Abstract
The parallel expression of activation products of the coagulation, fibrinolysis, and complement systems has long been observed in both clinical and experimental settings. Several interconnections between the individual components of these cascades have also been described, and the list of shared regulators is expanding. The coexistence and interplay of hemostatic and inflammatory mediators in the same microenvironment typically ensures a successful host immune defense in compromised barrier settings. However, dysregulation of the cascade activities or functions of inhibitors in one or both systems can result in clinical manifestations of disease, such as sepsis, systemic lupus erythematosus, or ischemia–reperfusion injury, with critical thrombotic and/or inflammatory complications. An appreciation of the precise relationship between complement activation and thrombosis may facilitate the development of novel therapeutics, as well as improve the clinical management of patients with thrombotic conditions that are characterized by complement-associated inflammatory responses.
Keywords: Anaphylatoxin, Coagulation, Complement, Fibrinolysis, Inflammation, Sepsis
Introduction
In recent years, an increasing body of evidence has demonstrated that regulatory enzymes participate in tightly organized processes that control a wide range of biological functions, apart from protein catabolism. Among the enzymatic cascades, major roles are attributed to the complement system [1, 2], the coagulation cascade [3, 4], and the fibrinolytic system [5, 6]. One common characteristic of these three protein networks is that they consist mainly of serine proteinases with trypsin-like activity, together with their activators and inhibitors [7–9]. More specifically, both the complement and the coagulation serine proteinase cascades have been associated with functions of the immune and cardiovascular systems. Description of the components and roles of these systems began as early as in the seventeenth and eighteenth centuries (reviewed in [4, 10] for coagulation and [2, 11] for complement).
The individual constituents of the complement and coagulation systems are finely orchestrated to form two distinct multi-component protein networks, which can have several crossover points linking these two cascades. The common role of these systems is to present a first line of defense against pathogens and other invaders that may enter the circulation. The common interactions of cascade components and their impact on inflammatory responses will be discussed in the following sections. In addition, we will discuss the possibility of using well-known anticoagulant or anti-inflammatory compounds as the basis for developing therapeutics that can target the pathways shared by the complement and coagulation systems.
Coagulation and fibrinolysis
Hemostasis, defined as the cessation of bleeding in the body, takes place continuously at a very low level and slightly favors a balanced anticoagulant state, with procoagulant factors becoming activated under tightly controlled conditions [4]. It is now well established that hemostasis involves a combination of processes supporting blood clotting (coagulation) in sites at which the vascular integrity has been compromised and a subsequent dissolution of blood clots (fibrinolysis) [12]. Hemostasis is dependent on the functions of blood cells, the vascular system, several soluble plasma proteins, and low molecular weight components occurring in a strictly organized and timely fashion at the site of vascular injury. Platelets play a major role in the regulation of hemostasis, their activation resulting in platelet aggregation at the site of injury [13]. Activated platelets provide a negatively charged surface in areas in which the clotting cascade is initiated, resulting in regulation of mediators vital to effective hemostatic responses.
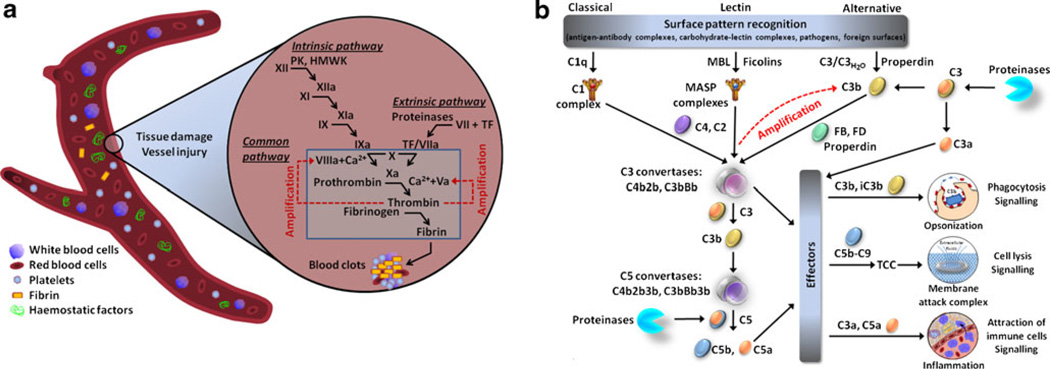
The concept of the stepwise activation of coagulation was introduced in 1964 [14]. Blood coagulation has been considered to be the result of the activation of two different systems, the intrinsic and extrinsic cascades, depending on the factor that acts as the initiator of the cascade (Fig. 1a). The instigation of both coagulation cascades occurs at a cell surface (platelets, foreign micro-particles, biomaterials or activated endothelium) [4], resulting in proteolytic activation of soluble coagulation factors (serine proteinases) by other components of the cascade. Although this abundance of activated enzymes raises the possibility of functional duplication, each component of the cascade has a specific role in the system. Both cascades culminate in the release of activated factor X (Xa), ultimately leading to the proteolytic activation and conversion of prothrombin to thrombin. Recently, it has been suggested that one common coagulation pathway, dependent on the activity of tissue factor (TF; extrinsic pathway), can be activated on different surfaces in vivo, while the importance of the intrinsic pathway at in vivo settings is not fully elucidated [4, 15].
Fig. 1.
Coagulation and complement cascades. a The coagulation branch of the hemostatic system. Coagulation takes place via the intrinsic and extrinsic pathways. The intrinsic pathway is initiated in vitro by contact activation of factor XII, in a plasma kallikrein-high molecular weight kininogen-dependent fashion (contact system). Triggering of the extrinsic pathway, which is considered the primary mode of in vivo coagulation, is tissue factor (TF) dependent and takes place on the surface of activated cells. The two pathways are based on the sequential activation of the coagulation factors, which converge in the catalytic generation of active thrombin by its zymogen proteinase. Thrombin is responsible for the proteolytic transformation of fibrinogen to fibrin and the subsequent generation of fibrin clots. The designation “a” following the various clotting factors represents a state of activation of the factors. Activated platelets have a major role in this process, as they can provide their negatively charged phospholipid area as a surface for initiation of coagulation. b Complement activation pathways. The components of complement system can be organized into three major pathways: the classical pathway is mainly initiated by the binding of C1q to antigen–antibody complexes, whereas the lectin pathway is triggered by binding of mannose-binding lectin (MBL) or ficolins to glycosylated surfaces on microbial cell walls. Both pathways lead to the formation of a common C3 convertase, an enzyme complex with serine proteinase trypsin-like specificity. The alternative pathway, on the other hand, can be triggered by spontaneous hydrolysis of the internal thioester bond of C3, leading to the formation of C3H2O. This non-proteolytically activated form of C3 can lead to the formation of the alternative pathway C3 convertase by interacting with factors B and D. This convertase formation can be further induced and stabilized by properdin. C3 convertases generated by all pathways are able to cleave C3 into C3a and C3b, the latter of which forms additional convertases, thereby rapidly amplifying complement response. C3b vitally contributes to the clearance of pathogens by phagocytes (macrophages and neutrophils) and is a major component of the C5 convertase, which in turn cleaves C5 to C5a and C5b. The anaphylatoxins C3a and C5a mediate the inflammatory responses of complement. C5b subsequently takes the lead in formation of the terminal C5b-9 complement complex, also called MAC, ultimately resulting into cell lysis. Potential roles in the proteolytic activation of C3 and C5 have also been assigned to non-complement proteinases, including enzymes of the coagulation and fibrinolysis cascades
Coagulation is resolved by fibrinolysis, which involves a distinct enzymatic cascade that leads to the removal of fibrin deposits [6]. The key component of the fibrinolytic system is plasminogen, the zymogen of the serine proteinase plasmin. Plasmin is chiefly generated by two other serine peptidases, the tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA) [16]. tPA is the activator that is mainly involved in fibrinolysis, in which generation of active plasmin is triggered on the fibrin surface, while uPA is responsible for the generation of soluble plasmin. tPA is clinically used to dissolve intravascular clots in the very early stages of acute myocardial ischemia [17] and in patients with stroke [18].
Apart from their straightforward role in activating downstream components of the enzymatic cascade, the coagulation and fibrinolysis systems can be viewed as intermediaries that convert mechanical information (fibrin deposits, blood clots) from a damaged tissue or leaky vessel into biochemical signals that trigger cell responses. In this regard, many of the effector serine proteinases of the two systems can act as ligands to confer pro- or anti-inflammatory properties on cells via a family of G-protein-coupled receptors, the proteinase-activated receptors (PARs) [19, 20]. The prototype of this signaling is found in thrombin, which activates PAR1, resulting in vascular biological and inflammatory responses, especially those involving platelet aggregation [21]. Another novel proteinase-triggered inflammatory mechanism has been discovered for plasmin; in addition to regulating PAR activity [22], plasmin can cleave the annexin A2 hetero-tetramer, triggering chemotactic responses by monocytes and macrophages [23, 24].
Complement system
The complement cascade was first described in the late 1800s (reviewed in [11]) and so named to reflect its capacity to enhance antibacterial activity of humoral components [2, 25, 26]. The complement protein network was later shown to be an essential component of the innate immune system, the first line of defense against microbial invaders (for review, see [2, 25]). Complement is also responsible for the enhancement of humoral immune responses, which are dependent on the production and binding of antibodies to foreign substances in order to facilitate their elimination by the immune system [2, 27, 28]. Recent studies have also suggested that complement participates in several non-inflammatory processes, such as coagulation, hematopoiesis, reproduction, liver regeneration, apoptosis, homeostasis, metabolism, and central nervous system development [2].
The complement system consists of numerous plasma and membrane-bound proteins [2]. This protein network is characterized by a level of complexity dictated by the diverse structural and functional properties of its components. Because of the specific structural nature of the initiators of the complement pathways, activation does not readily occur unless a change in the microenvironment triggers an appropriate surface pattern (pathogen or damage associated) and the subsequent binding of complement factors [2, 27]. The complement pathways are stratified (Fig. 1b; [2, 25, 29]), according to their respective surface recognition patterns, into three major pathways: (a) the classical pathway, which is mainly initiated by antigen–antibody complexes; (b) the lectin pathway, which is initiated by mannose-containing glycoproteins or carbohydrates on microbial surfaces; and (c) the alternative pathway, which is triggered by spontaneous activation of complement component 3 (C3) by hydrolysis (C3H2O) or by binding of C3b molecules to properdin [25, 30]. All three of these protein networks are activated through a series of ion-dependent proenzyme-to-active serine proteinase conversions, resulting in the formation of a C3 convertase [2, 31, 32]. The convertase can in turn trigger activation of more C3 molecules amplifying the generation of C3b (amplification phase). Subsequent steps of the cascade lead to production of the effector molecules of complement. The main biological activities of complement activation are as follows [2]: (a) the opsonization of pathogens mediated by the cleavage products of C3 (i.e., C3b and iC3b) and C4 (C4b); (b) the recruitment and activation of inflammatory cells by the anaphylatoxins C3a and C5a, which are proteolytically released from C3 and C5, respectively; (c) the direct elimination of pathogens by means of phagocytosis via complement receptors or by cell lysis as a result of formation of the membrane attack complex (MAC, C5b-9); and (d) the tuning of adaptive immunity by downstream stimulation of B and T cells.
