Abstract
Background:
Haemophilus Influenzae type b (Hib) is an important cause of morbidity and mortality in children. Although its burden is considerably preventable by vaccine, routine vaccination against Hib has not been defined in the National Immunization Program of Iran. This study was performed to assess the cost-benefit and cost-utility of running an Hib vaccination program in Iran.
Methods:
Based on a previous systematic review and meta-analysis for vaccine efficacy, we estimated the averted DALYs (Disability adjusted life years) and cost-benefit of vaccination. Different acute invasive forms of Hib infection and the permanent sequels were considered for estimating the attributed DALYs. We used a societal perspective for economic evaluation and included both direct and indirect costs of alternative options about vaccination. An annual discount rate of 3% and standard age-weighting were used for estimation. To assess the robustness of the results, a sensitivity analysis was performed.
Results:
The incidence of Hib infection was estimated 43.0 per 100000, which can be reduced to 6.7 by vaccination. Total costs of vaccination were estimated at US$ 15,538,129. Routine vaccination of the 2008 birth cohort would prevent 4079 DALYs at a cost per averted-DALY of US$ 4535. If we consider parents’ loss of income and future productivity loss of children, it would save US$ 8,991,141, with a benefit-cost ratio of 2.14 in the base-case analysis. Sensitivity analysis showed a range of 0.78 to 3.14 for benefit-to-cost ratios.
Conclusion:
Considering costs per averted DALY, vaccination against Hib is a cost-effective health intervention in Iran, and allocating resources for routine vaccination against Hib seems logical.
Keywords: Cost-benefit analysis, cost-utility analysis, Haemophilus Influenzae
INTRODUCTION
Invasive Haemophilus Influenzae type b (Hib) is an important cause of meningitis and pneumonia in children. It is estimated that at least three million serious cases of disease worldwide are caused by Hib each year, with an annual mortality of 380,000 to 700,000 in under-five-year-old children.[1–4]
Although safe and effective vaccines against Hib are available they are under-utilized in the developing countries, where a vast majority of Hib deaths occur. Among the complications of Hib infection, pneumonia accounts for a larger number of deaths than meningitis. However, Hib meningitis is also a serious problem in the developing countries with a higher mortality rate; it leaves 15 to 35% of the survivors with permanent disabilities, such as, mental retardation or deafness.[1–4]
There were some systematic reviews for the assessment of Hib conjugate vaccine efficacy.[5,6] We updated these reviews using a highly sensitive search, according to the instructions of Cochrane,[6,7] in another study. Nine eligible articles were enrolled in the updated systematic review and the pooled vaccine efficacy was estimated to be 84% (95% CI: 69–92%),[8] which was very similar to the previous estimations.
Routine use of Hib conjugate vaccine has virtually eliminated Hib in industrialized countries,[1–6] however, introduction of Hib conjugate vaccine in developing countries has progressed more slowly, because of its relatively high price.[1,3]
The World Health Organization (WHO) estimates that in the developed countries, 92% of the eligible population is vaccinated against Hib; however, the average coverage is 42% in developing countries.[3]
Although the Eastern Mediterranean (EMR) is a diverse region, studies show a moderate-to-high burden of Hib disease.[1,2,4] About half of the countries in the EMR have introduced Hib into their National Immunization Program; in Iran it has not been entered to the program yet.[4]
We performed this study to assess the costs of Hib vaccination and its benefits and to compare the strategy of routine Hib vaccination of all infants with a non-vaccination program from the societal point of view.
METHODS
In this economic evaluation of the vaccination program, we performed both cost-benefit and cost-utility analyses. We estimated the net benefits, benefit-cost ratio, and costs per averted Disability-adjusted life years (DALYs).
A model was developed to follow the 1387 A.H. birth cohort (21 March, 2008 to 20 March, 2009) under two scenarios: (1) Performing vaccination against Hib in three doses, as a routine immunization program, and (2) Not-performing vaccination against Hib.
