Abstract
Objectives:
The onset of multiple sclerosis in the majority of the cases occurs as a clinically isolated syndrome (CIS). We sought to assess serum levels of 25-hydroxyvitamin D (25-OHD) in CIS patients and healthy controls.
Methods:
In this cross-sectional study 40 patients (36 women and 4 men) with CIS manifesting as a single isolated optic neuritis and 40 Age- and sex-matched healthy controls (35 women and 5 men) were enrolled between late October 2010 and early March 2011. General vitamin D deficiency was defined as serum 25-OHD levels of lower than 20 ng/ml and was classified as mild (15 < 25-OHD <20 ng/ml), moderate (8 < 25-OHD <15 ng/ml), and severe (25-OHD <8 ng/ml).
Results:
We found no difference in the median interquartile range [IQR] between CIS patients and controls (17.95 [10.40-29.13] vs. 17.00 [12.25-31.00]; P=0.57). However, when stratified by the levels of deficiency, among CIS patients a significantly higher proportion had severe vitamin D deficiency in comparison to healthy controls (20% vs. 2.5%; P=0.034). Nevertheless, the frequency of general (62.5% vs. 60%, P=0.82), mild (25% vs. 30%, P=0.80), and moderate (17.5% vs. 27.5%, P=0.42) vitamin D deficiency were not different between the two groups.
Conclusions:
Our findings do not indicate any significant difference of serum 25-OHD between CIS patients and healthy controls. However, in our series severe vitamin D deficiency was more frequent among CIS patients.
Keywords: 25-Hydroxyvitamin D, Vitamin D deficiency, optic neuritis, multiple sclerosis, risk factor, prevention, Isfahan, Iran
INTRODUCTION
Multiple sclerosis (MS) is a common cause of disability among young adults. The disease is generally believed to be triggered by a combination of genetic and environmental components, although, its prevention is mostly limited to a control of the latter item. This category generally points toward past infections, smoking, and vitamin D.[1–3] In prospective investigations, an inverse relation was found between MS risk and vitamin D. Serum levels of 25-hydroxyvitamin D (25-OHD) serve as a good indicator of vitamin D intake, whether nutritional or by skin-related synthesis. To date, several lines of evidence support the association of MS and serum 25-OHD.[4–6] With regard to this point, the question that could be raised is, whether vitamin D deficiency is present at the very initial stages of MS. Accordingly, a Finnish survey on the early phase MS cases and healthy controls showed lower serum 25-OHD levels in MS cases in summer.[7] However, we can determine the earlier points for such an examination, that is, when the diagnosis is still not fully established. The onset of MS in 85% of the cases occurs as a ‘clinically isolated syndrome’ (CIS), which is a term for patients who have experienced a solitary clinical event of demyelination, but not fulfilled the diagnostic criteria for MS or any other related disease.[8] Indeed, CIS can be addressed as the earliest stage to assess the relation of vitamin D and clinical MS.
In this cross-sectional study, we sought to assess whether the serum levels of 25-hydroxyvitamin D are different in CIS cases, in comparison to healthy controls. By answering this question, we would have a better insight into the proper time for MS prevention.
METHODS
Study population
Between late October 2010 and early March 2011, we consecutively enrolled 40 patients (four male, 36 female) with CIS and 40 apparently healthy controls (five male, 35 female) into the study.
The general exclusion criteria for both CIS cases and controls were: Any kind of vitamin D dietary supplementation or medication interfering with calcium or vitamin D metabolism, hyperparathyroidism, hyperthyroidism, hypercortisolism, and glucocorticoid treatment whether oral or intravenous, in the preceding 60 days.[4]
Patients with CIS manifesting as a single isolated optic neuritis (SION) were recruited from two main hospitals of the Isfahan Province (the Feiz Eye Hospital and the Al-Zahra Hospital). SION was defined as follows: Patients with acute or subacute vision loss in one eye with unilateral relative afferent pupillary defect and diminished color vision and visual acuity (measured by Ishihara color plates and Snellen chart).[9] These patients were checked to have normal baseline magnetic resonance imaging (MRI). Patients fulfilling the diagnostic criteria for MS[10] after clinical and radiological evaluations, were excluded. We also excluded patients who had any previous demyelinative episode, patients with retinal or macular pathology, and those with accompanying diseases that could potentially cause or mimic SION.
