; Scola et al. 2009). Assuming that C5aR and C5L2 are situated in two distinctly different locales in or on PMNs, an important question is: How does C5a/C5ades Arg get into an intracellular position in order to bind to cytosolic C5L2 and affect cell function and is there any interaction (such as heterodimerization) between ligated C5aR and C5L2?
There is a consensus that Ca2+ signaling does not occur after C5a interaction with C5L2. Beyond this point, there is little agreement regarding the biological and biochemical responses to C5L2. The Toronto group finds that C5L2−/− macrophages or PMNs exposed to C5a have defective phosphorylation of ERK1/2 (Chen et al. 2007), while the Boston group claims that ERK1/2 activation is fully intact in C5L2−/− phagocytic cells (Okinaga et al. 2003). C5L2 was originally described as a “default” receptor, implying that it competes with C5aR for C5a binding but in the absence of subsequent cell signaling events in C5L2−/− cells (Gerard et al. 2005; Okinaga et al. 2003). In the setting of IgGIC-induced ALI, it has been reported that ALI and PMN accumulation are depressed in C5L2−/− mice (Chen et al. 2007), while another group reports that under similar conditions C5L2−/− mice show substantially intensified injury (defined by increased vascular permeability) as well as enhanced PMN accumulation in lung after IgGIC ALI. On the other hand, it has been recently reported by both groups (Toronto and Boston) that asthmatic lung responses in C5L2−/− mice are substantially reduced when compared to Wt mice (Chen et al. 2007; Zhang et al. 2010). How to explain such divergent observations is very difficult at present, unless one assumes that the genetic backgrounds of C5aR−/− and C5L2−/− mice used in the different laboratories are disparate, although all mice are on a C57BL/6 background.
5 Evidence for Regulatory Roles of C5, C3a, C5a, C3aR and C5aR in Inflammation
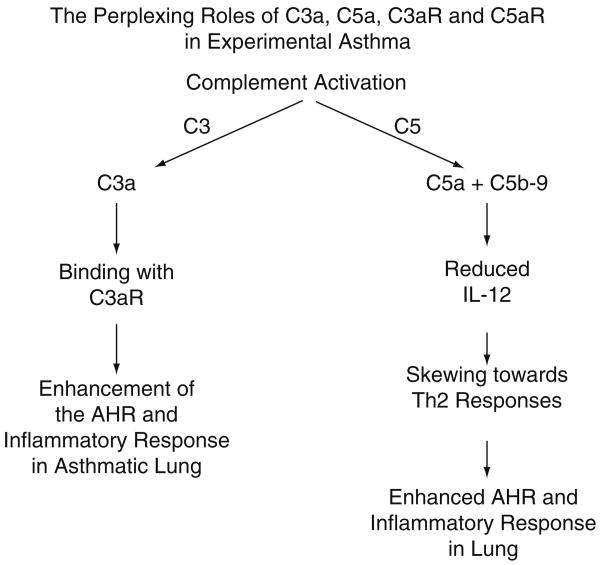
As indicated above, there is abundant evidence for the proinflammatory roles of C5a and C5aR in a variety of inflammatory responses (e.g., antigen-induced arthritis, collagen-induced arthritis, acute vascular injury after systemic activation of complement, IgGIC injury of venules, ALI, ARDS, etc.). There has been recently emerging evidence that in some circumstances C5, C5a and C5aR may regulate the inflammatory response (see Fig. 2). Several years ago it was found that mice deficient in C5 developed more intense asthmatic responses when compared to C5-intact mice, under conditions using active immunization and repetitive antigenic challenge of the lung (Karp et al. 2000), suggesting that C5 or its activation products somehow suppressed immune/inflammatory responses tied with development of experimental asthma. When C5aR was blocked with a mAb to C5aR, this resulted in exacerbation of airway hyperreactivity (AHR), whereas blockade of IL-17A reversed the enhanced AHR in mice in which C5aR was blocked (Lajoie et al. 2010). Such data suggested that, in experimental allergic asthma, AHR is mediated by IL-17A via enhanced IL-13-driven responses and that such responses are regulated by C5aR and C5a. Another interesting observation was that C3aR−/− mice had fewer Th17 cells and reduced AHR after antigen challenge (Lajoie et al. 2010). Accordingly, it appears that C5a and C5aR negatively inhibit asthmatic responses whereas C3a and C3aR have the opposite effects, suggesting a very complicated relationship between complement derived anaphylatoxins and their receptors in the setting of experimental asthma.