A major part in facilitating the inflammatory role of complement effector molecules is played by cell-surface proteins that recognize and bind to the complement components [2]. For example, the anaphylatoxins C3a and C5a can signal to cells and tissues via two members of the G-protein-coupled receptor family, the C3a receptor (C3aR) and C5a receptor (C5aR) [33]. Furthermore, C5a receptor-like 2 (C5L2) has been described as a putative receptor for ASP (the desArg derivative of C3a) [34, 35], while it has also been proposed to act as a decoy/synergistic cell-surface receptor that can regulate the C5a and C5adesArg signals directed toward C5aR [36–38]. More recently, a link between the complement lectin and thrombin-associated PAR pathways has been identified, in the form of the complement mannose-binding lectin (MBL)-associated serine proteinase 1 (MASP-1), which seems to be involved in PAR4-dependent activation of endothelial cells [39].
Related proteinases present in blood
In addition to the complement and coagulation components, several other pro- or anti-inflammatory proteins are highly expressed by tissues and cells in close proximity to the circulatory system or are derived from pathogenic microorganisms disseminated in blood. Among these are the serine proteinases that are secreted by leukocytes, endothelial, and epithelial cells. In particular, the neutrophil-released proteinases cathepsin-G, proteinase-3, and elastase can mediate the inactivation of IL-6, while elastase and cathepsin-G can proteolytically inactivate tumor necrosis factor (TNF), indicating a potential regulatory role for these proteinases in neutrophil-dependent inflammatory responses [40]. An equally important role in immune responses is played by proteinases of microbial origin, which are released during bacterial infection and play a major role in bacterial immune invasion strategies [2, 25]. For example, Pseudomonas aeruginosa elastase (an elastolytic metalloproteinase) can disarm PAR2 [41] and degrade plasma C3 [42], thereby attenuating the inflammatory capacity of host tissues. Porphyromonas gingivalis has also been shown to release proteolytic enzymes with degradative activity for C5aR [43], while at the same time, these enzymes may be able to regulate the availability of active anaphylatoxins [44, 45]. The potential interplay of these microbial proteinase networks with the coagulation and complement enzymes and their capacity to modulate inflammatory responses make them important players in the vascular/inflammatory niche.
Coagulation and complement
As mentioned above, coagulation and complement are two distinct systems with unique pathophysiological roles. Nevertheless, these networks have several common functional attributes, which are often overlooked. (a) Both systems serve as innate defenses against external threats (microbial invasion). (b) The presence of foreign or altered cellular surfaces is required for initiation of both pathways. This requirement ensures tight orchestration of a rapid but controlled initiation of the cascade in terms of its spatiotemporal localization. Such localization, for example, in close proximity to the vascular endothelium, lowers the kinetic requirements for the reaction to occur because it causes a local increase in otherwise minimal local concentrations of the appropriate trigger. (c) The cascade reactions of both systems can be organized into three phases: initiation, amplification, and propagation (Fig. 1), often occurring not only coincidentally but also in a self-reinforcing manner. The cascade organization allows for multiple points of activation, amplification, negative or positive regulation, and interaction with other systems. (d) Regulatory molecules (i.e., natural inhibitors or cofactors) are present at the same settings and can physiologically regulate both systems. Restraint of the coagulation and complement systems occurs at two levels: inhibition of the actual enzyme activities and/or restriction of the binding capacity of a cascade component. (e) Certain components of each cascade interact with cell-surface receptors mediating the downstream biological effects. These common characteristics of the coagulation and complement systems have allowed for multiple instances of cross-talk and can explain the association of both systems with several clinical inflammatory and thrombotic conditions.
Interplay between the coagulation and complement cascades
Early observations have pointed to the existence of significantly higher levels of complement activation products in human serum than in anti-coagulated blood, strongly suggesting the development of complement activation during blood clotting [46]. The most important interconnections of the complement and coagulation systems have emerged as evidence for cross-talk between the components of the two cascades (Fig. 2). For instance, early work has shown that the rabbit orthologue of plasma kallikrein is able to release the anaphylatoxin C5a as a result of the limited proteolysis of C5 [47]. Furthermore, human plasma kallikrein has been reported to affect the generation of active C3 fragments, either directly of via activation of factor B [48–50].
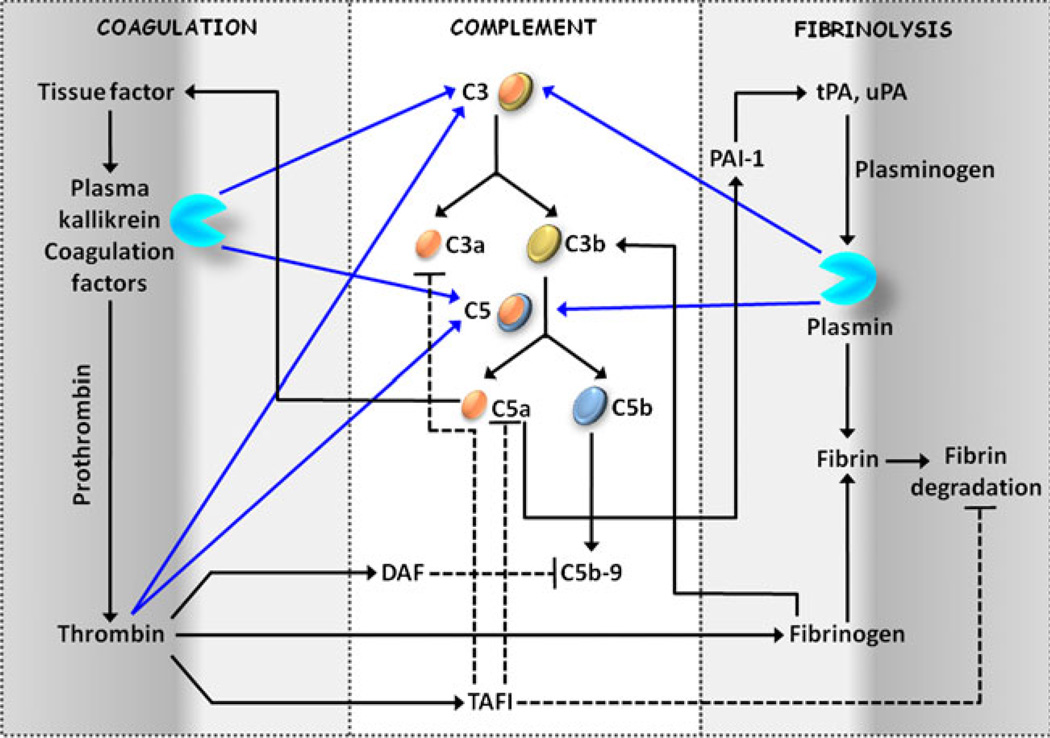
Fig. 2.
Interconnections between coagulation and complement. Components of the coagulation, fibrinolysis, and complement cascades are highlighted to demonstrate the potential vital intercommunication that the three systems may exhibit in vivo. More details on these interactions are discussed in the text. Proteolytic cleavages are represented by blue lines, while black-colored lines depict non-proteolytic interactions. Related inhibitory actions on the pathway components are shown by dotted lines
Nevertheless, the potential initiation of complement cascade by proteinases of the coagulation cascade has only lately come into sharper focus. Recent work has shown that C5a can be generated in C3-deficient mice as a result of the proteolytic activation of C5 by thrombin [51]. Furthermore, C5a released by the enzymatic action of thrombin possessed significant chemotactic activity for neutrophils, indicating that a participation of thrombin in an inflammatory pathway alternative to the activation of PAR1 may occur. Subsequent investigation has revealed that both C3 and C5 can be proteolytically activated by several components of the coagulation cascade in addition to thrombin [52]. More specifically, incubation of C3 or C5 with FIXa, FXa, FXIa (where the designation a denotes the active form of the coagulation factor), thrombin, and plasmin resulted in the release of C3a and C5a, respectively, while TF, FVII, FVIIa, and activated protein C (APC) were unable to proteolytically generate these anaphylatoxins. Apart from the coagulation factors, plasmin can, therefore, serve as another common bridge between innate immunity and hemostasis. Farther downstream in the coagulation cascade, platelet activation and subsequent expression of P-selectin have been associated with complement activation, supporting a novel mechanism for local inflammation at the site of vascular injury [53].
Activation of complement by components of the coagulation cascade can also occur upstream of the C3 and C5 convertases. For instance, coagulation factor XIIa is able to activate the complement complex C1, leading to the initiation of the classical complement pathway [54, 55]. Chondroitin sulfate on platelet surface can also contribute to binding of C1q, as well as the complement regulators C1 inhibitor (C1INH), C4b-binding protein (C4BP), and factor H [56]. Furthermore, the interaction of fibrinogen/fibrin with recognition molecules that trigger initiation of the lectin pathway can modulate the activation of complement components C3 and C4 and the complement deposition on the surface of microbial pathogens [57].
On the other hand, supporting evidence for a procoagulant effect of complement suggests that the coagulation cascade is activated by complement either directly or indirectly; the direct procoagulant activity of complement can be mediated on different levels. For example, MASP-2, a component of the lectin complement pathway, plays a role in the activation of thrombin and subsequent generation of the fibrin mesh [58, 59].
Complement effectors can also facilitate biochemical and morphological changes in the endothelium, resulting in modulation of the clotting propensity of blood. For instance, C5a in combination with antibodies against endothelial cells can trigger the release of the endothelial surface proteoglycan heparan sulfate, independent of the formation of the MAC and the lysis of endothelial cells [60]. Heparan sulfate can regulate the conformational activation of antithrombin (AT) [61], an inhibitor of several components of the coagulation cascade. Heparan sulphate has also been shown to facilitate the properdin-mediated C3 deposition onto proximal tubular epithelial cells, suggesting a potential role in complement-associated renal disease [62].
Considerable experimental evidence indicates that, in addition to regulating the individual cascade components, complement activity can also modulate the aggregative properties of platelets. An early report has indicated that combinations of C3 and MAC components enhance the thrombin-mediated platelet aggregation and serotonin secretion [63], although there may be important differences related to the species from which platelets are harvested. Formation of complement C5b-9 on cell surfaces can trigger changes in the membrane-associated components, affecting the activation of platelets and initiation of coagulation [64]. Stimulation of platelets with sublytic concentrations of the MAC can cause transient membrane depolarization [65], granule secretion [66], and induction of platelet-catalyzed thrombin generation and clotting [67]. Formation of MAC mediates the release of membrane microparticles from platelets [68] and endothelial cells [69], which can expose binding sites for factor Va and serve as a basis for the proteolytic generation of thrombin from its proenzyme by the prothrombinase complex. Platelet activation has also been reported after the binding of C1q to its receptor on platelet surfaces, a process that can further induce the aggregation of platelets via a P-selectin-dependent pathway [70, 71].