Age- and sex-matched controls were recruited during the same months and concurrently from the same hospitals’ personnel volunteers or their relatives. We particularly excluded healthy controls with a family history of MS in their first-degree relatives.
The study protocol was approved by the Institutional Ethics Committee and all the participants (CIS cases and controls) provided a voluntary signed informed consent before the inclusion in the study.
Background variables
The collected background variables from both CIS cases and controls at baseline were as follows: Age, gender, history of any significant disease (of most importance: Previous neurological symptoms and the disorders that interfered with vitamin D metabolism), diet program, and precise previous medications. Data collection was performed using a questionnaire.
Blood sampling and laboratory features
From both CIS cases and controls, fasting (>8 hours) venous blood samples were collected concurrently. CIS cases, in particular, were sampled, after at least 40 days of the onset of SION symptoms, and of course, after intravenous corticosteroid pulse therapy. The entire process of sampling was performed by a trained phlebotomist. All samples were protected from sunlight throughout the interval (<1 hour in all subjects) between sampling and assessments, which were carried out in common laboratories by the same staff and kits. Serum 25-OHD was evaluated by a commercially available radioimmunoassay kit (DiaSorin, Stillwater, Minnesota, USA).
25-OHD analysis
Overall vitamin D deficiency was defined as serum 25-OHD levels of lower than 20 ng/ml. We determined a local cut-point as 32 ng/ml. According to these limits, all the specimens were categorized into four sub-groups: (i) Lower than local cut-point (20 < 25-OHD <32 ng/ml) (ii) mild vitamin D deficiency (15 < 25-OHD <20 ng/ml), (iii) moderate vitamin D deficiency (8 < 25-OHD <15 ng/ml), and (iv) severe vitamin D deficiency (25-OHD <8 ng/ml).[11,12]
Statistical analysis
Data were analyzed by the Predictive Analytics Software (PASW), version 18.00. Results have been reported as a mean±1SD, median interquartile range [IQR], and number (percent). Due to the distribution of any variable, P values were calculated with Independent Sample T-test, Mann-Whitney or Chi-square. All tests were two-tailed, and a P value of <0.05 was considered as the significance threshold.
RESULTS
Clinically isolated syndrome cases and controls had no differences in females: Male ratio (35:5 vs. 36:4, P=1) and mean age±SD (29.02 ± 5.31 vs. 29.76 ± 4.54, P=0.51). No significant differences (P=0.43) were observed in the median [IQR] of serum levels of vitamin D, between the two groups (17.95(10.4 – 28.75) vs. 17.00(13.5 – 31)). However, when stratified by the levels of deficiency, among CIS patients a significantly higher proportion had severe vitamin D deficiency in comparison to healthy controls (20% vs. 2.5%; P=0.034). Nevertheless, the frequency of general (62.5% vs. 60%, P=0.82), mild (25% vs. 30%, P=0.80), and moderate (17.5% vs. 27.5%, P=0.42) vitamin D deficiency were not different between the two groups. In addition when using the local cut-point to stratify patients vitamin D deficiency was frequent in both groups with no significant differences (82.5% vs. 77.5%, P=0.78). Table 1 summarizes the findings of this study, while Figure 1 illustrates the scatter plot of the two groups for different levels of vitamin D deficiency.
Table 1.
Details of group sex-/age-matching and the comparisons between clinically isolated syndrome cases and healthy controls
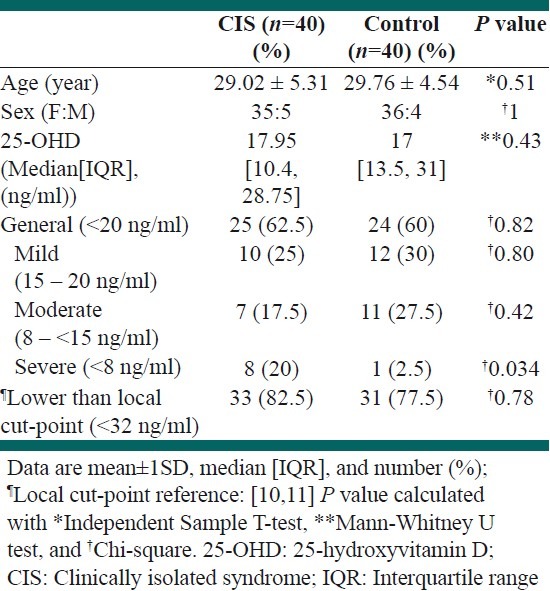
Figure 1.