Fig. 2. Roles of C3a, C5a and their receptors in development of experimental asthma in mice.
In work by another group, blockade of C5aR promoted Th2 sensitization and development of the asthmatic response whereas, using multi-antigenic challenges, the same type of blockade suppressed the allergen triggered acute inflammatory response in lung (Kohl et al. 2006). Other work has suggested that C5a suppresses IL-12 production (Hawlisch et al. 2005), thus skewing the immune response in lung in the direction of Th2 type responses, resulting in the asthmatic response and suppressing the cell mediated Th1 type response. Based on the complexity of the effects of C5a and C3a and their receptors, and the differences in effects of antigen based on when it was administered into lung and how often (see above), such data have greatly complicated the issue as to whether blockade of either C5a or C3a, or their receptors, would be safe and effective for the treatment of humans with asthma. Finally, there are data suggesting that IL-10 production from LPS-stimulated macrophages can be enhanced in the presence of C5a (Bosmann and Ward [unpublished results]; Zhang et al. 2007). Since IL-10 is a well known for its anti-inflammatory properties, this could be another example in which generation of C5a results in suppression of the inflammatory response by induction of an anti-inflammatory cytokine.
6 Roles of C3, C5 and C5a Receptors in Polymicrobial Sepsis
Extensive work has been done in order to define the roles of C3, C5, C5a and C5a receptors in the setting of polymicrobial sepsis following cecal ligation and puncture (CLP) in rodents (Table 1). Several years ago (Prodeus et al. 1997) and more recently (Flierl et al. 2008), it was shown that C3−/− mice are extremely susceptible to CLP-induced sepsis, possibly related to the loss of protective innate immune factors such as C3b and iC3b which enhance phagocytosis and removal of bacteria from tissues. If one accepts a substantial body of literature suggesting that C5a and its receptors have major negative impacts in the setting of CLP (Ward 2010), the absence of C5 would be expected to be protective. While C3−/− CLP mice had a much higher mortality rate compared to Wt mice, C5−/− mice after CLP were largely indistinguishable from Wt mice, which raises questions as to why C5−/− mice could not be distinguished from C5+/+ mice in the setting of CLP (Flierl et al. 2008). After CLP, Wt mice depleted of C6 with a neutralizing antibody, showed an almost 400-fold increase in bacterial colony forming units in blood when compared to C5+/+ mice, suggesting that products from C5 and C6, such as C5b-9, might be regulating the level of bacteremia after CLP, perhaps related to the ability of C5b-9 to cause lysis of gram-negative bacteria. On the other hand, it has been reported that C6−/− rats or rats depleted of C5 with an antibody had improved survival and reduced bacterial burden in various tissues. On the basis of such conflicting data, it is difficult to determine to what extent C5b-9 plays a significant role in the outcome of polymicrobial sepsis.
Table 1. Examples of adverse outcomes linked to C5a and C5a receptors during polymicrobial sepsis. (Described in greater detail in Ward 2010).