Finally, activation products of C3, namely, the anaphylatoxin C3a and its derivative C3adesArg, can directly induce platelet activation and aggregation [72]. Such aggregative properties of complement are further supported by recent studies that demonstrated delayed thrombosis after vessel wall injury in C3-deficient mice [73] and platelet hyper-reactivity in mice deficient in the negative regulator of MAC, CD59b (a highly active mouse variant of CD59) [74]. Binding of the non-proteolytically activated form of C3 (C3H2O) to the activated platelet surface has also been reported; however, the impact of this interaction on platelet aggregation was not investigated [75].
Regulation of TF expression on endothelial cells is another direct way in which complement activation can affect coagulation. Under conditions in which the integrity of the blood vessels is compromised, TF induction on endothelial cell surfaces allows coagulation factors to initiate activation of the extrinsic coagulation pathway (Fig. 1a). C5a can trigger the induction of TF expression and activity in human endothelial cells [76] and neutrophils [77] (Fig. 2). This latter finding may explain the procoagulant properties of bronchoalveolar lavage fluid in patients with acute respiratory distress syndrome [78]. A cytolytically inactive form of the terminal MAC is also known to induce TF expression on human endothelial cells [79].
In addition to platelets and endothelial cells, mast cells play an important role in the regulation of coagulation. Mast cells are often present at sites of inflammation and can prevent thrombosis through the expression of tPA, which generates the fibrinolytic proteinase plasmin [80]. Treating mast cells and basophils in vitro with C5a causes an upregulation in plasminogen activator inhibitor 1 (PAI-1), a vital regulatory component of the fibrinolysis cascade that is able to neutralize the enzymatic activity of tPA [81]. By inducing the expression of PAI-1, C5a can abolish the fibrinolytic activity of mast cells, in favor of a procoagulant phenotype [82].
Less direct effects of complement activation on hemostasis have also been described. These can occur via the regulation of specific cytokine networks, which may determine the balance between anticoagulant and procoagulant pathways [83, 84]. For example, the C5a and C3a anaphylatoxins are thought to influence the production and secretion of tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) from Kupffer liver cells [85, 86]. TNF-α and IL-6 may subsequently trigger TF expression from blood cells and endothelial cells [84, 87, 88], while IL-6 may influence the clotting propensity of platelets [87, 89]. Inflammatory cytokines can also modulate the levels of antithrombotic proteins, such as thrombomodulin [83]. Furthermore, the C1q-dependent production of monocyte chemo-attractant protein 1 (MCP-1), IL-6, IL-8, and macrophage inflammatory protein 1β (MIP-1β) from gingival and periodontal ligament fibroblasts has also been reported [90], although their role in the activation of coagulation components remains unknown.
The interplay of coagulation and complement cannot only trigger the activation of both cascades but also exert a negative regulatory effect on their biological activity. For instance, thrombin can induce the expression of the complement regulator decay accelerating factor (DAF) in a PAR1-dependent manner [91]. This induction results in greatly reduced C3 deposition and the complement-mediated lysis of endothelial cells in vitro. In a similar manner, thrombin-activatable fibrinolysis inhibitor (TAFI, also known as carboxypeptidase R or plasma carboxypeptidase B), generated by a thrombomodulin–thrombin complex, can play a dual role in the inhibition of plasmin-mediated fibrinolysis and the inactivation of C3a and C5a [92, 93].
Coagulation and complement in clinical settings
In clinical conditions, systemic inflammation often occurs together with coagulation abnormalities of varying severity [94]. The coagulation system seems to play an important role in host–pathogen interactions and the immune responses of the host. Localized formation of the fibrin mesh offers a physical barrier to potentially invading bacteria at sites of trauma [95]. It has been suggested that at the same time complement activation occurs immediately after trauma, and the level of activation can determine the clinical outcome of the injured patient [96]. This work has indicated C3a as a marker of trauma severity and a prognostic indicator of survival.
Several investigators have noted that activation of the complement cascade is correlated with thrombosis and the development of multiple organ failure. The most highly studied clinical condition in which coagulation and complement-related inflammation coexist is sepsis. However, several other inflammatory settings have also been connected to thrombotic events and vice versa. In the next few paragraphs, we will discuss several of these conditions.
Systemic Inflammatory Response Syndrome and Disseminated Intravascular Coagulation
Sepsis is a multifaceted medical condition that is characterized by an overwhelming systemic inflammatory response [97–99]. Worldwide, 13 million people become septic each year, and about one-third of these individuals will eventually succumb to the disease [100]. In the USA alone, septicemia accounts for approximately 215,000 deaths among the 750,000 reported cases per year, with mortality rates being higher in septic patient with advanced disease severity. As the average age of the world’s population continues to increase, accompanied by a higher incidence of related co-morbidities and immunosuppressive conditions, sepsis is rapidly becoming a serious and increasingly common clinical problem.
Despite the complexity of sepsis, there is good evidence to suggest that hyper-activation of the complement and innate immune systems may be linked to the excessive septic inflammatory response, referred to as the systemic inflammatory response syndrome (SIRS) [101, 102]. Sparked by an initial condition such as bacterial infection, immune cells can produce a variety of inflammatory mediators, including cytokines, chemokines, and complement activation products that embody the septic proinflammatory microenvironment. Failure of the regulatory safety switches to contain the inflammatory hyperactivation during sepsis can result in persistent inflammation; this unrestricted inflammation can eventually lead to tissue/organ damage and, possibly, death.
In addition to trauma, sepsis is one of the most common causes of disseminated intravascular coagulation (DIC) and multiple organ dysfunction syndrome (MODS) [103]. Coagulation in sepsis cause disseminated consumptive coagulopathy, in which organ dysfunction can be the result of intravascular fibrin deposition following local coagulation activation, inhibition of anticoagulants (such as AT), or blockage of fibrinolysis [101, 104]. As sepsis progresses, immune and endothelial cells are stimulated by proinflammatory cytokines. The resulting expression of TF can lead to a systemic activation of coagulation. DIC is characterized by an increased incidence of thrombin formation or hemorrhagic diathesis (increased bleeding tendency) as a result of the consumption of platelets and coagulation proteins in the circulation [97, 105]. DIC contributes to MODS, a clinical complication that adversely affects the survival rates of patients with sepsis. MODS is the leading cause of death in septic patients admitted to intensive care units [106, 107] and is accompanied by hemostatic changes, complement and platelet activation, and release of proinflammatory mediators [105, 108, 109].
Activation of platelets is a common event during sepsis, often associated with consumptive coagulopathy and thrombocytopenia [110, 111]. Activated platelets can release a serine/threonine protein kinase that is able to phosphorylate C3 [112, 113]. This modification can potentially result in the generation of a phosphorylated C3b fragment that is resistant to further proteolytic processing into iC3b by factor I. It has also been suggested that phosphorylation can increase deposition of C3b and binding to its receptor CR1 [113]. Therefore, phosphorylation of C3/C3b could potentially perpetuate the formation of the alternative pathway convertase and cause persistent complement activation.
The nature of the direct interactions between the coagulation and complement systems in sepsis has been a matter of conjecture. Complement activation products, such as C3a, C4a, and C5a, are elevated in patients with sepsis [109, 114]. Furthermore, increased anaphylatoxin levels are correlated with an adverse outcome in sepsis [114]. Specifically, C5a is a major player in the pathogenesis of disease (Fig. 3; [102]), possibly exerting its deleterious effects via its receptors C5aR and C5L2 [101, 115]. C5a can also cause activation of the coagulation cascade and Toll-like receptor pathways [45, 101, 116]. It also modulates the release of various inflammatory components, such as chemokines/cytokines, macrophage migration-inhibitory factor (MIF), and high mobility globulin B1 (HMGB1), which can have pleiotropic effects [101, 102, 115–117]. Therefore, complement may promote procoagulant activity or induce cytokine production, while the hemostatic changes can in turn affect the level of complement activation as outlined in the previous sections.
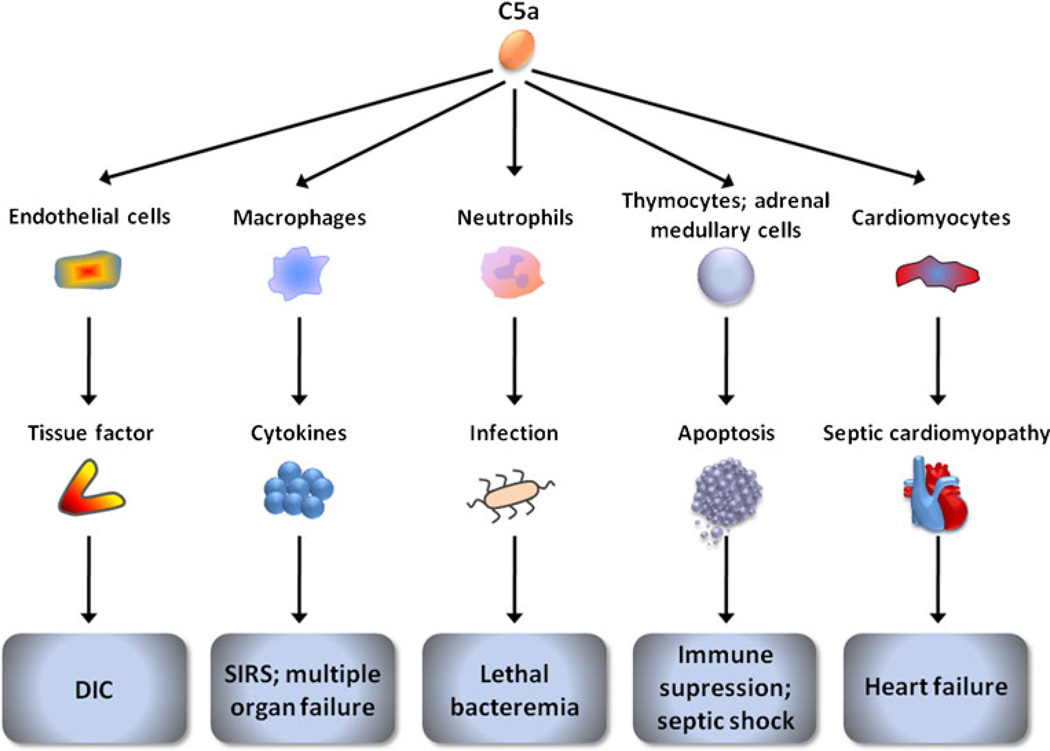
Fig. 3.
A potential cardinal role for the complement anaphylatoxin C5a in septicemia. In patients with sepsis, systemic activation of complement and persistent release of C5a can induce the upregulation of tissue factor (TF) by immune and endothelial cells, leading to the development of disseminated intravascular coagulation (DIC). DIC, in cooperation with the C5a-mediated release of pro-inflammatory cytokines, can lead to the multiple organ dysfunction that characterizes the systemic inflammatory response syndrome (SIRS). In advanced sepsis, C5a can elicit neutrophil dysfunction, adversely affecting the host’s immune response to opportunistic infections. C5a can also cause apoptosis of thymocytes, possibly lymphocytes, and adrenal medullary cells, resulting in immunosuppression or septic shock. C5a has further been implicated in the development of septic cardiomyopathy leading to heart failure. The aforementioned outcomes of C5a induction can potentially be triggered by cell signaling via its two receptors, C5aR and C5L2 (reviewed in [101, 102, 115])
The interconnections between coagulation and complement may be significant for the management of septic patients, in whom amplification of the complement and coagulation cascades parallels the clinical progression of the disease. Support for this hypothesis comes from the potential of regimens that regulate coagulation, such as AT or APC, to improve the overall survival of patients with sepsis, which will be discussed hereafter.