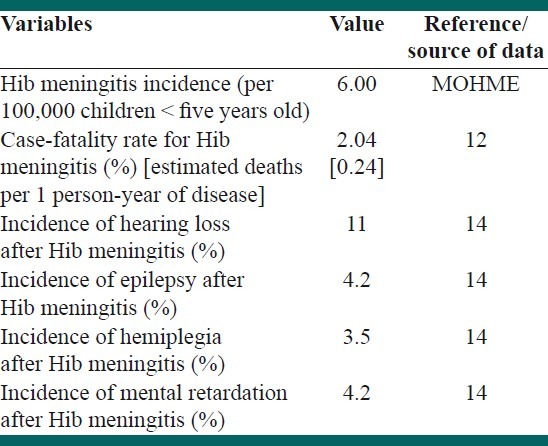
We used the same methodology used in the burden of disease study in Iran to estimate utilities (averted DALYs) of the vaccination;[9] The state of health of the birth cohort regarding the Hib disease was modeled using the DisMod-II software (version 1.05, World Health Organization, 2001-9). Table 1 shows the main input data for disease modeling.
Table 1.
Summary of the variable used to estimate the epidemiological indices of Haemophilus influenza type b (Hib) in Iran-input data of the model
Direct and indirect costs were estimated for both scenarios using a societal view; we estimated direct medical costs for both acute care and long-term care of patients with permanent sequels. Non-medical direct costs, such as, the work-absence of parents and indirect costs such as the costs of productivity loss were estimated. All costs were inflated 5% per year, and all costs and benefits in the future were discounted at a 3% annual rate, for the base case analysis in the reference year.
We used a decision tree diagram for economic evaluations [Figure 1]. We assumed a national coverage of 95% for vaccination in the birth cohort.
Figure 1.
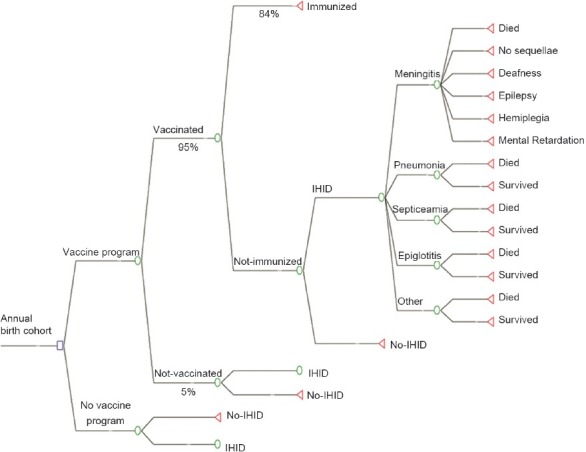
The decision tree used for the economic analysis of vaccination against Hib in Iranian children (IHID: Invasive Hib Disease)
Data on the incidence of invasive Hib meningitis was obtained from a prospective 2.5-year pilot surveillance undertaken in five provinces of Iran from September 2003 to February 2006 (the data was collected by personal communication with experts in the Ministry of Health and Medical Education (MOHME)). There was limited data about the incidence of other types of invasive Hib diseases, so we used the ratio of meningitis to pneumonia cases, to estimate the incidence of Hib pneumonia. This strategy has been suggested in the international guidelines.[10–12] A ratio of five Hib pneumonia cases to one Hib meningitis was considered based on the previous studies.[12]
Proportional frequencies of the other complications of Hib were obtained from the literature[1,13] and their incidence rates were estimated based on the incidence of Hib meningitis data in Iran. The case-fatality rates for meningitis and pneumonia were considered 2.05 and 6.02%, respectively, based on the estimations made by Feikin et al.[12] Our estimate of pneumonia incidence by this method was compatible with that estimated by the WHO child health epidemiology reference group.[14]
We considered possibilities of long-term consequences of Hib meningitis, including mental retardation, hearing loss, epilepsy, and spasticity/hemiplegia in our model. The probabilities of these outcomes were obtained from a meta-analysis that reported the proportional frequencies of deafness as 11%, epilepsy as 4.20%, mental retardation as 4.20%, spasticity/hemiplegia as 3.50%, and no-sequels in 78% of survivors.[15]
We applied the estimations of incidence and mortality rates to the entire birth cohort of -2008, to drive at the expected number of cases that would occur in the absence of a Hib vaccination program.