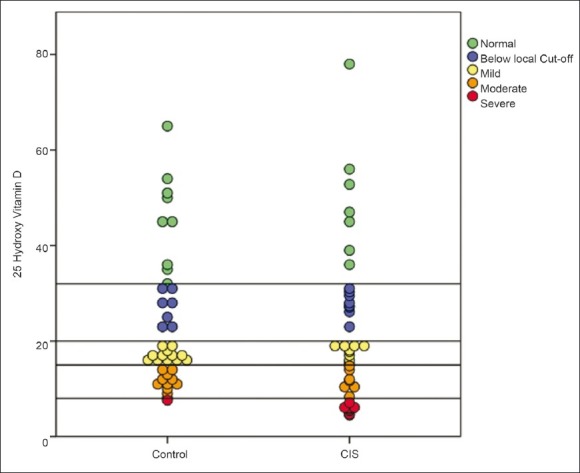
Levels of 25-OHD among the study cohort. Horizontal lines represent different cut-points for 25-OHD levels (ng/ml)
DISCUSSION
In this study, our main goal was to evaluate the status of vitamin D deficiency at the earliest stage of MS manifestation (i.e., CIS) and our findings did not indicate any significant differences in serum levels of 25-OHD between the two groups. However, severe deficiency was more frequent in CIS patients and this finding would be relatively novel. Interestingly, vitamin D deficiency was present in a majority of subjects in both the groups. This could be due to two main facts: First, the study was carried out in winter, when the serum levels of 25-hydroxy vitamin D were known to be lower. Second, vitamin D deficiency was reported to be very commonplace in Isfahan. During recent years, the incidence and the prevalence of MS had dramatically increased in Isfahan and this fact highlighted the importance of preventive trends in this province of Iran.
One of the most important explanations for the cause of such a sharp increase is vitamin D deficiency,[13] although, it cannot be ruled out that in Isfahan, many seemingly healthy people are living with severe vitamin D deficiency and of course they will never develop the disease. Indeed, the etiologic background of MS is complex and vitamin D should interact with many other factors to trigger the disease, among which, the role of genetics is of crucial importance. In other words, MS occurs in genetically susceptible cases that are exposed to other risk factors.[1,2,5,6]
From another point of view, the effectiveness of the environmental risk factors of MS is likely to be limited to a certain period of time. Investigations on the migration of populations showed that immigrants to a place after the age 15 years, present similar prevalence figures to the area of origin, and not the target.[14] We conclude that the role of vitamin D deficiency in the development of MS is distributable to childhood or at least years before the presentation of CIS, and this can explain why we cannot show any significant differences in the median [IQR] of the serum levels of 25-OHD, between the two groups. One of the most important studies that can provide evidence for this explanation is a prospective investigation by Munger et al. in USA. In this study, serum sampling was performed a few years before any clinically detectable neurological disorder.[15] Interestingly, subjects with the highest levels of 25-OHD (99 – 152 nmol/l) were significantly at a lower risk of MS and vice versa (i.e., lowest levels: 15 – 63 nmol/l). We showed that the frequency of severe 25-OHD deficiency was significantly more in CIS cases. This finding was almost compatible with the previous results, although, not completely parallel. Based on the results of the Munger et al. study, it was postulated that 70% of the disease incidence was preventable only if the 25-OHD serum levels exceeded 100 nmol/L.[6,15]
Our present experience is the first cross-sectional survey about vitamin D that exclusively focuses on CIS patients. To date, only one study by Mowry et al. has included CIS cases in a prospective design, although, these cases have been concurrently sampled with pediatric-onset MS patients.[16] Notably, there are some limitations in our study. Of importance, we mention the seasonal implementation that imposed the role of winter on the status of 25-OHD.