Other clinical manifestations of thrombotic complement activity linked to intravascular thrombosis
Apart from SIRS, coagulation is activated in various other clinical conditions that are manifested together with complement-related inflammation [118]. C5a-mediated induction of TF in peripheral blood neutrophils has been described in patients with antiphospholipid syndrome [77] and acute respiratory distress syndrome [78]. Atypical hemolytic uremic syndrome (aHUS), paroxysmal nocturnal hemoglobinuria (PNH), and hereditary angioedema (HAE) are other clinical examples of dysfunctional complement regulation that lead to extravagant complement function associated with thrombotic disturbances [2, 118]. Studies have suggested that the mechanisms of thrombosis in PNH [119], aHUS [120], and HAE [121] are related to a complement-induced platelet hyperactivation. In patients with glomerulonephritis or vasculitis, the presence of downstream effectors of the complement system such as C3b is associated with intravascular fibrin deposition [118, 122]. Furthermore, in glomerular-associated renal failure, uPA, acting via its specific receptor (uPAR), induces the expression of C5aR [123]. A role for consumptive complement activation has also been suggested in thrombotic diseases such as ischemic stroke [124] and ischemia–reperfusion injury [125]. More specifically, in ischemic stroke, an MBL deficiency has been found to be associated with a more favorable clinical outcome after acute stroke in both mice and humans [126].
Furthermore, systemic lupus erythematosus (SLE) is a diverse autoimmune disease that has been mainly associated with a deficiency in C1q and a subsequently enhanced interferon-alpha production [127]. A loss in other complement components, namely, C1r, C1s, C4, and C2, has also been associated with SLE, but these deficiencies are less frequent [128]. Patients with SLE are at great risk for coagulation-related pathologies, including venous thrombosis and atherosclerosis [129].
An emerging field of research studying conditions where complement and coagulation synergize is the biomaterial-induced inflammation [130]. Medical devices and extracorporeal circuits commonly used for therapeutics, as well as vehicles used for drug delivery can potentially facilitate the activation of complement and coagulation systems. In this regard, a recent study has examined the procoagulant activity mediated by complement in long-term hemodialyzed patients [131]. Biomaterial-induced generation of the complement anaphylatoxin C5a was found to increase active TF expression in peripheral blood neutrophils, pointing to an increased risk of hemodialysis patients for thrombotic complications. This effect was abolished by the use of the complement inhibitor compstatin in an ex vivo model of hemodialysis.
Similar activation may also occur in the case of organ transplantation, in which the fate of the graft may be dependent on both complement and coagulation activity, as it has been suggested by work on an ex vivo heart model of xenotransplantation [132]. Consistent with this hypothesis, a recent proteomic analysis has pointed to 18 plasma proteins related to inflammation, complement activation, blood coagulation, and wound healing that may be associated with acute graft rejection [133].
The role of complement in other inflammatory conditions in which hemostatic dysfunction has been implicated is only now beginning to unfold. For example, rheumatoid arthritis [134], inflammatory bowel disease [135], and the inflammatory skin conditions urticaria and psoriasis [136] can be characterized by a thrombotic phenotype. Some of this work has pointed to a role for complement activation in the pathogenesis of acute dextran sulfate sodium (DSS)-induced colitis [135], supporting the potential involvement of complement and coagulation in inflammatory bowel disease (IBD). In principle, complement activation in these conditions can facilitate the release of proinflammatory modulators such as C5a and TNF-α, which can in turn also induce a TF-dependent initiation of coagulation [77, 78].
Furthermore, activation of the serine proteinases participating in both the complement and coagulation cascades can potentially induce immune cell activation and inflammation via PAR signaling [19, 39]. The parallel release of other active proteolytic enzymes from immune cells and inflamed tissues can amplify the potential enzymatic cascade interactions and signaling events that may take place within a shared inflammatory-thrombotic niche.
Mutual endogenous and therapeutic regulators of coagulation and complement
The optimal functional level of any enzymatic cascade depends on the orchestrated timing of its specific proteolytic activities. The control of excessive proteolysis is mainly dependent on zymogen activation and enzyme deactivation, achieved through degradation or interaction with proteinase inhibitors or factors that can alter the active structural conformation of enzymes. Zymogen activation in both the coagulation and complement cascades is normally maintained under tight control involving the binding of initiators such as TF and MBL on cell surfaces, as discussed above. However, to counterbalance downstream excessive cascade activities, natural inhibitors or de-activators are recruited to sites of reaction immediately upon activation.
In an effort to manipulate the levels of complement activation in disease settings, several complement inhibitors have been developed, some of which are under clinical investigation [26, 137, 138]. Among the available complement inhibitors, the anti-C5 antibody eculizumab acts as an inhibitor of C5 activation, thereby preventing both the generation of C5a and the assembly of MAC [139]. Administration of eculizumab has been shown to reduce the risk of clinical thromboembolism in patients with PNH, supporting the use of complement inhibitors in thrombotic diseases. Similarly, pexelizumab, an eculizumab fragment antibody, has been studied as an adjunctive therapy in ischemic heart disease [140].
Given the prominent role of C5a in sepsis, interventions targeting complement at the level of C5 may have significant value for the treatment of septic patients [98, 101, 115]. Inhibition of the complement-mediated inflammatory response with an antibody against C5a has resulted in reduced coagulation/fibrinolytic dysregulation in a rat model of sepsis [103]. More recent work has shown that complement lytic activity has a major role in the control of bacteremia in septic mice [141]; this finding led the authors to suggest that inhibition of the complement cascade at the level of C5a would be an effective strategy for the treatment of sepsis, while the apparently favorable formation of MAC, along with opsonization and phagocytosis, would remain intact.
Compstatin, a synthetic cyclic tridecapeptide and a selective C3 inhibitor, has been successfully applied in several experimental disease models [142], such as a primate model of early onset macular degeneration, where the formation of subretinal lipid/protein deposits (drusen) was reduced and partially reversed [143]. Compstatin has been successfully investigated in phase I clinical trials for the treatment of age-related macular degeneration [144] and is now being pursued in phase II clinical trials [137]. Several recent studies have also suggested that the compound may be efficacious in the management of systemic thrombosis, an attribute which may be of clinical value in sepsis. For instance, in a non-human primate model of Escherichia coli-induced sepsis accompanied by multiple organ failure, administration of compstatin reduced the levels of TF and the PAI-1-mediated coagulopathic response and preserved endothelial anticoagulant properties [145]. Furthermore, in an ex vivo model of hemodialysis, inhibition of C3 activity with similar compstatin analogues reduced the TF-dependent procoagulant activity of neutrophils [131], which may prove beneficial for dialysis patients with high risk of thrombosis.
Although no reports of synthetic anticoagulants capable of negatively regulating the complement system have yet appeared, several natural antithrombotic regimens have demonstrated potent anti-inflammatory activity. For instance, it was observed that the anticoagulant, dicoumarin, can decrease the lethality/virulence of streptococcal bacteria after subcutaneous injection into rabbits [146]. Furthermore, TAFI, the metallocarboxypeptidase that inhibits fibrinolysis by preventing the binding of plasminogen to fibrin clots, is also able to control the inflammatory response by inactivation of the anaphylatoxins [92].
Heparin has been reported to block both the classical and alternative complement activation pathways, an effect that is enhanced in the presence of AT [147]. Therefore, the role of heparin as an anti-inflammatory agent has been widely discussed. Heparin acts as an inhibitor of IFN-γ responses and also inhibits the transendothelial migration and arterial recruitment of memory T cells [148]. Furthermore, heparin has been associated with intravascular myeloperoxidase mobilization and improved endothelial nitric oxide bioavailability [149]. Interestingly, the anti-inflammatory pharmacological attributes of heparin seem to be for the most part independent of its antithrombotic activity [150]. Notably, heparin-coated biomaterial surfaces have been successfully utilized to protect against the host immune response [130].
C1INH is another proteinase inhibitor that interferes at the level of both complement and hemostasis [151, 152]. Decreased expression of C1INH or the presence of a dysfunctional C1INH protein can be found in plasma of patients with HAE. Purified or recombinant forms of C1INH have been, therefore, successfully used for the treatment of HAE [152, 153]. C1INH controls the activation of the classical complement pathway by inactivating the proteinases C1r and C1s, and it also regulates the activation of the lectin pathway potentially by inactivating MASPs 1 and 2 [151, 154, 155]. C1INH was also suggested to negatively regulate the activation of the alternative pathway by binding to C3b [156]. On the other hand, C1INH regulates the activation of the intrinsic coagulation pathway via the inhibition of plasma kallikrein and coagulation factors XIa and XIIa [151]. A negative role for C1INH in inflammation and coagulation has been also demonstrated in baboons experiencing lethal E. coli sepsis, with inhibitor-treated animals exhibiting reduced amounts of several cytokines such as TNF, IL-10, IL-6, and IL-8 [157]. Interestingly, C1INH administration has recently been shown to ameliorate DSS-induced colitis via a mechanism that involves suppression of leukocyte infiltration [135]. However, this study failed to single out the direct inhibition of the complement or coagulation cascade as the major cause of the C1INH-mediated favorable outcome of this disease. Nevertheless, overall, the findings suggested that complement plays a role in the development of DSS-induced colitis and that blockade of the complement system might be useful in the acute phase of IBD treatment.
The value of antithrombotic inhibitors for the treatment of sepsis in several experimental studies and clinical trials of thrombomodulin, TF pathway inhibitor (TFPI), AT, and APC is under ongoing investigation [158–160]. Soluble recombinant thrombomodulin has been used in septic models due to its ability to cause a substantial increase in APC levels, as well as block the coagulant activity of thrombin [158]. While preclinical studies have suggested that treatment with thrombomodulin attenuates some of the sepsis-related coagulation and inflammatory effects, the treatment of septic patients with DIC did not result in any 28-day mortality rate improvement, as shown by a phase III clinical trial [159, 161, 162]. TFPI, another protein that has been used to attenuate the coagulation cascade, has also not as yet demonstrated clear survival benefits for septic patients [158].
In the case of AT, a clinical trial involving patients with severe sepsis did not point to a clear benefit of AT in the survival of patients [163], in which the “gold standard” set by the FDA is survival at 28 days. However, the survival outcome could have been adversely influenced by the concomitant administration of heparin in a subgroup of patients [158, 163, 164], as heparin is known to competitively block the cell surface binding of AT [165]. The use of AT in the absence of heparin is, therefore, under clinical evaluation [166].
With respect to the antithrombotic inhibitor APC, several clinical trials have investigated its efficacy for increasing the survival of septic patients [158, 160, 167]. While some of these studies pointed to a potential clinical value for APC in the management of patients with high disease severity, APC administration was associated with an increased risk of bleeding [168]. As in the case of AT, a potential adverse confounding effect of the heparin administration was recognized [168], which was, however, not indicated by a subsequent clinical trial [169].