| 1. | Early in sepsis, upregulation on PMNs of CD11b/CD18 and chemokine receptors that are usually expressed at very low levels. |
| 2. | Later in sepsis, paralysis of MAPK signaling pathways in blood PMNs, resulting in loss of innate immune responses of PMNs (phagocytosis, chemotaxis, onset of respiratory burst and activation of NADPH oxidase). |
| 3. | Upregulation on endothelial cells of ICAM-1 and tissue factor. |
| 4. | Enhancement of cytokine/chemokine storm. |
| 5. | Apoptosis of lymphoid cells. |
| 6. | Activation of consumptive coagulopathy and fibrinolytic cascades. |
It is clear that C5a and C5a receptors play a harmful role in the setting of CLP both in rats and in mice. Neutralization of C5a resulted in dramatically improved survival after CLP (Czermak et al. 1999), together with largely intact innate immune responses of blood PMNs from CLP rats (Huber-Lang et al. 2001, 2002b), greatly reduced intensity of activation of the clotting and fibrinolytic cascades (Laudes et al. 2002), substantially reduced evidence of apoptosis of thymocytes (Riedemann et al. 2002), greatly reduced intensity of the cytokine storm (Rittirsch et al. 2008b), reduced evidence of defects in contractility and relaxation responses of cardiomyocytes after CLP (Niederbichler et al. 2006), and reduced levels of plasma/serum biomarkers of multiorgan failure (Huber-Lang et al. 2001, 2002a, b). Using survival of CLP mice as the main endpoint, it was concluded that both C5aR and C5L2 contribute to the adverse outcomes of sepsis. This was determined by use of receptor KO mice (C5aR−/−, C5L2−/−) as well as by the use of blocking antibodies to C5aR and C5L2 (Rittirsch et al. 2008b). One of the interesting facets of these studies was the observation that production of the pleiotropic mediator, HMGB1, which is known to be an important harmful mediator in CLP-induced sepsis (Lotze and Tracey 2005; Qin et al. 2006), was derived from engagement of the C5L2 receptor on macrophages (Rittirsch et al. 2008b). Based on the absence of C5a receptors or antibody-induced inhibition of C5aR and/or C5L2, the cytokine storm was dependent on both receptors, since absence or blockade of either receptor substantially reduced the cytokine storm. This observation suggested that the cytokine storm after CLP may depend on sequential engagement of C5aR and C5L2.
7 Possible Translational Applications to Human Sepsis
Taken together, these data suggest in the setting of polymicrobial sepsis that the sequences of adverse events can be collectively linked to complement activation, production of C5a and engagement of C5a with both C5aR and C5L2. The appearance of C5a (10–100 nM) in serum/plasma from septic human patients, as well as the development of the defects in blood PMNs of these patients, resulting in defective innate immune responses, apoptosis of lymphoid cells resulting in immunodeficiency and intense consumptive coagulopathy and activation of the fibrinolytic system, demonstrate striking parallels between experimental polymicrobial sepsis (CLP) and human sepsis. It is possible that the extensive data obtained in polymicrobial sepsis of rodents may have translational implications for the treatment of septic humans.
Acknowledgments
This work was supported by the National Institutes of Health grants HL-31963, GM-29507, GM-61656.
References
- Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Bruckner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M. Molecular intercommunication between the complement and coagulation systems. Journal of Immunology. 2010;185:5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C, Gerard NP. The C5a Receptor (C5aR) C5L2 Is a Modulator of C5aR-mediated Signal Transduction. Journal of Biological Chemistry. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton PA, Warren JS. Complement component C5 modulates the systemic tumor necrosis factor response in murine endotoxic shock. Infection & Immunity. 1993;61:1474–1481. doi: 10.1128/iai.61.4.1474-1481.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain SA, Monk PN. The orphan receptor C5L2 has high affinity binding sites for complement fragments C5a and C5a des-Arg(74) Journal of Biological Chemistry. 2002;277:7165–7169. doi: 10.1074/jbc.C100714200. [DOI] [PubMed] [Google Scholar]