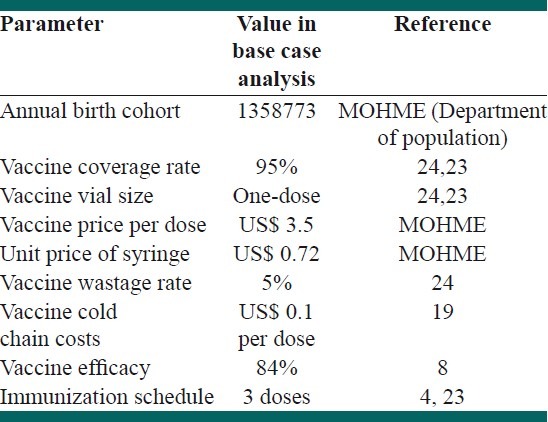
Table 2 shows the parameters used in economic evaluation. Direct health care and direct non-medical and indirect costs were estimated from the point of view of society. Direct health care costs included costs of acute care of invasive Hib diseases and additional life-long costs for management of sequels after Hib meningitis. The costs of outpatient and inpatient care were included.
Table 2.
Parameters used for economic evaluation of Hib vaccination in the base case analysis
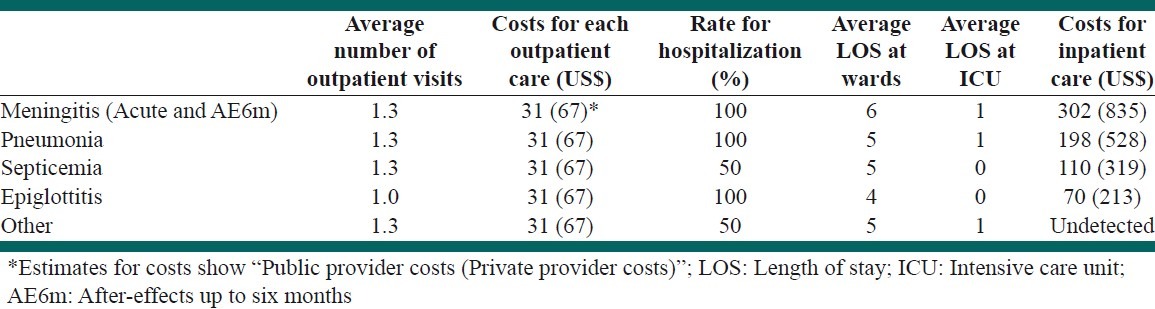
To calculate the medical costs of acute care, we considered frequency and average costs of outpatient visits, average duration of hospitalization, bed-day costs of services in the pediatric and intensive-care units (ICU) of the hospitals (estimated for hospitals of private and public sectors separately) and other costs, including drugs, medical supplies, and paraclinical examination tests. We used both the tariffs of the MOHME and the data extracted from a private and a public hospital.[16] To estimate the costs for the national level, we used the ratio of public/private service usage from the study of healthcare utilization in Iran.[9] So, we assumed that the proportion of people who received their inpatient and outpatient services from public health service providers were 84.0 and 67.7%, respectively.[9] The average costs of acute care in public and private hospitals are given in Table 3.
Table 3.
Costs of acute care of Hib sequels and their assumptions
Finally, the total cost for acute care was estimated for the birth cohort of 2008.
Other direct costs included costs for long-term care for patients with permanent sequels (deafness, spasticity/hemiplegia, epilepsy, and mental retardation); these people required additional care including consultations with specialists, special education, hearing-aid devices, and tests such as computer tomography (CT) and magnetic resonance imaging (MRI).
Average annual costs for special education (50 sessions per year) per case of deafness was estimated to be 1206 US$ for the first two years. The annual treatment costs for the first two years, for cases of epilepsy were estimated to be US$ 224.
The annual costs of long-term care and special education for individuals with mental retardation and hemiplegia were estimated to be US$ 2434 and US$ 2181, respectively. We estimated the costs of rehabilitative services based on the data collected by personal communication with experts in the National State Welfare Organization.
According to these data, the average costs of rehabilitative services are much higher during the few years after the incidence of sequel, rather than the following years, due to less number of medical investigations and therapies needed in the following years.
For children with deafness a cost of US$ 333 per hearing-aid was included; each patient would need to replace the hearing-aid every five years for 60 years.
Total costs were inflated by 5% (to have a conservative estimation, we did not use high inflation rates of the recent years in Iran) and discounted 3% annually.
We estimated the productivity loss (for both lives lost prematurely and permanent disability), the indirect costs associated with parents who had to miss work and stay at home to care for sick children.