The beneficial effects of APC during systemic inflammation can be independent of its anticoagulant properties, but be rather based on its potential anti-inflammatory effects and its interaction with the fibrinolytic system [167], as well as on the degradation of cytotoxic extracellular histones [170]. Interestingly, recent work has suggested a role for APC in the regulation of complement activity in an LPS-induced model of factor B expression in human monocytes [171].
Collectively, the clinical studies on septic patients have thus far proven uncertain regarding the use of antithrombotic regimens to attenuate the clotting cascade in sepsis. More information is clearly needed regarding the molecular, i.e., inflammatory and/or thrombotic, mechanisms that are unleashed during sepsis to resolve these issues. In line of these efforts, the outcome of ongoing experimental and clinical studies addressing the issues evidenced by the clinical trials discussed above is highly anticipated. Notably, the potential use of APC mutants exhibiting reduced anticoagulant but enhanced cytoprotective properties [172, 173] would be a valuable asset to future clinical trials involving septic patients.
Future avenues of research
Inflammation and thrombosis are two responses that are linked through a number of mechanisms. Emerging biochemical, biological, and clinical findings indicate that the complement and coagulation systems are interconnected at various levels in vivo. These interactions point to alternative ways in which complement or coagulation components can potentially become activated. In this regard, they can also suggest attractive targets for the development of novel therapeutic interventions.
The equilibrium between the complement and coagulation components and their interacting mutual inhibitors can determine the overall biological activity and the outcome of a disease. Investigation of the levels of these factors in disease settings in which complement-mediated immune responses and thrombotic events clinically manifest together is vital. Understanding this fine balance is imperative for approaching patients with diseases, such as sepsis, in which traditional therapeutics directed toward immune components can further compromise the innate immune response of the host and result in progression of the disease. These investigations can also provide the basis for the development of common therapeutic regimens and potentially single out diagnostic or prognostic markers for specific inflammation-associated thrombotic diseases.
Acknowledgements
We thank D. McClellan for editorial assistance. This work was supported by U.S. National Institutes of Health grants AI68730, AI30040 and GM62134 (to J.D.L.), and GM29507, GM61656 (to P.A.W.). K.O. is recipient of a Natural Sciences and Engineering Research Council of Canada postdoctoral fellowship.
Abbreviations
- aHUS
Atypical hemolytic uremic syndrome
- C1INH
C1 inhibitor
- C4BP
C4b-binding protein
- C3aR
C3a receptor
- C5aR
C5a receptor
- C5L2
C5a receptor-like 2
- DAF
Decay accelerating factor
- DIC
Disseminated intravascular coagulation
- DSS
Dextran sulphate sodium
- HAE
Hereditary angioedema
- HMGB1
High mobility globulin B1
- IBD
Inflammatory bowel disease
- IL
Interleukin
- LPS
Lipopolysaccharide
- MAC
Membrane-attack complex
- MASP
Mannose-binding lectin-associated serine proteinase
- MBL
Mannose-binding lectin
- MCP-1
Monocytes chemoattractant protein 1
- MIF
Migration-inhibitory factor
- MIP-1
Macrophage inflammatory protein 1
- PAI-1
Plasminogen activator inhibitor 1
- PAR
Proteinase-activated receptor
- PNH
Paroxysmal nocturnal hemoglobinuria
- SIRS
Systemic inflammatory response syndrome
- SLE
Systemic lupus erythematosus
- TAFI
Thrombin activatable fibrinolysis inhibitor
- TF
Tissue factor
- TNF
Tumor necrosis factor
- tPA
Tissue plasminogen activator
- uPA
Urokinase plasminogen activator
- uPAR
Urokinase plasminogen activator receptor
Contributor Information
Katerina Oikonomopoulou, Department of Pathology & Laboratory Medicine, School of Medicine, University of Pennsylvania, 401 Stellar Chance Laboratories, 422 Curie Boulevard, Philadelphia, PA 19104-6100, USA.
Daniel Ricklin, Department of Pathology & Laboratory Medicine, School of Medicine, University of Pennsylvania, 401 Stellar Chance Laboratories, 422 Curie Boulevard, Philadelphia, PA 19104-6100, USA.
Peter A. Ward, Department of Pathology, University of Michigan Medical School, Ann Arbor, MI 48109-5602, USA
John D. Lambris, Email: lambris@upenn.edu, Department of Pathology & Laboratory Medicine, School of Medicine, University of Pennsylvania, 401 Stellar Chance Laboratories, 422 Curie Boulevard, Philadelphia, PA 19104-6100, USA.
References
- 1.Reid KB, Porter RR. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- 2.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson CM, Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- 4.Adams RL, Bird RJ. Review article: coagulation cascade and therapeutics update: relevance to nephrology. Part 1: Overview of coagulation, thrombophilias and history of anticoagulants. Nephrology (Carlton) 2009;14:462–470. doi: 10.1111/j.1440-1797.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- 5.Francis CW, Marder VJ. Physiologic regulation and pathologic disorders of fibrinolysis. Hum Pathol. 1987;18:263–274. doi: 10.1016/s0046-8177(87)80009-6. [DOI] [PubMed] [Google Scholar]
- 6.Kane KK. Fibrinolysis—a review. Ann Clin Lab Sci. 1984;14:443–449. [PubMed] [Google Scholar]
- 7.Rawlings ND, Barrett AJ, Bateman A. MEROPS: the peptidase database. Nucleic Acids Res. 2010;38:D227–D233. doi: 10.1093/nar/gkp971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawlings ND. Peptidase inhibitors in the MEROPS database. Biochimie. 2010;92:1463–1483. doi: 10.1016/j.biochi.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Barrett AJ, Rawlings ND, Woessner JF. Handbook of proteolytic enzymes. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- 10.Macfarlane RG. Normal and abnormal blood coagulation: a review. J Clin Pathol. 1948;1:113–143. doi: 10.1136/jcp.1.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lachmann P. Complement before molecular biology. Mol Immunol. 2006;43:496–508. doi: 10.1016/j.molimm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman R, Benz E, Jr., Shattil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P. Hematology: basic principles and practice. Philadelphia: Elsevier Churchill Livingstone; 2005. [Google Scholar]
- 13.Walsh PN. Platelet coagulation–protein interactions. Semin Thromb Hemost. 2004;30:461–471. doi: 10.1055/s-2004-833481. [DOI] [PubMed] [Google Scholar]
- 14.Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman M. Remodeling the blood coagulation cascade. J Thromb Thrombolysis. 2003;16:17–20. doi: 10.1023/B:THRO.0000014588.95061.28. [DOI] [PubMed] [Google Scholar]
- 16.Rakic JM, Maillard C, Jost M, Bajou K, Masson V, Devy L, Lambert V, Foidart JM, Noel A. Role of plasminogen activator-plasmin system in tumor angiogenesis. Cell Mol Life Sci. 2003;60:463–473. doi: 10.1007/s000180300039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van de Werf FJ, Topol EJ, Sobel BE. The impact of fibrinolytic therapy for ST-segment-elevation acute myocardial infarction. J Thromb Haemost. 2009;7:14–20. doi: 10.1111/j.1538-7836.2008.03195.x. [DOI] [PubMed] [Google Scholar]
- 18.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 19.Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, Luger TA, Hollenberg MD. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- 20.Riewald M, Ruf W. Proteinase-activated receptor activation by coagulation proteinases. Drug Develop Res. 2003;59:400–407. [Google Scholar]
- 21.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 22.Kuliopulos A, Covic L, Seeley SK, Sheridan PJ, Helin J, Costello CE. Plasmin desensitization of the PAR1 thrombin receptor: kinetics, sites of truncation, and implications for thrombolytic therapy. Biochemistry. 1999;38:4572–4585. doi: 10.1021/bi9824792. [DOI] [PubMed] [Google Scholar]
- 23.Laumonnier Y, Syrovets T, Burysek L, Simmet T. Identification of the annexin A2 heterotetramer as a receptor for the plasmin-induced signaling in human peripheral monocytes. Blood. 2006;107:3342–3349. doi: 10.1182/blood-2005-07-2840. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Laumonnier Y, Syrovets T, Simmet T. Plasmin triggers cytokine induction in human monocyte-derived macrophages. Arterioscler Thromb Vasc Biol. 2007;27:1383–1389. doi: 10.1161/ATVBAHA.107.142901. [DOI] [PubMed] [Google Scholar]
- 25.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6:132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le FG, Kemper C. Complement: coming full circle. Arch Immunol Ther Exp (Warsz) 2009;57:393–407. doi: 10.1007/s00005-009-0047-4. [DOI] [PubMed] [Google Scholar]
- 28.Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 29.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemper C, Atkinson JP, Hourcade DE. Properdin: emerging roles of a pattern-recognition molecule. Annu Rev Immunol. 2010;28:131–155. doi: 10.1146/annurev-immunol-030409-101250. [DOI] [PubMed] [Google Scholar]
- 31.Pangburn MK, Muller-Eberhard HJ. The C3 convertase of the alternative pathway of human complement. Enzymic properties of the bimolecular proteinase. Biochem J. 1986;235:723–730. doi: 10.1042/bj2350723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volanakis JE. C3 convertases of complement. Molecular genetics, structure and function of the catalytic domains, C2 and B. Year Immunol. 1989;4:218–230. [PubMed] [Google Scholar]
- 33.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalant D, MacLaren R, Cui W, Samanta R, Monk PN, Laporte SA, Cianflone K. C5L2 is a functional receptor for acylation-stimulating protein. J Biol Chem. 2005;280:23936–23944. doi: 10.1074/jbc.M406921200. [DOI] [PubMed] [Google Scholar]
- 35.Ward PA. Functions of C5a receptors. J Mol Med. 2009;87:375–378. doi: 10.1007/s00109-009-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Lith LH, Oosterom J, Van EA, Zaman GJ. C5a-stimulated recruitment of beta-arrestin2 to the nonsignaling 7-transmembrane decoy receptor C5L2. J Biomol Screen. 2009;14:1067–1075. doi: 10.1177/1087057109341407. [DOI] [PubMed] [Google Scholar]