Regarding the current status of MS in Isfahan and the promising abilities of vitamin D,[13,17,18] it seems mandatory to plan a screening program for individuals who are at the highest risks. Generally, females between 20 and 40 years of age should be considered as first-line candidates. Also particular candidates are: (i) Cases of radiologically isolated syndrome, (ii) first-degree relatives of MS patients, and (iii) young adults with infectious mononucleosis in their recent history.[5] Moreover, it seems that the proper time for MS prevention is related to the years before CIS, namely, at ages of early high school or even earlier. Thus, in an effective perspective, such a trend is more suitable in Isfahan when applied to lower ages. If we assume that our evaluation is unbiased; CIS might be the subject of intervention by supplement of adequate 25-OHD, especially, in cases with severe deficiency. However, this needs more prospective confirmatory results.
ACKNOWLEDGMENT
We are very grateful to individuals who made this project possible, for their patience and their precious contribution. We particularly salute the endeavors of Dr. Afsane Khandan (Internist) who devoted her life to health development and medical research. The results of this study are dedicated to MS patients who bravely cope with the illness.
Footnotes
Source of Support: This study was funded by the Isfahan Multiple Sclerosis Society (IMSS) as a private health caring sector and the Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Milo R, Kahana E. Multiple sclerosis: Geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9:A387–94. doi: 10.1016/j.autrev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. Multiple sclerosis: Risk factors, prodromes, and potential causal pathways. Lancet Neurol. 2010;9:727–39. doi: 10.1016/S1474-4422(10)70094-6. [DOI] [PubMed] [Google Scholar]
- 3.Ascherio A, Munger K. Epidemiology of multiple sclerosis: From risk factors to prevention. Semin Neurol. 2008;28:17–28. doi: 10.1055/s-2007-1019126. [DOI] [PubMed] [Google Scholar]
- 4.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Pierrot-Deseilligny C, Souberbielle JC. Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis? Brain. 2010;133:1869–88. doi: 10.1093/brain/awq147. [DOI] [PubMed] [Google Scholar]
- 6.Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol. 2010;9:599–612. doi: 10.1016/S1474-4422(10)70086-7. [DOI] [PubMed] [Google Scholar]
- 7.Soilu-Hänninen M, Airas L, Mononen I, Heikkilä A, Viljanen M, Hänninen A. 25-Hydroxyvitamin D levels in serum at the onset of multiple sclerosis. Mult Scler. 2005;11:266–71. doi: 10.1191/1352458505ms1157oa. [DOI] [PubMed] [Google Scholar]
- 8.Miller D, Barkhof F, Montalban X, Thompson A, Filippi M. Clinically isolated syndromes suggestive of multiple sclerosis, part I: Natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol. 2005;4:281–8. doi: 10.1016/S1474-4422(05)70071-5. [DOI] [PubMed] [Google Scholar]
- 9.Hickman SJ, Dalton CM, Miller DH, Plant GT. Management of acute optic neuritis. Lancet. 2002;360:1953–62. doi: 10.1016/s0140-6736(02)11919-2. [DOI] [PubMed] [Google Scholar]
- 10.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 11.Hickey L, Gordon CM. Vitamin D deficiency: New perspectives on an old disease. Curr Opin Endocrinol Diabetes. 2004;11:18–25. [Google Scholar]
- 12.Moussavi M, Heidarpour R, Aminorroaya A, Pournaghshband Z, Amini M. Prevalence of Vitamin D Deficiency in Isfahani High School Students in 2004. Horm Res. 2005;64:144–8. doi: 10.1159/000088588. [DOI] [PubMed] [Google Scholar]
- 13.Etemadifar M, Maghzi AH. Sharp increase in the incidence and prevalence of multiple sclerosis in Isfahan, Iran. Mult Scler. 2011;17:1022–7. doi: 10.1177/1352458511401460. [DOI] [PubMed] [Google Scholar]
- 14.Detels R, Visscher BR, Haile RW, Malmgren RM, Dudley JP, Coulson AH. Multiple sclerosis and age at migration. Am J Epidemiol. 1978;108:386–93. doi: 10.1093/oxfordjournals.aje.a112636. [DOI] [PubMed] [Google Scholar]
- 15.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 16.Mowry EM, Krupp LB, Milazzo M, Chabas D, Strober JB, Belman AL, et al. Vitamin D status is associated with relapse rate in pediatric-onset MS. Ann Neurol. 2010;67:618–24. doi: 10.1002/ana.21972. [DOI] [PubMed] [Google Scholar]
- 17.Prentice A. Vitamin D deficiency: A global perspective. Nutr Rev. 2008;66:S153–64. doi: 10.1111/j.1753-4887.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF, Chen TC. Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]