To estimate the monetary costs of parent missing work due to acute care, we considered the length of care for sick children. Those were 38 days for meningitis, 19 days for pneumonia, 15 days for epiglottitis, and 30 days for septicemia.[17,18]
For children with permanent sequels or premature death, we estimated the loss of income for ages between 20 and (average age of getting first job) 65 years. The disability weights were used to calculate loss of potential income for children with sequels.
To estimate the economic value of productivity, the human capital method was used.[17–22]
An individual's monetary value of life was considered as his or her future average production potential. The present value of the future years was calculated using an annual discount rate of 3%. The proportion of economically active population was considered to be 50% for calculation. The required data were extracted from the data provided by the World Bank[23] and the Central Bank of Iran.
Costs of Hib vaccine delivery were calculated according to the WHO guidelines for estimating costs of new vaccines.[4,24] A 10% increase in income was considered for every five years; the estimate for per capita income for year 2008 was $ 10840, based on the purchasing power parity.[23]
We assumed that the Hib vaccines will be administered by the public healthcare providers, similar to the other immunization programs in our country. There are several formulations of Hib vaccine such as Hib vaccine combined with DTP (Diphtheria-Tetanus-Pertussis), Hepatitis-B vaccine or polio vaccine.[24] However, in the present study, we considered the Hib monovalent vaccine in the three-dose schedule, concurrent with DTP vaccination schedule. The number of immunizations needed with Hib conjugate vaccine was three to four.[1,4,24]
The coverage rates were considered to be 95% for each dose. We assumed that the Hib vaccine Monovalant would be to introduce in a one-dose vial, which would result in a low vaccine wastage rate.[25]
Items that were considered in estimating the costs of the immunization program included the vaccine price, maintaining cold chain, equipment of injection, and treatment of adverse reactions.[4,24] On account of the concurrent provision of Hib vaccine with routine DTP, we did not consider the opportunity costs for vaccinators or the costs for the time dedicated by the caregivers to the vaccination. Also, transportation of children and caregivers to health facility centers would not involve any additional costs.
We considered the cost of maintaining a cold chain as US$ 0.1 per dose of Hib vaccine, similar to the other programs.[20]
The parameters for estimating the costs and efficacy of Hib vaccination are summarized in Table 2.
However, if Hib immunization is adopted as one of the routine immunizations and administrated in public clinics, the costs would decrease.
The Hib immunization schedule is the same as the DTP immunization schedule, which can decrease the administrative cost. It has been considered as a 30 – 50% reduction of administrative costs in most countries.[17–22] We assumed a reduction of 40% in our study, for administrative costs.
Conjugate vaccines have been found to be generally well-tolerated.[5,6,8]
Adverse events associated with it do not occur in a significantly higher proportion compared to other routine vaccines that were administered at the same time.[5,6,8]
Therefore, the associated costs were not included.
Disability-adjusted life years: We estimated that DALYs attributed to Hib infection in Iran for two alternative scenarios; incidence rate, case-fatality, and remission rate for meningitis, pneumonia, epiglottitis, and septicemia were used as the input data of the model and the output of DisMod (Incidence, duration of disease, average age at onset of disease, and mortality rate) were used to calculate the years of lives lost (YLL) and years lived with disability (YLD). Then we added the YLL and YLD to calculate the DALYs. We considered a 3% discount rate and age-weightings for DALY calculations. Disability weights were equivalent to the global burden of disease study or Dutch weights.[26,27] We used the life expectancy data reported by MOHME for Iran, for comparison. Costs per averted DALYs were estimated as a measure of utility.
RESULTS
Introduction of Hib vaccine would reduce the estimated incidence of HIB disease from 43.0 to 6.7 per 100,000 under-five-year-old children, in Iran.
Our model predicted that Hib vaccination in the 2008 birth cohort would prevent 2301 new cases of Hib disease and 113 related deaths. Frequencies of different outcomes of Hib are seen in Table 4. The intervention would prevent 4079 DALYs, which is the difference between DALYs attributed to Hib disease under the two scenarios.
Table 4.