- 37.Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C, Gerard NP. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scola AM, Johswich KO, Morgan BP, Klos A, Monk PN. The human complement fragment receptor, C5L2, is a recycling decoy receptor. Mol Immunol. 2009;46:1149–1162. doi: 10.1016/j.molimm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Megyeri M, Mako V, Beinrohr L, Doleschall Z, Prohaszka Z, Cervenak L, Zavodszky P, Gal P. Complement protease MASP-1 activates human endothelial cells: PAR4 activation is a link between complement and endothelial function. J Immunol. 2009;183:3409–3416. doi: 10.4049/jimmunol.0900879. [DOI] [PubMed] [Google Scholar]
- 40.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 41.Dulon S, Leduc D, Cottrell GS, D’Alayer J, Hansen KK, Bunnett NW, Hollenberg MD, Pidard D, Chignard M. Pseudomonas aeruginosa elastase disables proteinase-activated receptor 2 in respiratory epithelial cells. Am J Respir Cell Mol Biol. 2005;32:411–419. doi: 10.1165/rcmb.2004-0274OC. [DOI] [PubMed] [Google Scholar]
- 42.Schmidtchen A, Holst E, Tapper H, Bjorck L. Elastase-producing Pseudomonas aeruginosa degrade plasma proteins and extracellular products of human skin and fibroblasts, and inhibit fibroblast growth. Microb Pathog. 2003;34:47–55. doi: 10.1016/s0882-4010(02)00197-3. [DOI] [PubMed] [Google Scholar]
- 43.Jagels MA, Travis J, Potempa J, Pike R, Hugli TE. Proteolytic inactivation of the leukocyte C5a receptor by proteinases derived from Porphyromonas gingivalis. Infect Immun. 1996;64:1984–1991. doi: 10.1128/iai.64.6.1984-1991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wingrove JA, DiScipio RG, Chen Z, Potempa J, Travis J, Hugli TE. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J Biol Chem. 1992;267:18902–18907. [PubMed] [Google Scholar]
- 45.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mollnes TE, Garred P, Bergseth G. Effect of time, temperature and anticoagulants on in vitro complement activation: consequences for collection and preservation of samples to be examined for complement activation. Clin Exp Immunol. 1988;73:484–488. [PMC free article] [PubMed] [Google Scholar]
- 47.Wiggins RC, Giclas PC, Henson PM. Chemotactic activity generated from the fifth component of complement by plasma kallikrein of the rabbit. J Exp Med. 1981;153:1391–1404. doi: 10.1084/jem.153.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DiScipio RG. The activation of the alternative pathway C3 convertase by human plasma kallikrein. Immunology. 1982;45:587–595. [PMC free article] [PubMed] [Google Scholar]
- 49.Hiemstra PS, Daha MR, Bouma BN. Activation of factor B of the complement system by kallikrein and its light chain. Thromb Res. 1985;38:491–503. doi: 10.1016/0049-3848(85)90182-3. [DOI] [PubMed] [Google Scholar]
- 50.Thoman ML, Meuth JL, Morgan EL, Weigle WO, Hugli TE. C3d-K, a kallikrein cleavage fragment of iC3b is a potent inhibitor of cellular proliferation. J Immunol. 1984;133:2629–2633. [PubMed] [Google Scholar]
- 51.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 52.Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Bruckner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185:5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Conde I, Crúz MA, Zhang H, López JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J Exp Med. 2005;201:871–879. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghebrehiwet B, Silverberg M, Kaplan AP. Activation of the classical pathway of complement by Hageman factor fragment. J Exp Med. 1981;153:665–676. doi: 10.1084/jem.153.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghebrehiwet B, Randazzo BP, Dunn JT, Silverberg M, Kaplan AP. Mechanisms of activation of the classical pathway of complement by Hageman factor fragment. J Clin Invest. 1983;71:1450–1456. doi: 10.1172/JCI110898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamad OA, Nilsson PH, Lasaosa M, Ricklin D, Lambris JD, Nilsson B, Ekdahl KN. Contribution of chondroitin sulfate A to the binding of complement proteins to activated platelets. PLoS One. 2010;5:e12889. doi: 10.1371/journal.pone.0012889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Endo Y, Nakazawa N, Iwaki D, Takahashi M, Matsushita M, Fujita T. Interactions of ficolin and mannose-binding lectin with fibrinogen/fibrin augment the lectin complement pathway. J Innate Immun. 2009;2:33–42. doi: 10.1159/000227805. [DOI] [PubMed] [Google Scholar]
- 58.Krarup A, Wallis R, Presanis JS, Gal P, Sim RB. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS One. 2007;2:e623. doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gulla KC, Gupta K, Krarup A, Gal P, Schwaeble WJ, Sim RB, O’Connor CD, Hajela K. Activation of mannan-binding lectin-associated serine proteases leads to generation of a fibrin clot. Immunology. 2010;129:482–495. doi: 10.1111/j.1365-2567.2009.03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Platt JL, Dalmasso AP, Lindman BJ, Ihrcke NS, Bach FH. The role of C5a and antibody in the release of heparan sulfate from endothelial cells. Eur J Immunol. 1991;21:2887–2890. doi: 10.1002/eji.1830211135. [DOI] [PubMed] [Google Scholar]
- 61.Marcum JA, Atha DH, Fritze LM, Nawroth P, Stern D, Rosenberg RD. Cloned bovine aortic endothelial cells synthesize anticoagulantly active heparan sulfate proteoglycan. J Biol Chem. 1986;261:7507–7517. [PubMed] [Google Scholar]
- 62.Zaferani A, Vives RR, van der PP, Hakvoort JJ, Navis GJ, van GH, Daha MR, Lortat-Jacob H, Seelen MA, van den BJ. Identification of tubular heparan sulfate as a docking platform for the alternative complement component properdin in proteinuric renal disease. J Biol Chem. 2011;286:5359–5367. doi: 10.1074/jbc.M110.167825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Polley MJ, Nachman R. The human complement system in thrombin-mediated platelet function. J Exp Med. 1978;147:1713–1726. doi: 10.1084/jem.147.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sims PJ, Wiedmer T. The response of human platelets to activated components of the complement system. Immunol Today. 1991;12:338–342. doi: 10.1016/0167-5699(91)90012-I. [DOI] [PubMed] [Google Scholar]
- 65.Wiedmer T, Sims PJ. Effect of complement proteins C5b-9 on blood platelets. Evidence for reversible depolarization of membrane potential. J Biol Chem. 1985;260:8014–8019. [PubMed] [Google Scholar]
- 66.Ando B, Wiedmer T, Hamilton KK, Sims PJ. Complement proteins C5b-9 initiate secretion of platelet storage granules without increased binding of fibrinogen or von Willebrand factor to newly expressed cell surface GPIIb-IIIa. J Biol Chem. 1988;263:11907–11914. [PubMed] [Google Scholar]
- 67.Wiedmer T, Esmon CT, Sims PJ. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrom-binase. Blood. 1986;68:875–880. [PubMed] [Google Scholar]
- 68.Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988;263:18205–18212. [PubMed] [Google Scholar]
- 69.Hamilton KK, Hattori R, Esmon CT, Sims PJ. Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J Biol Chem. 1990;265:3809–3814. [PubMed] [Google Scholar]
- 70.Peerschke EI, Reid KB, Ghebrehiwet B. Platelet activation by C1q results in the induction of alpha IIb/beta 3 integrins (GPIIb-IIIa) and the expression of P-selectin and procoagulant activity. J Exp Med. 1993;178:579–587. doi: 10.1084/jem.178.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skoglund C, Wettero J, Tengvall P, Bengtsson T. C1q induces a rapid up-regulation of P-selectin and modulates collagen-and collagen-related peptide-triggered activation in human platelets. Immunobiology. 2010;215:987–995. doi: 10.1016/j.imbio.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 72.Polley MJ, Nachman RL. Human platelet activation by C3a and C3a des-arg. J Exp Med. 1983;158:603–615. doi: 10.1084/jem.158.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gushiken FC, Han H, Li J, Rumbaut RE, fshar-Kharghan V. Abnormal platelet function in C3-deficient mice. J Thromb Haemost. 2009;7:865–870. doi: 10.1111/j.1538-7836.2009.03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qin X, Krumrei N, Grubissich L, Dobarro M, Aktas H, Perez G, Halperin JA. Deficiency of the mouse complement regulatory protein mCd59b results in spontaneous hemolytic anemia with platelet activation and progressive male infertility. Immunity. 2003;18:217–227. doi: 10.1016/s1074-7613(03)00022-0. [DOI] [PubMed] [Google Scholar]
- 75.Hamad OA, Nilsson PH, Wouters D, Lambris JD, Ekdahl KN, Nilsson B. Complement component C3 binds to activated normal platelets without preceding proteolytic activation and promotes binding to complement receptor 1. J Immunol. 2010;184:2686–2692. doi: 10.4049/jimmunol.0902810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost. 1997;77:394–398. [PubMed] [Google Scholar]
- 77.Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, Rafail S, Kartalis G, Sideras P, Lambris JD. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 78.Kambas K, Markiewski MM, Pneumatikos IA, Rafail SS, Theodorou V, Konstantonis D, Kourtzelis I, Doumas MN, Magotti P, DeAngelis RA, Lambris JD, Ritis KD. C5a and TNF-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J Immunol. 2008;180:7368–7375. doi: 10.4049/jimmunol.180.11.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tedesco F, Pausa M, Nardon E, Introna M, Mantovani A, Dobrina A. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185:1619–1627. doi: 10.1084/jem.185.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sillaber C, Baghestanian M, Bevec D, Willheim M, Agis H, Kapiotis S, Fureder W, Bankl HC, Kiener HP, Speiser W, Binder BR, Lechner K, Valent P. The mast cell as site of tissue-type plasminogen activator expression and fibrinolysis. J Immunol. 1999;162:1032–1041. [PubMed] [Google Scholar]
- 81.Wojta J, Kaun C, Zorn G, Ghannadan M, Hauswirth AW, Sperr WR, Fritsch G, Printz D, Binder BR, Schatzl G, Zwirner J, Maurer G, Huber K, Valent P. C5a stimulates production of plasminogen activator inhibitor-1 in human mast cells and basophils. Blood. 2002;100:517–523. doi: 10.1182/blood.v100.2.517. [DOI] [PubMed] [Google Scholar]
- 82.Wojta J, Huber K, Valent P. New aspects in thrombotic research: complement induced switch in mast cells from a profibrinolytic to a prothrombotic phenotype. Pathophysiol Haemost Thromb. 2003;33:438–441. doi: 10.1159/000083842. [DOI] [PubMed] [Google Scholar]