- Chen NJ, Mirtsos C, Suh D, Lu YC, Lin WJ, McKerlie C, Lee T, Baribault H, Tian H, Yeh WC. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature. 2007;446:203–207. doi: 10.1038/nature05559. [DOI] [PubMed] [Google Scholar]
- Collard CD, Lekowski R, Jordan JE, Agah A, Stahl GL. Complement activation following oxidative stress. Molecular Immunology. 1999;36:941–948. doi: 10.1016/s0161-5890(99)00116-9. [DOI] [PubMed] [Google Scholar]
- Czermak BJ, Sarma V, Pierson CL, Warner RL, Huber-Lang M, Bless NM, Schmal H, Friedl HP, Ward PA. Protective effects of C5a blockade in sepsis. Nature Medicine. 1999;5:788–792. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- Donnelly SC, Haslett C, Reid PT, Grant IS, Wallace WA, Metz CN, Bruce LJ, Bucala R. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nature Medicine. 1997;3:320–323. doi: 10.1038/nm0397-320. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ward PA. Functions of the complement components C3 and C5 during sepsis. Faseb Journal. 2008;22:3483–3490. doi: 10.1096/fj.08-110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon DE, Varani J, Phan SH, Ward JH, Kaplan J, Till GO, Simon RH, Ryan US, Ward PA. Source of iron in neutrophil-mediated killing of endothelial cells. Laboratory Investigation. 1987;57:37–44. [PubMed] [Google Scholar]
- Gao H, Neff T, Ward PA. Regulation of lung inflammation in the model of IgG immune-complex injury. Annual Review Of Pathology. 2006;1:215–242. doi: 10.1146/annurev.pathol.1.110304.100155. [DOI] [PubMed] [Google Scholar]
- Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991;349:614–617. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- Gerard NP, Lu B, Liu P, Craig S, Fujiwara Y, Okinaga S, Gerard C. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. Journal of Biological Chemistry. 2005;280:39677–39680. doi: 10.1074/jbc.C500287200. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt DE, Weaver LJ, Hudson LD, Craddock PR, Jacob HS. Association ofcomplement activation and elevated plasma-C5a with adult respiratory distress syndrome. Pathophysiological relevance and possible prognostic value. Lancet. 1980;1:947–949. doi: 10.1016/s0140-6736(80)91403-8. [DOI] [PubMed] [Google Scholar]
- Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Kohl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of C5a in the absence of C3: a new complement activation pathway. Nature Medicine. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- Huber-Lang MS, Riedeman NC, Sarma JV, Younkin EM, McGuire SR, Laudes IJ, Lu KT, Guo RF, Neff TA, Padgaonkar VA, Lambris JD, Spruce L, Mastellos D, Zetoune FS, Ward PA. Protection of innate immunity by C5aR antagonist in septic mice. FASEB Journal. 2002a;16:1567–1574. doi: 10.1096/fj.02-0209com. [DOI] [PubMed] [Google Scholar]
- Huber-Lang MS, Sarma JV, McGuire SR, Lu KT, Guo RF, Padgaonkar VA, Younkin EM, Laudes IJ, Riedemann NC, Younger JG, Ward PA. Protective effects of anti-C5a peptide antibodies in experimental sepsis. FASEB Journal. 2001;15:568–570. doi: 10.1096/fj.00-0653fje. [DOI] [PubMed] [Google Scholar]
- Huber-Lang MS, Younkin EM, Sarma JV, McGuire SR, Lu KT, Guo RF, Padgaonkar VA, Curnutte JT, Erickson R, Ward PA. Complement-induced impairment of innate immunity during sepsis. Journal of Immunology. 2002b;169:3223–3231. doi: 10.4049/jimmunol.169.6.3223. [DOI] [PubMed] [Google Scholar]
- Kalant D, Cain SA, Maslowska M, Sniderman AD, Cianflone K, Monk PN. The chemoattractant receptor-like protein C5L2 binds the C3a des-Arg(77)/acylation-stimulating protein. Journal of Biological Chemistry. 2003;278:11123–11129. doi: 10.1074/jbc.M206169200. [DOI] [PubMed] [Google Scholar]
- Kalant D, MacLaren R, Cui W, Samanta R, Monk PN, Laporte SA, Cianflone K. C5L2 is a functional receptor for acylation-stimulating protein. Journal of Biological Chemistry. 2005;280:23936–23944. doi: 10.1074/jbc.M406921200. [DOI] [PubMed] [Google Scholar]
- Karp CL, Grupe A, Schadt E, Ewart SL, Keane-Moore M, Cuomo PJ, Köhl J, Wahl L, Kuperman D, Germer S, Aud D, Peltz G, Wills-Karp M. Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nature Immunology. 2000;1:221–226. doi: 10.1038/79759. [DOI] [PubMed] [Google Scholar]