Frequency of different outcomes of Haemophilus influenzae type b in 2008 birth cohort of Iran with and without vaccination
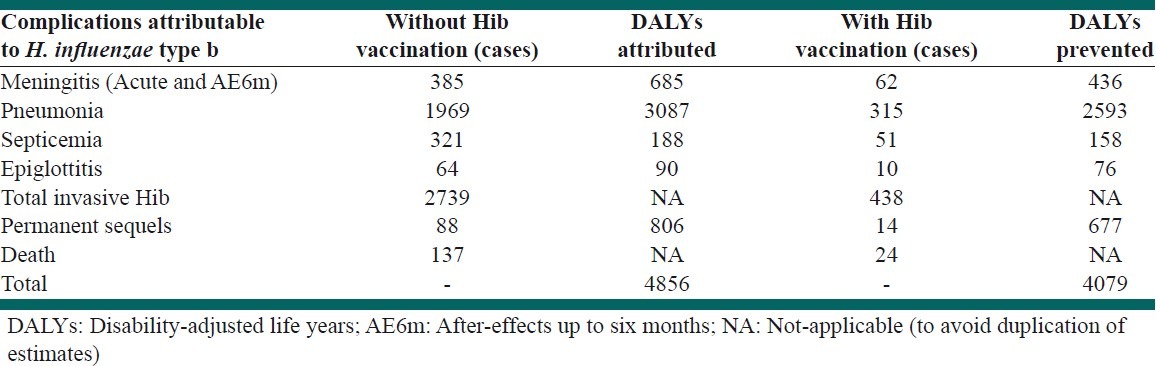
Table 5 shows the estimated costs and expected benefits of the vaccination program. We estimated that the Hib vaccination program spends US$ 6754 to prevent one invasive Hib case, US$ 136,300 to prevent one Hib-related death, and US$ 4535 per averted-DALY.
Table 5.
Estimated costs and expected benefits of Hib vaccination of 2008 birth cohort in Iran
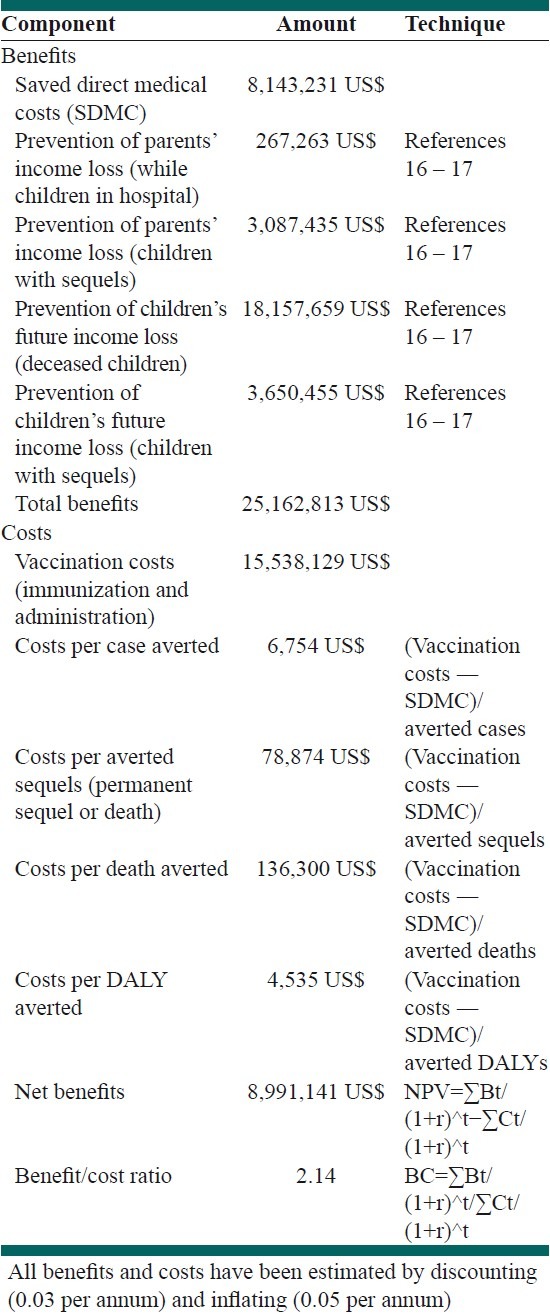
The HIB vaccination program would reduce direct costs of Hib disease acute care from US$ 9,694,322 to US$ 1,551,091 [Table 5]. Considering the monetary values of life by the human capital method, the program would net save net present value (NPV) US $ 8,991,141. The Benefit-cost ratio would be 2.14 for the Hib vaccination program.