- 83.Levi M, van der Tom P. Inflammation and coagulation. Crit Care Med. 2010;38:S26–S34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 84.Levi M, van der Tom P. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med. 2005;15:254–259. doi: 10.1016/j.tcm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 85.Markiewski MM, DeAngelis RA, Lambris JD. Liver inflammation and regeneration: two distinct biological phenomena or parallel pathophysiologic processes? Mol Immunol. 2006;43:45–56. doi: 10.1016/j.molimm.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 86.Markiewski MM, DeAngelis RA, Strey CW, Foukas PG, Gerard C, Gerard N, Wetsel RA, Lambris JD. The regulation of liver cell survival by complement. J Immunol. 2009;182:5412–5418. doi: 10.4049/jimmunol.0804179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shebuski RJ, Kilgore KS. Role of inflammatory mediators in thrombogenesis. J Pharmacol Exp Ther. 2002;300:729–735. doi: 10.1124/jpet.300.3.729. [DOI] [PubMed] [Google Scholar]
- 88.Szotowski B, Antoniak S, Poller W, Schultheiss HP, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96:1233–1239. doi: 10.1161/01.RES.0000171805.24799.fa. [DOI] [PubMed] [Google Scholar]
- 89.Burstein SA, Peng J, Friese P, Wolf RF, Harrison P, Downs T, Hamilton K, Comp P, Dale GL. Cytokine-induced alteration of platelet and hemostatic function. Stem Cells. 1996;14(Suppl 1):154–162. doi: 10.1002/stem.5530140720. [DOI] [PubMed] [Google Scholar]
- 90.Verardi S, Page RC, Ammons WF, Bordin S. Differential chemokine response of fibroblast subtypes to complement C1q. J Periodontal Res. 2007;42:62–68. doi: 10.1111/j.1600-0765.2006.00916.x. [DOI] [PubMed] [Google Scholar]
- 91.Lidington EA, Haskard DO, Mason JC. Induction of decay-accelerating factor by thrombin through a protease-activated receptor 1 and protein kinase C-dependent pathway protects vascular endothelial cells from complement-mediated injury. Blood. 2000;96:2784–2792. [PubMed] [Google Scholar]
- 92.Campbell W, Okada N, Okada H. Carboxypeptidase R is an inactivator of complement-derived inflammatory peptides and an inhibitor of fibrinolysis. Immunol Rev. 2001;180:162–167. doi: 10.1034/j.1600-065x.2001.1800114.x. [DOI] [PubMed] [Google Scholar]
- 93.Bajzar L, Morser J, Nesheim M. TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex. J Biol Chem. 1996;271:16603–16608. doi: 10.1074/jbc.271.28.16603. [DOI] [PubMed] [Google Scholar]
- 94.Levi M, Opal SM. Coagulation abnormalities in critically ill patients. Crit Care. 2006;10:222. doi: 10.1186/cc4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Degen JL, Bugge TH, Goguen JD. Fibrin and fibrinolysis in infection and host defense. J Thromb Haemost. 2007;5(Suppl 1):24–31. doi: 10.1111/j.1538-7836.2007.02519.x. [DOI] [PubMed] [Google Scholar]
- 96.Hecke F, Schmidt U, Kola A, Bautsch W, Klos A, Kohl J. Circulating complement proteins in multiple trauma patients—correlation with injury severity, development of sepsis, and outcome. Crit Care Med. 1997;25:2015–2024. doi: 10.1097/00003246-199712000-00019. [DOI] [PubMed] [Google Scholar]
- 97.Markiewski MM, DeAngelis RA, Lambris JD. Complexity of complement activation in sepsis. J Cell Mol Med. 2008;12:2245–2254. doi: 10.1111/j.1582-4934.2008.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ward PA, Gao H. Sepsis, complement and the dysregulated inflammatory response. J Cell Mol Med. 2009;13:4154–4160. doi: 10.1111/j.1582-4934.2009.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Castellheim A, Brekke OL, Espevik T, Harboe M, Mollnes TE. Innate immune responses to danger signals in systemic inflammatory response syndrome and sepsis. Scand J Immunol. 2009;69:479–491. doi: 10.1111/j.1365-3083.2009.02255.x. [DOI] [PubMed] [Google Scholar]
- 100.Daniels R, Nutbeam T. ABC of Sepsis. Chichester: Blackwell Publishing Ltd; 2010. [Google Scholar]
- 101.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133–142. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 103.Laudes IJ, Chu JC, Sikranth S, Huber-Lang M, Guo RF, Riedemann N, Sarma JV, Schmaier AH, Ward PA. Anti-c5a ameliorates coagulation/fibrinolytic protein changes in a rat model of sepsis. Am J Pathol. 2002;160:1867–1875. doi: 10.1016/S0002-9440(10)61133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Knoebl P. Blood coagulation disorders in septic patients. Wien Med Wochenschr. 2010;160:129–138. doi: 10.1007/s10354-009-0738-9. [DOI] [PubMed] [Google Scholar]
- 105.Gando S. Microvascular thrombosis and multiple organ dysfunction syndrome. Crit Care Med. 2010;38:S35–S42. doi: 10.1097/CCM.0b013e3181c9e31d. [DOI] [PubMed] [Google Scholar]
- 106.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barie PS, Hydo LJ, Pieracci FM, Shou J, Eachempati SR. Multiple organ dysfunction syndrome in critical surgical illness. Surg Infect (Larchmt) 2009;10:369–377. doi: 10.1089/sur.2009.9935. [DOI] [PubMed] [Google Scholar]
- 108.Ten Cate H, Schoenmakers SH, Franco R, Timmerman JJ, Groot AP, Spek CA, Reitsma PH. Microvascular coagulopathy and disseminated intravascular coagulation. Crit Care Med. 2001;29:S95–S97. doi: 10.1097/00003246-200107001-00030. [DOI] [PubMed] [Google Scholar]
- 109.Younger JG, Bracho DO, Chung-Esaki HM, Lee M, Rana GK, Sen A, Jones AE. Complement activation in emergency department patients with severe sepsis. Acad Emerg Med. 2010;17:353–359. doi: 10.1111/j.1553-2712.2010.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Semple JW, Freedman J. Platelets and innate immunity. Cell Mol Life Sci. 2010;67:499–511. doi: 10.1007/s00018-009-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gawaz M, Dickfeld T, Bogner C, Fateh-Moghadam S, Neumann FJ. Platelet function in septic multiple organ dysfunction syndrome. Intensive Care Med. 1997;23:379–385. doi: 10.1007/s001340050344. [DOI] [PubMed] [Google Scholar]
- 112.Ekdahl KN, Nilsson B. Phosphorylation of complement component C3 and C3 fragments by a human platelet protein kinase. Inhibition of factor I-mediated cleavage of C3b. J Immunol. 1995;154:6502–6510. [PubMed] [Google Scholar]
- 113.Nilsson-Ekdahl K, Nilsson B. Phosphorylation of C3 by a casein kinase released from activated human platelets increases opsonization of immune complexes and binding to complement receptor type 1. Eur J Immunol. 2001;31:1047–1054. doi: 10.1002/1521-4141(200104)31:4<1047::aid-immu1047>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 114.Hack CE, Nuijens JH, Felt-Bersma RJ, Schreuder WO, Eerenberg-Belmer AJ, Paardekooper J, Bronsveld W, Thijs LG. Elevated plasma levels of the anaphylatoxins C3a and C4a are associated with a fatal outcome in sepsis. Am J Med. 1989;86:20–26. doi: 10.1016/0002-9343(89)90224-6. [DOI] [PubMed] [Google Scholar]
- 115.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Kohl J, Gerard C, Sarma JV, Ward PA. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Kohl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 117.Riedemann NC, Guo RF, Gao H, Sun L, Hoesel M, Hollmann TJ, Wetsel RA, Zetoune FS, Ward PA. Regulatory role of C5a on macrophage migration inhibitory factor release from neutrophils. J Immunol. 2004;173:1355–1359. doi: 10.4049/jimmunol.173.2.1355. [DOI] [PubMed] [Google Scholar]
- 118.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 119.Wiedmer T, Hall SE, Ortel TL, Kane WH, Rosse WF, Sims PJ. Complement-induced vesiculation and exposure of membrane prothrombinase sites in platelets of paroxysmal nocturnal hemoglobinuria. Blood. 1993;82:1192–1196. [PubMed] [Google Scholar]
- 120.Karpman D, Manea M, Vaziri-Sani F, Stahl AL, Kristoffersson AC. Platelet activation in hemolytic uremic syndrome. Semin Thromb Hemost. 2006;32:128–145. doi: 10.1055/s-2006-939769. [DOI] [PubMed] [Google Scholar]
- 121.Coppola L, Guastafierro S, Verrazzo G, Coppola A, De LD, Tirelli A. C1 inhibitor infusion modifies platelet activity in hereditary angioedema patients. Arch Pathol Lab Med. 2002;126:842–845. doi: 10.5858/2002-126-0842-CIIMPA. [DOI] [PubMed] [Google Scholar]
- 122.Nangaku M, Couser WG. Mechanisms of immune-deposit formation and the mediation of immune renal injury. Clin Exp Nephrol. 2005;9:183–191. doi: 10.1007/s10157-005-0357-8. [DOI] [PubMed] [Google Scholar]
- 123.Shushakova N, Tkachuk N, Dangers M, Tkachuk S, Park JK, Hashimoto K, Haller H, Dumler I. Urokinase-induced activation of the gp130/Tyk2/Stat3 pathway mediates a pro-inflammatory effect in human mesangial cells via expression of the anaphylatoxin C5a receptor. J Cell Sci. 2005;118:2743–2753. doi: 10.1242/jcs.02409. [DOI] [PubMed] [Google Scholar]
- 124.Mocco J, Wilson DA, Komotar RJ, Sughrue ME, Coates K, Sacco RL, Elkind MS, Connolly ES., Jr Alterations in plasma complement levels after human ischemic stroke. Neurosurgery. 2006;59:28–33. doi: 10.1227/01.NEU.0000219221.14280.65. [DOI] [PubMed] [Google Scholar]
- 125.Banz Y, Rieben R. Role of complement and perspectives for intervention in ischemia-reperfusion damage. Ann Med. 2011 doi: 10.3109/07853890.2010.535556. [DOI] [PubMed] [Google Scholar]
- 126.Cervera A, Planas AM, Justicia C, Urra X, Jensenius JC, Torres F, Lozano F, Chamorro A. Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke. PLoS One. 2010;5:e8433. doi: 10.1371/journal.pone.0008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lood C, Gullstrand B, Truedsson L, Olin AI, Alm GV, Ronnblom L, Sturfelt G, Eloranta ML, Bengtsson AA. C1q inhibits immune complex-induced interferon-alpha production in plasmacytoid dendritic cells: a novel link between C1q deficiency and systemic lupus erythematosus pathogenesis. Arthritis Rheum. 2009;60:3081–3090. doi: 10.1002/art.24852. [DOI] [PubMed] [Google Scholar]
- 128.Robson MG, Walport MJ. Pathogenesis of systemic lupus erythematosus (SLE) Clin Exp Allergy. 2001;31:678–685. doi: 10.1046/j.1365-2222.2001.01147.x. [DOI] [PubMed] [Google Scholar]
- 129.Palatinus A, Adams M. Thrombosis in systemic lupus erythematosus. Semin Thromb Hemost. 2009;35:621–629. doi: 10.1055/s-0029-1242716. [DOI] [PubMed] [Google Scholar]