- Kohl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang LH, Best J, Herman NS, Sproles AA, Zwirner J, Whitsett JA, Gerard C, Sfyroera G, Lambris JD, Wills-Karp M. A regulatory role for the C5a anaphylatoxin in, type 2 immunity in asthma. Journal of Clinical Investigation. 2006;116:783–796. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nature Immunology. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen GL, Mitchell BC, Henson PM. The pulmonary response of C5 sufficient and deficient mice to immune complexes. American Review of Respiratory Disease. 1981;123:434–439. doi: 10.1164/arrd.1981.123.4.434. [DOI] [PubMed] [Google Scholar]
- Laudes IJ, Chu JC, Sikranth S, Huber-Lang M, Guo RF, Riedemann N, Sarma JV, Schmaier AH, Ward PA. Anti-C5a ameliorates coagulation/fibrinolytic protein changes in a rat model of sepsis. American Journal of Pathology. 2002;160:1867–1875. doi: 10.1016/S0002-9440(10)61133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Whitfeld PL, Mackay CR. Receptors for complement C5a. The importance of C5aR and the enigmatic role of C5L2. Immunology & Cell Biology. 2008;86:153–160. doi: 10.1038/sj.icb.7100166. [DOI] [PubMed] [Google Scholar]
- Li S, Boackle SA, Holers VM, Lambris JD, Blatteis CM. Complement component C5a is integral to the febrile response of mice to lipopolysaccharide. Neuroimmuno-modulation. 2005;12:67–80. doi: 10.1159/000083578. [DOI] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB): Nuclear weapon in the immune arsenal. Nature Reviews Immunology. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Makita H, Nishimura M, Miyamoto K, Nakano T, Tanino Y, Hirokawa J, Nishihira J, Kawakami Y. Effect of anti-macrophage migration inhibitory factor antibody on lipopolysaccharide-induced pulmonary neutrophil accumulation. American Journal of Respiratory & Critical Care Medicine. 1998;158:573–579. doi: 10.1164/ajrccm.158.2.9707086. [DOI] [PubMed] [Google Scholar]
- Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends in Immunology. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Mulligan MS, Schmid E, Beck-Schimmer B, Till GO, Friedl HP, Brauer RB, Hugli TE, Miyasaka M, Warner RL, Johnson KJ, Ward PA. Requirement and role of C5a in acute lung inflammatory injury in rats. Journal of Clinical Investigation. 1996;98:503–512. doi: 10.1172/JCI118818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederbichler AD, Hoesel LM, Westfall MV, Gao HW, Ipaktchi KR, Sun L, Zetoune FS, Su GL, Arbabi S, Sarma JV, Wang SC, Hemmila MR, Ward PA. An essential role for complement C5a in the pathogenesis of septic cardiac dysfunction. Journal of Experimental Medicine. 2006;203:53–61. doi: 10.1084/jem.20051207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihira J. Macrophage migration inhibitory factor (MIF): its essential role in the immune system and cell growth. Journal of Interferon & Cytokine Research. 2000;20:751–762. doi: 10.1089/10799900050151012. [DOI] [PubMed] [Google Scholar]
- Okinaga S, Slattery D, Humbles A, Zsengeller Z, Morteau O, Kinrade MB, Brodbeck RM, Krause JE, Choe HR, Gerard NP, Gerard C. C5L2, a nonsignaling C5A binding protein. Biochemistry. 2003;42:9406–9415. doi: 10.1021/bi034489v. [DOI] [PubMed] [Google Scholar]
- Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: prognostic and pathogenetic significance. American Journal of Respiratory & Critical Care Medicine. 1997;155:1187–1205. doi: 10.1164/ajrccm.155.4.9105054. [DOI] [PubMed] [Google Scholar]
- Prodeus AP, Zhou XN, Maurer M, Galli SJ, Carroll MC. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- Qin SX, Wang HC, Yuan RQ, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin XC, Sherry B, Kumar A, LaRosa G, Newman W, Tracey KJ, Yang H. Role of HMGB1 in apoptosis-mediated sepsis lethality. Journal of Experimental Medicine. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch JV, Clynes RA. Divergent roles for Fc receptors and complement in vivo. Annual Review of Immunology. 1998;16:421–432. doi: 10.1146/annurev.immunol.16.1.421. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Laudes IJ, Keller K, Sarma VJ, Padgaonkar V, Zetoune FS, Ward PA. C5a receptor and thymocyte apoptosis in sepsis. FASEB Journal. 2002;16:887–888. doi: 10.1096/fj.02-0033fje. [DOI] [PubMed] [Google Scholar]