We undertook several one-way and two-way sensitivity analyses to evaluate the robustness of the findings, to make changes in the assumptions. We changed the incidence rate of meningitis and the discount rate and case fatality rate of pneumonia [Table 6].
Table 6.
Sensitivity/uncertainty analysis
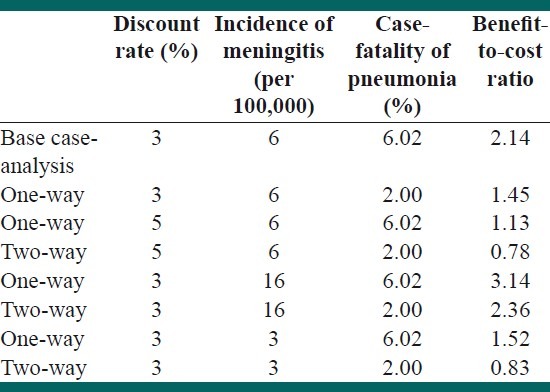
We re-analyzed the data assuming two different conditions for the Hib meningitis incidence rate: Half of the primary estimation (3.0 per 100000) and minimum estimated incidence for the eastern Mediterranean region (EMR), which was 16.0 per 100,000 (more than 2.5 times the estimation for Iran). For the discount rate, we used a 5% rate instead of 3%. As the estimates of the pneumonia cases, primarily based on a study on HIB meningitis cases, and considering that the CFR of pneumonia in other countries was usually lower than meningitis CFR, we performed a sensitivity analysis with a pneumonia CFR of 2%. The range of benefit to cost ratio was between 0.78 and 3.14.
DISCUSSION
Making decisions for the introduction of Hib to the National Immunization Program is mainly a consideration of “costs and administration” not “efficacy and safety”.[28,29] Results of the current study showed that introduction of Hib vaccination in Iran could reduce the total cost to society. We found that the ot per averted-DALY was about US$ 4535. The WHO considers an intervention as cost-effective when the cost per discounted DALY averted is one to three times the gross domestic product per-capita;[30] so, we could consider Hib vaccination as a cost-effective intervention in the situation of Iran.
On the other hand, the benefit-cost ratio of this intervention seems favorable (>1) and would be accompanied with a positive economic benefit for the society.[17]
The sensitivity analysis showed that under special circumstances (such as low CFR of pneumonia), this ratio could be lower than 1. Unfortunately, there was no official national system for health technology assessment. There was little information about the economic evaluation of other health interventions in Iran, so we could not compare Hib vaccination with other interventions, to decide the resource allocation.
We used some conservative assumptions; if we ignored these assumptions, the situation would be pro-vaccination. We did not consider herd-immunity in the analysis as in the previous studies; however, it would lead to decreased costs of the vaccination program and increased benefit-cost ratio. Also the estimated vaccine efficacy in this study was lower than the estimates in the previous studies, which were generally higher than 90%.
We considered three doses of vaccination based on the recommendations of the National Expert Committee. In a four-dose scenario, the benefit-to-cost ratio would be decreased.
Pneumonia is the most common type of Hib disease in developing countries.[1,2,17–22]
In our study, the largest part of the Hib burden was due to pneumonia (3087 DALYs). Findings from Hib vaccine trials have explained reduction in radiologically proven pneumonia cases.[1,10–12]
The incidence of Hib disease is a major determinant of the economic burden of the disease. We obtained incidence of Hib meningitis from five states not at the national level. Also, the estimates for other types of Hib invasive diseases and sequels of meningitis were extrapolated from the studies in other settings; that was the most important limitation in this study.
Iran is one of the countries in the Eastern Mediterranean. The WHO reported Hib disease as “a serious threat for children globally and in the Eastern Mediterranean Region” (EMR).[1,2,31] The Hib meningitis incidence in the middle east is estimated to be 16–31 per 100,000 children under five years, which is higher than our estimate for Iran.[31] About half of the countries in the EMR have introduced Hib vaccine into their routine immunization. The WHO reports that the efficacy of the Hib vaccine in Bahrain, Qatar, Kuwait, Saudi Arabia, and United Arab Emirates has been confirmed.[31]
If we consider the potential productivity loss of cases of Hib, the benefit-to-cost ratio of vaccination against Haemophilus influenzae would be 2.14. Program costs per averted DALY were estimated to be US$ 4535, which could be categorized as a cost-effective health intervention. We conclude that adding this intervention to our routine national immunization program is a logical decision, based on the current best available evidence.