- 130.Nilsson B, Korsgren O, Lambris JD, Ekdahl KN. Can cells and biomaterials in therapeutic medicine be shielded from innate immune recognition? Trends Immunol. 2010;31:32–38. doi: 10.1016/j.it.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kourtzelis I, Markiewski MM, Doumas M, Rafail S, Kambas K, Mitroulis I, Panagoutsos S, Passadakis P, Vargemezis V, Magotti P, Qu H, Mollnes TE, Ritis K, Lambris JD. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood. 2010;116:631–639. doi: 10.1182/blood-2010-01-264051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brenner P, Keller M, Beiras-Fernandez A, Uchita S, Kur F, Thein E, Wimmer C, Hammer C, Schmoeckel M, Reichart B. Prevention of hyperacute xenograft rejection through direct thrombin inhibition with hirudin. Annals of Transplantation: quarterly of the Polish Transplantation Society. 2010;15:30–37. [PubMed] [Google Scholar]
- 133.Freue GV, Sasaki M, Meredith A, Gunther OP, Bergman A, Takhar M, Mui A, Balshaw RF, Ng RT, Opushneva N, Hollander Z, Li G, Borchers CH, Wilson-McManus J, McManus BM, Keown PA, McMaster WR. Proteomic signatures in plasma during early acute renal allograft rejection. Mol Cell Proteomics. 2010;9:1954–1967. doi: 10.1074/mcp.M110.000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ingegnoli F, Fantini F, Griffini S, Soldi A, Meroni PL, Cugno M. Anti-tumor necrosis factor alpha therapy normalizes fibrinolysis impairment in patients with active rheumatoid arthritis. Clin Exp Rheumatol. 2010;28:254–257. [PubMed] [Google Scholar]
- 135.Lu F, Fernandes SM, Davis AE., III The role of the complement and contact systems in the dextran sulfate sodium—induced colitis model: the effect of C1 inhibitor in inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2010;298:G878–G883. doi: 10.1152/ajpgi.00400.2009. [DOI] [PubMed] [Google Scholar]
- 136.Cugno M, Tedeschi A, Crosti C, Marzano AV. Activation of blood coagulation in autoimmune skin disorders. Expert Rev Clin Immunol. 2009;5:605–613. doi: 10.1586/eci.09.40. [DOI] [PubMed] [Google Scholar]
- 137.Wagner E, Frank MM. Therapeutic potential of complement modulation. Nat Rev Drug Discov. 2010;9:43–56. doi: 10.1038/nrd3011. [DOI] [PubMed] [Google Scholar]
- 138.Qu H, Ricklin D, Lambris JD. Recent developments in low molecular weight complement inhibitors. Mol Immunol. 2009;47:185–195. doi: 10.1016/j.molimm.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hillmen P, Muus P, Duhrsen U, Risitano AM, Schubert J, Luzzatto L, Schrezenmeier H, Szer J, Brodsky RA, Hill A, Socie G, Bessler M, Rollins SA, Bell L, Rother RP, Young NS. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110:4123–4128. doi: 10.1182/blood-2007-06-095646. [DOI] [PubMed] [Google Scholar]
- 140.Testa L, Van Gaal WJ, Bhindi R, Biondi-Zoccai GG, Abbate A, Agostoni P, Porto I, Andreotti F, Crea F, Banning AP. Pexelizumab in ischemic heart disease: a systematic review and meta-analysis on 15,196 patients. J Thorac Cardiovasc Surg. 2008;136:884–893. doi: 10.1016/j.jtcvs.2007.12.062. [DOI] [PubMed] [Google Scholar]
- 141.Flierl MA, Rittirsch D, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ward PA. Functions of the complement components C3 and C5 during sepsis. FASEB J. 2008;22:3483–3490. doi: 10.1096/fj.08-110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ricklin D, Lambris JD. Compstatin: a complement inhibitor on its way to clinical application. Adv Exp Med Biol. 2008;632:273–292. doi: 10.1007/978-0-387-78952-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chi ZL, Yoshida T, Lambris JD, Iwata T. Suppression of drusen formation by compstatin, a peptide inhibitor of complement C3 activation, on cynomolgus monkey with early-onset macular degeneration. Adv Exp Med Biol. 2010;703:127–135. doi: 10.1007/978-1-4419-5635-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Potentia. Potentia Pharmaceuticals announces initiation of Phase I clinical trials to evaluate its lead compound for age-related macular degeneration. Potentia Press Release. 2007 Apr 5; 2007. [Google Scholar]
- 145.Silasi-Mansat R, Zhu H, Popescu NI, Peer G, Sfyroera G, Magotti P, Ivanciu L, Lupu C, Mollnes TE, Taylor FB, Kinasewitz G, Lambris JD, Lupu F. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood. 2010;116:1002–1010. doi: 10.1182/blood-2010-02-269746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thuerer GR, Angevine DM. Influence of dicumarol on streptococcic infection in rabbits. Arch Pathol (Chic) 1949;48:274–277. [PubMed] [Google Scholar]
- 147.Weiler JM, Linhardt RJ. Antithrombin III regulates complement activity in vitro. J Immunol. 1991;146:3889–3894. [PubMed] [Google Scholar]
- 148.Ranjbaran H, Wang Y, Manes TD, Yakimov AO, Akhtar S, Kluger MS, Pober JS, Tellides G. Heparin displaces interferon-gamma-inducible chemokines (IP-10, I-TAC, and Mig) sequestered in the vasculature and inhibits the transendothelial migration and arterial recruitment of Tcells. Circulation. 2006;114:1293–1300. doi: 10.1161/CIRCULATIONAHA.106.631457. [DOI] [PubMed] [Google Scholar]
- 149.Baldus S, Rudolph V, Roiss M, Ito WD, Rudolph TK, Eiserich JP, Sydow K, Lau D, Szocs K, Klinke A, Kubala L, Berglund L, Schrepfer S, Deuse T, Haddad M, Risius T, Klemm H, Reichenspurner HC, Meinertz T, Heitzer T. Heparins increase endothelial nitric oxide bioavailability by liberating vessel-immobilized myeloperoxidase. Circulation. 2006;113:1871–1878. doi: 10.1161/CIRCULATIONAHA.105.590083. [DOI] [PubMed] [Google Scholar]
- 150.Rao NV, Argyle B, Xu X, Reynolds PR, Walenga JM, Prechel M, Prestwich GD, MacArthur RB, Walters BB, Hoidal JR, Kennedy TP. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin—induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am J Physiol Cell Physiol. 2010;299:C97–C110. doi: 10.1152/ajpcell.00009.2010. [DOI] [PubMed] [Google Scholar]
- 151.Davis AE., III The pathophysiology of hereditary angioedema. Clin Immunol. 2005;114:3–9. doi: 10.1016/j.clim.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 152.Kaplan AP. Enzymatic pathways in the pathogenesis of hereditary angioedema: the role of C1 inhibitor therapy. J Allergy Clin Immunol. 2010;126:918–925. doi: 10.1016/j.jaci.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 153.Antoniu SA. Therapeutic approaches in hereditary angioedema. Clin Rev Allergy Immunol. 2011 doi: 10.1007/s12016-011-8254-2. [DOI] [PubMed] [Google Scholar]
- 154.Petersen SV, Thiel S, Jensen L, Vorup-Jensen T, Koch C, Jensenius JC. Control of the classical and the MBL pathway of complement activation. Mol Immunol. 2000;37:803–811. doi: 10.1016/s0161-5890(01)00004-9. [DOI] [PubMed] [Google Scholar]
- 155.Matsushita M, Thiel S, Jensenius JC, Terai I, Fujita T. Proteolytic activities of two types of mannose-binding lectin-associated serine protease. J Immunol. 2000;165:2637–2642. doi: 10.4049/jimmunol.165.5.2637. [DOI] [PubMed] [Google Scholar]
- 156.Jiang H, Wagner E, Zhang H, Frank MM. Complement 1 inhibitor is a regulator of the alternative complement pathway. J Exp Med. 2001;194:1609–1616. doi: 10.1084/jem.194.11.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jansen PM, Eisele B, de Jang I, Chang A, Delvos U, Taylor FB, Jr, Hack CE. Effect of C1 inhibitor on inflammatory and physiologic response patterns in primates suffering from lethal septic shock. J Immunol. 1998;160:475–484. [PubMed] [Google Scholar]
- 158.Levi M, Lowenberg E, Meijers JC. Recombinant anticoagulant factors for adjunctive treatment of sepsis. Semin Thromb Hemost. 2010;36:550–557. doi: 10.1055/s-0030-1255449. [DOI] [PubMed] [Google Scholar]
- 159.Levi M, van der Tom P. The role of natural anticoagulants in the pathogenesis and management of systemic activation of coagulation and inflammation in critically ill patients. Semin Thromb Hemost. 2008;34:459–468. doi: 10.1055/s-0028-1092876. [DOI] [PubMed] [Google Scholar]
- 160.Crowther MA, Marshall JC. Continuing challenges of sepsis research. JAMA. 2001;286:1894–1896. doi: 10.1001/jama.286.15.1894. [DOI] [PubMed] [Google Scholar]
- 161.Hagiwara S, Iwasaka H, Matsumoto S, Hasegawa A, Yasuda N, Noguchi T. In vivo and in vitro effects of the anticoagulant, thrombomodulin, on the inflammatory response in rodent models. Shock. 2010;33:282–288. doi: 10.1097/SHK.0b013e3181b0ef7b. [DOI] [PubMed] [Google Scholar]
- 162.Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, Hirayama A, Matsuda T, Asakura H, Nakashima M, Aoki N. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost. 2007;5:31–41. doi: 10.1111/j.1538-7836.2006.02267.x. [DOI] [PubMed] [Google Scholar]
- 163.Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, Chalupa P, Atherstone A, Penzes I, Kubler A, Knaub S, Keinecke HO, Heinrichs H, Schindel F, Juers M, Bone RC, Opal SM. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286:1869–1878. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 164.Levi M, Schouten M, van der Tom P. Sepsis, coagulation, and antithrombin: old lessons and new insights. Semin Thromb Hemost. 2008;34:742–746. doi: 10.1055/s-0029-1145256. [DOI] [PubMed] [Google Scholar]
- 165.Opal SM. Therapeutic rationale for antithrombin III in sepsis. Crit Care Med. 2000;28:S34–S37. doi: 10.1097/00003246-200009001-00008. [DOI] [PubMed] [Google Scholar]
- 166.Kienast J, Juers M, Wiedermann CJ, Hoffmann JN, Ostermann H, Strauss R, Keinecke HO, Warren BL, Opal SM. Treatment effects of high-dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J Thromb Haemost. 2006;4:90–97. doi: 10.1111/j.1538-7836.2005.01697.x. [DOI] [PubMed] [Google Scholar]
- 167.Sarangi PP, Lee HW, Kim M. Activated protein C action in inflammation. Br J Haematol. 2010;148:817–833. doi: 10.1111/j.1365-2141.2009.08020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 169.Levi M, Levy M, Williams MD, Douglas I, Artigas A, Antonelli M, Wyncoll D, Janes J, Booth FV, Wang D, Sundin DP, Macias WL. Prophylactic heparin in patients with severe sepsis treated with drotrecogin alfa (activated) Am J Respir Crit Care Med. 2007;176:483–490. doi: 10.1164/rccm.200612-1803OC. [DOI] [PubMed] [Google Scholar]
- 170.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Goring K, Huang Y, Mowat C, Leger C, Lim TH, Zaheer R, Mok D, Tibbles LA, Zygun D, Winston BW. Mechanisms of human complement factor B induction in sepsis and inhibition by activated protein C. Am J Physiol Cell Physiol. 2009;296:C1140–C1150. doi: 10.1152/ajpcell.00071.2009. [DOI] [PubMed] [Google Scholar]
- 172.Mosnier LO, Yang XV, Griffin JH. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions. J Biol Chem. 2007;282:33022–33033. doi: 10.1074/jbc.M705824200. [DOI] [PubMed] [Google Scholar]
- 173.Mosnier LO, Gale AJ, Yegneswaran S, Griffin JH. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood. 2004;104:1740–1744. doi: 10.1182/blood-2004-01-0110. [DOI] [PubMed] [Google Scholar]