- Rittirsch D, Flierl MA, Day DE, Nadeau BA, McGuire SR, Hoesel LM, Ipaktchi K, Zetoune FS, Sarma JV, Leng L, Huber-Lang MS, Neff TA, Bucala R, Ward PA. Acute lung injury induced by lipopolysaccharide is independent of complement activation. Journal of Immunology. 2008a;180:7664–7672. doi: 10.4049/jimmunol.180.11.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Kohl J, Gerard C, Sarma JV, Ward PA. Functional roles for C5a receptors in sepsis. Nature Medicine. 2008b;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scola AM, Johswich KO, Morgan BP, Klos A, Monk PN. The human complement fragment receptor, C5L2, is a recycling decoy receptor. Molecular Immunology. 2009;46:1149–1162. doi: 10.1016/j.molimm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seekamp A, Mulligan MS, Till GO, Ward PA. Requirements for neutrophil products and L-arginine in ischemia-reperfusion injury. American Journal of Pathology. 1993;142:1217–1226. [PMC free article] [PubMed] [Google Scholar]
- Seekamp A, Ward PA. Ischemia-reperfusion injury. Agents & Actions - Supplements. 1993;41:137–152. [PubMed] [Google Scholar]
- Solomkin JS, Cotta LA, Satoh PS, Hurst JM, Nelson RD. Complement activation and clearance in acute illness and injury: evidence for C5a as a cell-directed mediator of the adult respiratory distress syndrome in man. Surgery. 1985;97:668–678. [PubMed] [Google Scholar]
- Sylvestre D, Clynes R, Ma M, Warren H, Carroll MC, Ravetch JV. Immuno-globulin G-mediated inflammatory responses develop normally in complement-deficient mice. Journal of Experimental Medicine. 1996;184:2385–2392. doi: 10.1084/jem.184.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till GO, Friedl HP, Ward PA. Lung injury and complement activation: role of neutrophils and xanthine oxidase. Free Radical Biology & Medicine. 1991;10:379–386. doi: 10.1016/0891-5849(91)90046-6. [DOI] [PubMed] [Google Scholar]
- Till GO, Johnson KJ, Kunkel R, Ward PA. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. Journal of Clinical Investigation. 1982;69:1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till GO, Morganroth ML, Kunkel R, Ward PA. Activation of C5 by cobra venom factor is required in neutrophil-mediated lung injury in the rat. American Journal of Pathology. 1987;129:44–53. [PMC free article] [PubMed] [Google Scholar]
- Varani J, Fligiel SE, Till GO, Kunkel RG, Ryan US, Ward PA. Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Laboratory Investigation. 1985;53:656–663. [PubMed] [Google Scholar]
- Ward PA. Rous-Whipple Award Lecture. Role of complement in lung inflammatory injury. American Journal of Pathology. 1996;149:1081–1086. [PMC free article] [PubMed] [Google Scholar]
- Ward PA. The Harmful Role of C5a on Innate Immunity in Sepsis. Journal of Innate Immunity. 2010;2:439–445. doi: 10.1159/000317194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PA, Hill JH. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. Journal of Immunology. 1970;104:535–543. [PubMed] [Google Scholar]
- Warren JS, Yabroff KR, Remick DG, Kunkel SL, Chensue SW, Kunkel RG, Johnson KJ, Ward PA. Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. Journal of Clinical Investigation. 1989;84:1873–1882. doi: 10.1172/JCI114374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watford WT, Ghio AJ, Wright JR. Complement-mediated host defense in the lung. American Journal of Physiology - Lung Cellular & Molecular Physiology. 2000;279:L790–798. doi: 10.1152/ajplung.2000.279.5.L790. [DOI] [PubMed] [Google Scholar]
- Watford WT, Wright JR, Hester CG, Jiang H, Frank MM. Surfactant protein A regulates complement activation. Journal of Immunology. 2001;167:6593–6600. doi: 10.4049/jimmunol.167.11.6593. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, Konig P, Gerard NP, Gerard C, Wills-Karp M, Kohl J. A Critical Role for C5L2 in the Pathogenesis of Experimental Allergic Asthma. Journal of Immunology. 2010;185:6741–6752. doi: 10.4049/jimmunol.1000892. [DOI] [PubMed] [Google Scholar]