ACKNOWLEDGMENT
This article is part of the results of a doctorate thesis of the Community Medicine Residency Program of the Iran University of Medical Sciences. The authors acknowledge the Council of Research of the Medical Faculty for approving the proposal. We are thankful to our colleagues in the Ministry of Health and Medical Education (deputy for health), Office of Immunization Programs, and the Social Welfare Organization for providing the input data for our model.
Footnotes
Source of Support: Grant number: 703P-1388-IUMS
Conflict of Interest: None declared.
REFERENCES
- 1.Peltola H. Worldwide Haemophilus Influenza type b disease at the beginning of the 21st century: Global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13:302–17. doi: 10.1128/cmr.13.2.302-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lolekha S, Cooksley G, Chan V, Isahak I, Ismael S, John J, et al. Steering Committee for Prevention and Control of Infectious Diseases in Asia.A review of Hib epidemiology in Asia. Southeast Asian J Trop Med Public Health. 2000;31:650–7. [PubMed] [Google Scholar]
- 3.Morris SK, Moss WJ, Halsey N. Haemophilus Influenzae type b conjugate vaccine use and effectiveness. Lancet. 2008;8:435–43. doi: 10.1016/S1473-3099(08)70152-X. [DOI] [PubMed] [Google Scholar]
- 4.The Department of Vaccines and Biologicals. Geneva: WHO; 2002. World Health Organization: Estimating the potential cost-effectiveness of using Haemophilus influenza e HIB Vaccine. [Google Scholar]
- 5.Obonyo CO, Lau J. Efficacy of Haemophilus Influenzae type b vaccination of children: A meta-analysis. Eur J Clin Microbial Infec Dis. 2006;25:90–7. doi: 10.1007/s10096-006-0092-4. [DOI] [PubMed] [Google Scholar]
- 6.Swingler G, Fransman D, Hussey G. Conjugate vaccines for preventing Haemophilus Influenza type b infections. Cochrane Database Syst Rev. 2007;(2):CD001729. doi: 10.1002/14651858.CD001729.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Green S, editors. The Cochrane Collaboration. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane handbook for systematic Reviews of interventions 4.2.5, [Web document] 2005. [Last accessed on 2010 Mar 26]. Available from: http://www.cochrane.org/resources/handbook/hbook.htm .
- 8.Shakerian S, Moradi-Lakeh M, Esteghamati A. Vaccine Efficacy against Haemophilus Influenzae type b in under 5 children: A Systematic Review and meta-analysis. J Isfahan Med School. 2010;28:437–8. [Google Scholar]
- 9.Naghavi M, Abolhassani F, Pourmalek F, Moradi Lakeh M, Jafari N, Vaseghi S, et al. Burden of disease and injuries in Iran 2003. Population Health Metrics. 2009;7:9. doi: 10.1186/1478-7954-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levien OS, Lagos R, Munoz A, Villaroel J, Alvares AM, Abrego P, et al. Defining the burden of pneumonia in children preventable by vaccination against Haemophilus Influenzae type b. Pediatr infect dis J. 1999;18:106–4. doi: 10.1097/00006454-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Mulholland K, Levine O, Nohynek H, Greenwood BM. Evaluation of vaccines for the prevention of pneumonia in children in developing countries. Epidemiol Rev. 1999;21:43–55. doi: 10.1093/oxfordjournals.epirev.a017987. [DOI] [PubMed] [Google Scholar]
- 12.Feikin DR, Nelson CB, Watt JP, Mohseni E, Wenger JD, Levine OS. Rapid assessment tool for Haemophilus Influenzae type b disease in developing countries. Emerg Infect Dis. 2004;10:1270–6. doi: 10.3201/eid1007.030737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.State government of Victoria, Australia, Department of Health: Health status of Victorians. [Last accessed on 2010 Mar 26]. Available from: http://www.health.vic.gov.au/healthstatus/bodvic/bod_current.htm#daly .
- 14.Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ. 2004;82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 15.Baraff LJ, Lee SI, Schriger DL. Outcome of bacterial meningitis in children; A meta-an alysis. Pediatr Infect Dis J. 1993;12:389–94. doi: 10.1097/00006454-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Abolhassani M. Tariffs of diagnostic and therapeutic services in governmental and non-governmental sectors.Office of policy-making and control of insurance services. Tehran: Ministry of Health and Medical Education; 2008. [Google Scholar]
- 17.Zhou F, Bisgard KM, Yusuf HR, Deuson RR, Bath SK, Murphy TV. Impact of universal Haemophilus Influenzae type b vaccination starting at 2 months of age in the United States: An economic analysis. Pediatrics. 2002;110:653–61. doi: 10.1542/peds.110.4.653. [DOI] [PubMed] [Google Scholar]
- 18.Shin S, Shin Y, Ki M. Cost-Benefit analysis of Haemophilus influenzae Type b immunization in Korea. J Korean Med Sci. 2008;23:176–84. doi: 10.3346/jkms.2008.23.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broughton EI. Economic evaluation of Haemophilus influenzae type b vaccination in Indonesia: A cost-effectiveness analysis. J Public Health. 2007;29:441–8. doi: 10.1093/pubmed/fdm055. [DOI] [PubMed] [Google Scholar]
- 20.Platonov AE, Griffiths UK, Voeykova MV, Platonova OV, Shakhanina IL, Chistyakova GG, et al. Economic evaluation of Haemophilus Influenzae type b vaccination in Moscow, Russian Federation. Vaccine. 2006;24:2367–76. doi: 10.1016/j.vaccine.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 21.Akumu AO, English M, Scott JA, Griffiths UK. Economic evaluation of delivering Haemophilus influenzae type b vaccine in routine immunization services in Kenya. Bull World Health Organ. 2007;85:511–8. doi: 10.2471/BLT.06.034686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pokorn M, Kopac S, Neubauer D, Cizman M. Economic evaluation of Haemophilus influenzae type b vaccination in Slovenia. Vaccine. 2001;19:3600–5. doi: 10.1016/s0264-410x(01)00071-8. [DOI] [PubMed] [Google Scholar]
- 23.World development indicators database: Gross national income per capita 2008, Atlas method and PPP. [Last accessed on 2010 Mar 26];World Bank. 2009 available from: http://siteresources.worldbank.org/DATASTATISTICS/Resources/GNIPC.pdf . [Google Scholar]
- 24.The Department of Vaccines and Biologicals. Geneva: World Heath Organization; 2002. World Heath Organization: Guidelines for estimating costs of introduction new vaccines into the national immunization system. [Google Scholar]
- 25.Drain PK, Nelson CM, Lloyd JS. Single-dose versus multi-dose vaccine vials for immunization programs in developing countries. Bull World Health Organ. 2003;81:726–31. [PMC free article] [PubMed] [Google Scholar]
- 26.Murray CJ, Lopez AD. The global burden of disease: A comprehensive assessment of mortality and disability from disease, injury and risk factors in 1990 and projected to 2020. Cambridge, MA: Harvard School of Public Health (Global burden of disease and injury series); 1996. [Google Scholar]
- 27.Stouthard ME, Essink-Bot ML, Bonsel GJ. Disability weights for diseases; A modified protocol and results for a Western European region. Eur J Public Health. 2000;10:24–30. [Google Scholar]
- 28.World Health Organization: WHO position paper on Haemophilus Influenzae type b conjugate vaccines. (Replaces WHO position paper on Hib vaccines previously published in the Weekly epidemiological record. Wkly Epidemiol Rec. 2006;81:445–52. [PubMed] [Google Scholar]
- 29.Lau YL. Haemophilus Influenzae type b diseases in Asia. Bull World Health Organ. 1999;77:867–8. [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization: Threshold values for intervention cost-effectiveness by Region. WHO-CHOICE. [Last accessed on 2010 Mar 26]. Available from: http://www.who.int/choice/costs/CER_levels/en .
- 31.The HIB initiative: Fact sheet: Haemophilus influenzae type b (Hib) in the Eastern Mediterranean Region (EMR) [Last accessed on 2010 Mar 26]. Available from: http://www.Hibaction.org/resources/HibinEMRO.pdf .


